Radiotherapy with Helium Ions Has the Potential to Improve Both Endocrine and Neurocognitive Outcome in Pediatric Patients with Ependymoma
Abstract
:Simple Summary
Abstract
1. Introduction
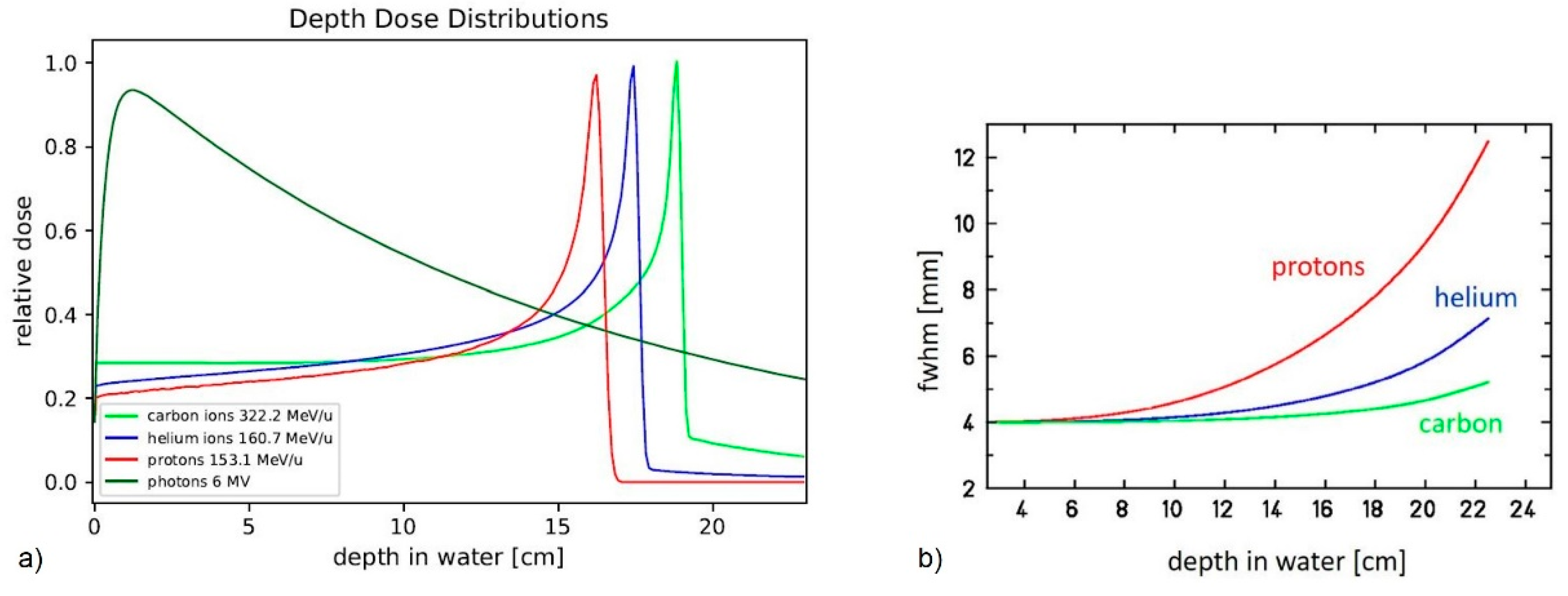
2. Materials and Methods
3. Results
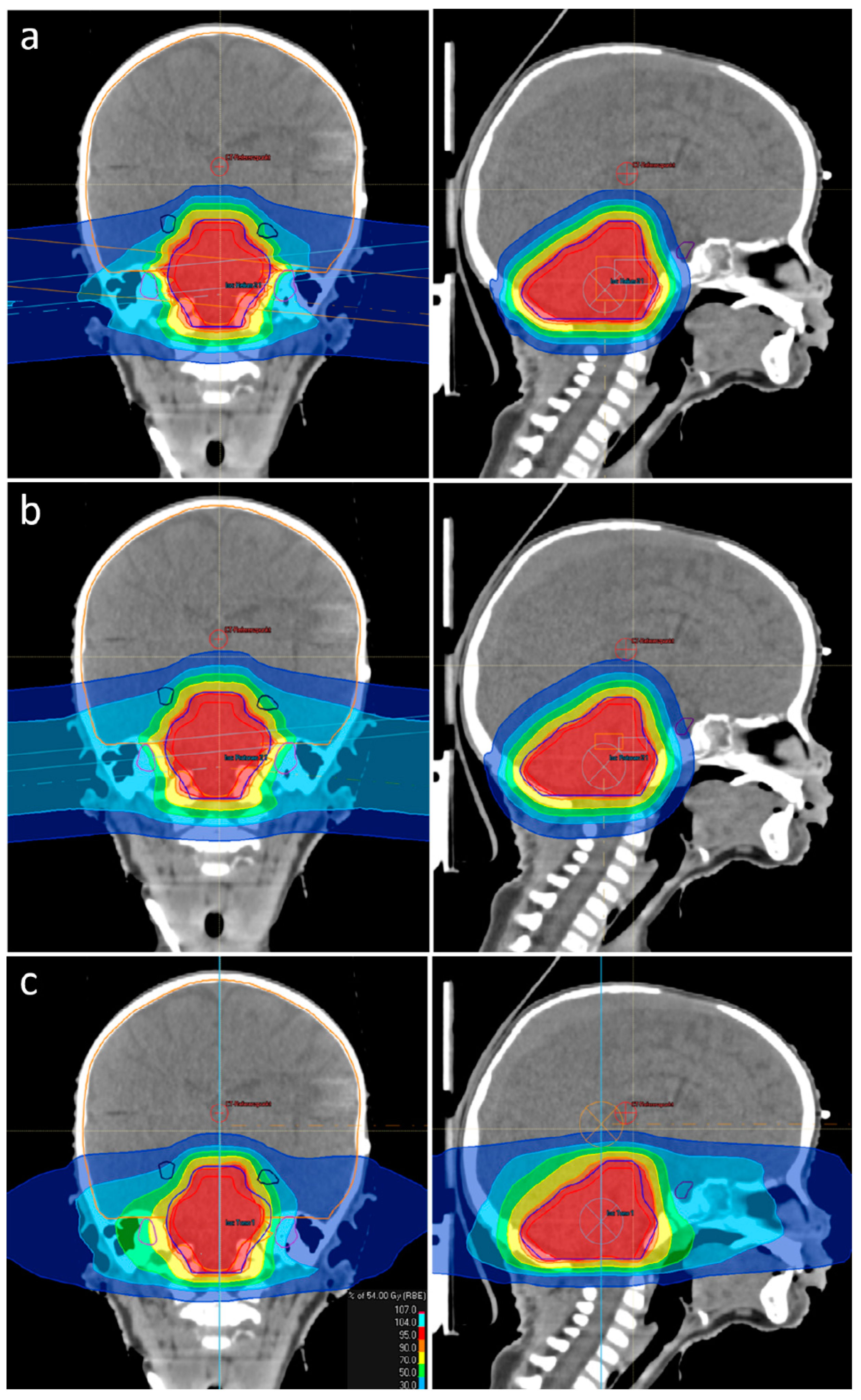
3.1. CTV Coverage
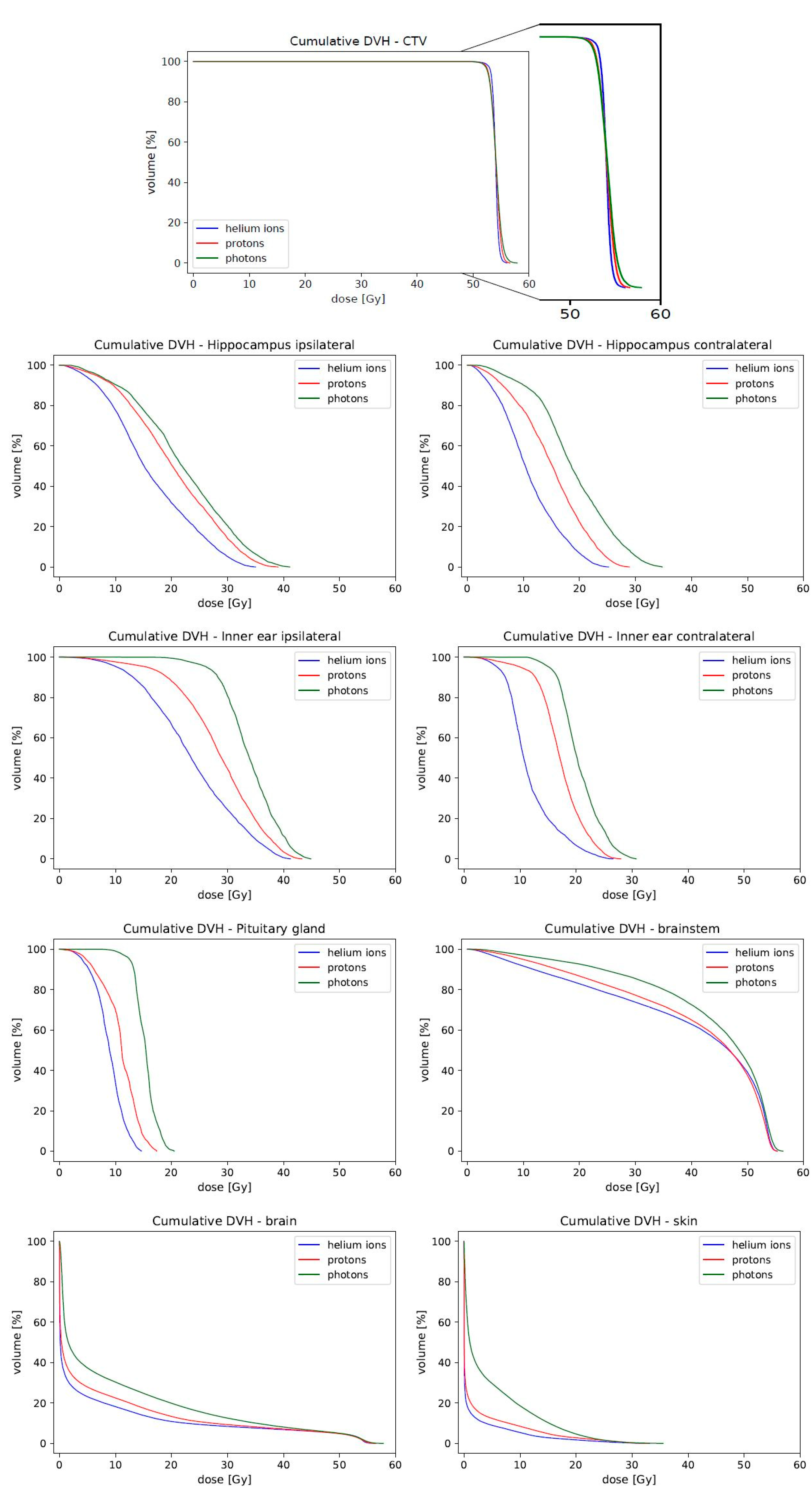
3.2. Sparing Organs at Risk
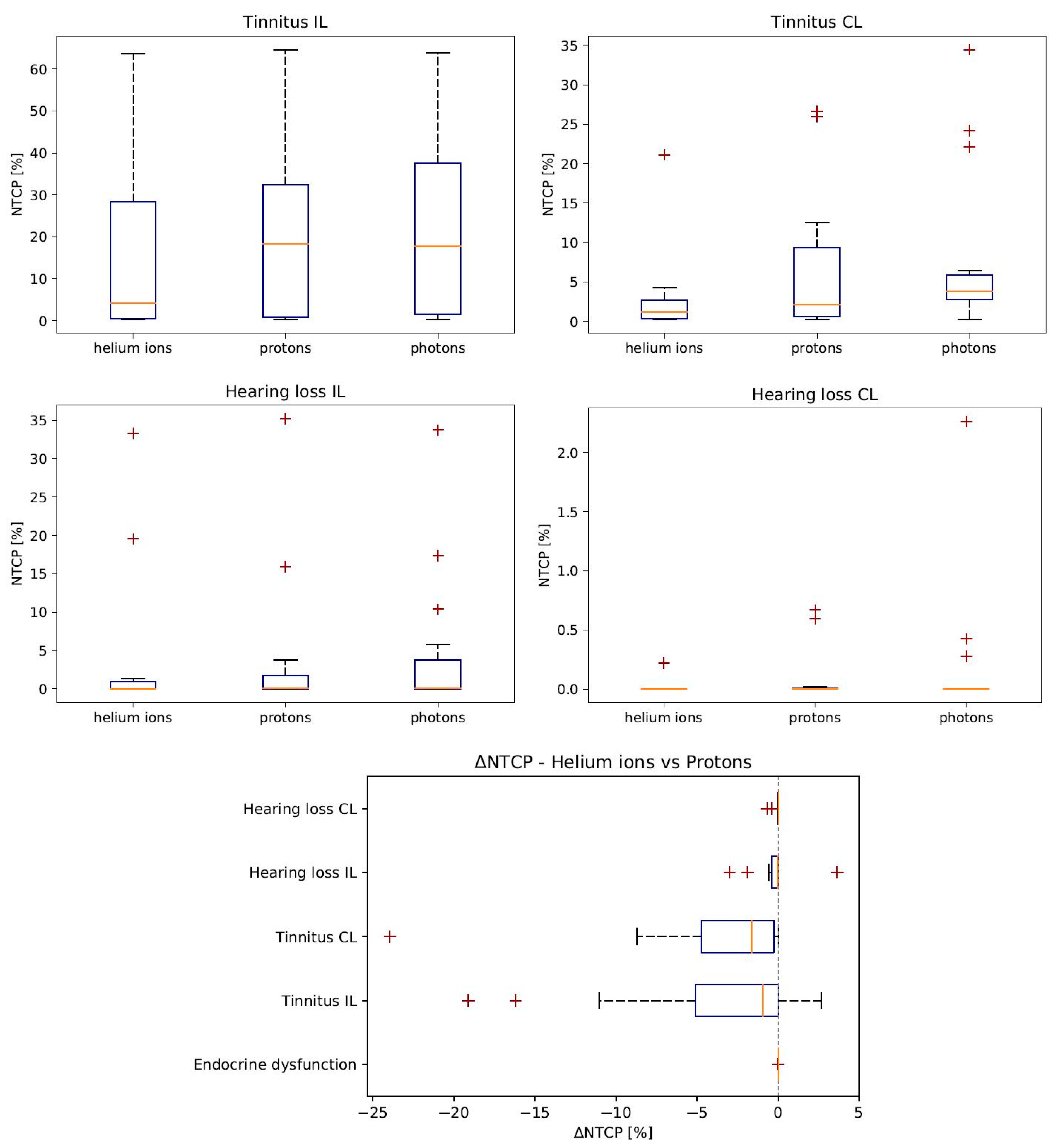
3.3. NTCP—Normal Tissue Complication Probabilty
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sato, M.; Gunther, J.R.; Mahajan, A.; Jo, E.; Paulino, A.C.; Adesina, A.M.; Jones, J.Y.; Ketonen, L.M.; Su, J.M.; Okcu, M.F.; et al. Progression-free survival of children with localized ependymoma treated with intensity-modulated radiation therapy or proton-beam radiation therapy. Cancer 2017, 123, 2570–2578. [Google Scholar] [CrossRef] [Green Version]
- Goldwein, J.W.; Leahy, J.M.; Packer, R.J.; Sutton, L.N.; Curran, W.J.; Rorke, L.B.; Schut, L.; Littman, P.; D’Angio, G.J. Intracranial ependymomas in children. Int. J. Radiat. Oncol. * Biol. * Phys. 1990, 19, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Li, C.; Xiong, X.; Kun, L.E.; Boop, F.A.; Sanford, R.A. Conformal radiotherapy after surgery for paediatric ependymoma: A prospective study. Lancet Oncol. 2009, 10, 258–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritzmann, T.A.; Chapman, R.J.; Macarthur, D.; Mallucci, C.; Kilday, J.P.; Thorp, N.; Modena, P.; Giagnacovo, M.; Dineen, R.; Jaspan, T.; et al. Epen-04. siop ependymoma i: Final results, long term follow-up and molecular analysis of the trial cohort: A biomeca consortium study. Neuro Oncol. 2021, 23 (Suppl. 1), i14. [Google Scholar] [CrossRef]
- Merchant, T.E. Three-dimensional conformal radiation therapy for ependymoma. Child’s Nerv. Syst. 2009, 25, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Leblond, P.; Massimino, M.; English, M.; Ritzmann, T.A.; Gandola, L.; Calaminus, G.; Thomas, S.; Pérol, D.; Gautier, J.; Grundy, R.G.; et al. Toward Improved Diagnosis Accuracy and Treatment of Children, Adolescents, and Young Adults With Ependymoma: The International SIOP Ependymoma II Protocol. Front Neurol. 2022, 13, 887544. [Google Scholar] [CrossRef]
- Palmer, J.D.; Hall, M.D.; Mahajan, A.; Paulino, A.C.; Wolden, S.; Constine, L.S. Radiotherapy and late effects. Pediatr. Clin. 2020, 67, 1051–1067. [Google Scholar] [CrossRef]
- Constine, L.S.; Woolf, P.D.; Cann, D.; Mick, G.; McCormick, K.; Raubertas, R.F.; Rubin, P. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N. Engl. J. Med. 1993, 328, 87–94. [Google Scholar] [CrossRef]
- Fong, R.S.; Beste, D.J.; Murray, K.J. Pediatric sensorineural hearing loss after temporal bone radiation. Am. J. Otol. 1995, 16, 793–796. [Google Scholar] [PubMed]
- Donahue, B. Short-and long-term complications of radiation therapy for pediatric brain tumors. Pediatr. Neurosurg. 1992, 18, 207–217. [Google Scholar] [CrossRef]
- Paulino, A.C. The local field in infratentorial ependymoma: Does the entire posterior fossa need to be treated? Int. J. Radiat. Oncol. * Biol. * Phys. 2001, 49, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Kahalley, L.S.; Peterson, R.; Ris, M.D.; Janzen, L.; Okcu, M.F.; Grosshans, D.R.; Ramaswamy, V.; Paulino, A.C.; Hodgson, D.; Mahajan, A.; et al. Superior intellectual outcomes after proton radiotherapy compared with photon radiotherapy for pediatric medulloblastoma. J. Clin. Oncol. 2020, 38, 454. [Google Scholar] [CrossRef] [PubMed]
- Vatner, R.E.; Niemierko, A.; Misra, M.; Weyman, E.A.; Goebel, C.P.; Ebb, D.H.; Jones, R.M.; Huang, M.S.; Mahajan, A.; Grosshans, D.R.; et al. Endocrine deficiency as a function of radiation dose to the hypothalamus and pituitary in pediatric and young adult patients with brain tumors. J. Clin. Oncol. 2018, 36, 2854. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, O. Physical advantages of particles: Protons and light ions. Br. J. Radiol. 2020, 93, 20190428. [Google Scholar] [CrossRef]
- Tessonnier, T.; Mairani, A.; Chen, W.; Sala, P.; Cerutti, F.; Ferrari, A.; Haberer, T.; Debus, J.; Parodi, K. Proton and helium ion radiotherapy for meningioma tumors: A monte carlo-based treatment planning comparison. Radiat. Oncol. 2018, 13, 2. [Google Scholar] [CrossRef]
- Dokic, I.; Mairani, A.; Niklas, M.; Zimmermann, F.; Chaudhri, N.; Krunic, D.; Tessonnier, T.; Ferrari, A.; Parodi, K.; Jäkel, O.; et al. Next generation multi-scale biophysical characterization of high precision cancer particle radiotherapy using clinical proton, helium-, carbon-and oxygen ion beams. Oncotarget 2016, 7, 56676. [Google Scholar] [CrossRef] [Green Version]
- Greenberger, B.A.; Pulsifer, M.B.; Ebb, D.H.; MacDonald, S.M.; Jones, R.M.; Butler, W.E.; Huang, M.S.; Marcus, K.J.; Oberg, J.A.; Tarbell, N.J.; et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int. J. Radiat. Oncol. * Biol. * Phys. 2014, 89, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Conklin, H.M.; Wu, S.; Lustig, R.H.; Xiong, X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J. Clin. Oncol. 2009, 27, 3691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaikh, A.; Calugaru, V.; Bondiau, P.Y.; Thariat, J.; Balosso, J. Impact of the ntcp modeling on medical decision to select eligible patient for proton therapy: The usefulness of eud as an indicator to rank modern photon vs proton treatment plans. Int. J. Radiat. Biol. 2018, 94, 789–797. [Google Scholar] [CrossRef]
- Langendijk, J.A.; Lambin, P.; De Ruysscher, D.; Widder, J.; Bos, M.; Verheij, M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: The model-based approach. Radiother. Oncol. 2013, 107, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Dell’Oro, M.; Wilson, P.; Short, M.; Hua, C.H.; Merchant, T.E.; Bezak, E. Normal tissue complication probability modeling to guide individual treatment planning in pediatric cranial proton and photon radiotherapy. Med. Phys. 2022, 49, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Vai, A.; Molinelli, S.; Rossi, E.; Iacovelli, N.A.; Magro, G.; Cavallo, A.; Pignoli, E.; Rancati, T.; Mirandola, A.; Russo, S.; et al. Proton radiation therapy for nasopharyngeal cancer patients: Dosimetric and ntcp evaluation supporting clinical decision. Cancers 2022, 14, 1109. [Google Scholar] [CrossRef] [PubMed]
- Mairani, A.; Mein, S.; Blakely, E.; Debus, J.; Durante, M.; Ferrari, A.; Fuchs, H.; Georg, D.; Grosshans, D.R.; Guan, F.; et al. Roadmap: Helium ion therapy. Phys. Med. Biol. 2022, 67, 15TR02. [Google Scholar] [CrossRef]
- Kataria, T.; Sharma, K.; Subramani, V.; Karrthick, K.P.; Bisht, S.S. Homogeneity index: An objective tool for assessment of conformal radiation treatments. J. Med. Phys. Assoc. Med. Phys. India 2012, 37, 207. [Google Scholar] [CrossRef]
- Claus, F.; Mijnheer, B.; Rasch, C.; Bortfeld, T.; Fraass, B.; De Gersem, W.; Wirtz, H.; Hoinkis, C.; Cho, B.C.; Kwong, L.W.D.; et al. Report of a study on imrt planning strategies for ethmoid sinus cancer. Strahlenther. Und Onkol. 2002, 178, 572–576. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, W.D.; Rosen, I.I. Nontumor integral dose variation in conventional radiotherapy treatment planning. Med. Phys. 2003, 30, 2065–2071. [Google Scholar] [CrossRef]
- Burman, C.; Kutcher, G.J.; Emami, B.; Goitein, M. Fitting of normal tissue tolerance data to an analytic function. Int. J. Radiat. Oncol. * Biol. * Phys. 1991, 21, 123–135. [Google Scholar] [CrossRef]
- De Marzi, L.; Feuvret, L.; Boulé, T.; Habrand, J.L.; Martin, F.; Calugaru, V.; Fournier-Bidoz, N.; Ferrand, R.; Mazal, A. Use of geud for predicting ear and pituitary gland damage following proton and photon radiation therapy. Br. J. Radiol. 2015, 88, 20140413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knäusl, B.; Fuchs, H.; Dieckmann, K.; Georg, D. Can particle beam therapy be improved using helium ions?—A planning study focusing on pediatric patients. Acta Oncol. 2016, 55, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Tessonnier, T.; Böhlen, T.T.; Ceruti, F.; Ferrari, A.; Sala, P.; Brons, S.; Haberer, T.; Debus, J.; Parodi, K.; Mairani, A. Dosimetric verification in water of a monte carlo treatment planning tool for proton, helium, carbon and oxygen ion beams at the heidelberg ion beam therapy center. Phys. Med. Biol. 2017, 62, 6579. [Google Scholar] [CrossRef]
- Tessonnier, T.; Mairani, A.; Brons, S.; Sala, P.; Cerutti, F.; Ferrari, A.; Haberer, T.; Debus, J.; Parodi, K. Helium ions at the heidelberg ion beam therapy center: Comparisons between fluka monte carlo code predictions and dosimetric measurements. Phys. Med. Biol. 2017, 62, 6784. [Google Scholar] [CrossRef] [PubMed]
- Mairani, A.; Dokic, I.; Magro, G.; Tessonnier, T.; Kamp, F.; Carlson, D.J.; Ciocca, M.; Cerutti, F.; Sala, P.R.; Ferrari, A.; et al. Biologically optimized helium ion plans: Calculation approach and its in vitro validation. Phys. Med. Biol. 2016, 61, 4283. [Google Scholar] [CrossRef]
- Ager, B.J.; Christensen, M.T.; Burt, L.M.; Poppe, M.M. The value of high-dose radiotherapy in intracranial ependymoma. Pediatr. Blood Cancer 2019, 66, e27697. [Google Scholar] [CrossRef]
- Child, A.E.; Warren, E.A.; Grosshans, D.R.; Paulino, A.C.; Okcu, M.F.; Ris, M.D.; Mahajan, A.; Orobio, J.; Cirino, P.T.; Minard, C.G.; et al. Long-term cognitive and academic outcomes among pediatric brain tumor survivors treated with proton versus photon radiotherapy. Pediatr. Blood Cancer 2021, 68, e29125. [Google Scholar] [CrossRef] [PubMed]
- Arvold, N.D.; Niemierko, A.; Broussard, G.P.; Adams, J.; Fullerton, B.; Loeffler, J.S.; Shih, H.A. Projected second tumor risk and dose to neurocognitive structures after proton versus photon radiotherapy for benign meningioma. Int. J. Radiat. Oncol. * Biol. * Phys. 2012, 83, e495–e500. [Google Scholar] [CrossRef]
- Harrabi, S.B.; Bougatf, N.; Mohr, A.; Haberer, T.; Herfarth, K.; Combs, S.E.; Debus, J.; Adeberg, S. Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlenther. Onkol. 2016, 192, 759–769. [Google Scholar] [CrossRef] [PubMed]
| Structure | Endpoint | TD50 [Gy] | m | n | |
|---|---|---|---|---|---|
| Cochlea [21] | Tinnitus | 46.52 | 0.35 | 1 | |
| Cochlea [21] | Hearing loss | 55.57 | 0.14 | 1 | |
| Pituitary [27] | Endocrine dysfunction | 60.6 | 0.08 | 1 | |
| Endpoint | helium | protons | photons | He vs. H+ | H+ vs. Ph |
| absolute difference | |||||
| Endocrine dysfunction | 0.0006 ± 0.0004 | 0.003 ± 0.002 | 0.006 ± 0.006 | −0.002 ± 0.002 | −0.003 ± 0.004 |
| Tinnitus Chochlea IL | 16 ± 5 | 20 ± 5 | 23 ± 6 | −4 ± 2 | −3 ± 1 |
| Tinnitus Cochlea CL | 3 ± 1 | 7 ± 2 | 8 ± 3 | −4 ± 2 | −1 ± 2 |
| Hearing loss Chochlea IL | 4 ± 2 | 4 ± 2 | 5 ± 2 | −0.2 ± 0.4 | −0.8 ± 0.5 |
| Hearing loss Chochlea CL | 0.01 ± 0.01 | 0.09 ± 0.06 | 0.2 ± 0.2 | −0.07 ± 0.05 | −0.1 ± 0.1 |
| CTV | Helium Ions | Protons | Photons | He vs. H+ | H+ vs. Ph | |
|---|---|---|---|---|---|---|
| rel. difference [%] | rel. difference [%] | |||||
| Dmean | 54.00 ± 0.03 | 54.03 ± 0.03 | 54.11 ± 0.03 | −0.07 ± 0.06 | −0.15 ± 0.06 * | |
| D1 | 55.3 ± 0.1 | 55.8 ± 0.1 | 56.5 ± 0.2 | −0.9 ± 0.3 * | −1.2 ± 0.4 * | |
| D95 | 53.1 ± 0.1 | 52.7 ± 0.1 | 52.61 ± 0.09 | 0.8 ± 0.2 * | 0.1 ± 0.3 | |
| D99 | 52.3 ± 0.2 | 51.9 ± 0.2 | 51.9 ± 0.1 | 0.6 ± 0.2 | 0.2 ± 0.4 | |
| HI | 3.2 ± 0.3 | 4.8 ± 0.3 | 5.6 ± 0.3 | −33 ± 7 * | −12 ± 8 | |
| IC | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.149 ± 0.008 | 1 ± 9 | −3 ± 9 | |
| OAR | helium ions | protons | photons | He vs. H+ | H+ vs. Ph | |
| Rel. difference [%] | Rel. difference [%] | |||||
| Hippocampus | Dmean | 17 ± 3 | 21 ± 3 | 22 ± 3 | −27 ± 6 * | −4 ± 9 |
| ipsilateral | D1 | 32 ± 5 | 36 ± 4 | 38 ± 4 | −17 ± 6 * | −1 ± 10 |
| D50 | 15 ± 3 | 20 ± 3 | 21 ± 3 | −33 ± 6 * | 8 ± 20 | |
| ID | 23 ± 3 | 28 ± 3 | 31 ± 4 | −27 ± 6 * | −4 ± 9 | |
| Hippocampus | Dmean | 11 ± 3 | 15 ± 3 | 19 ± 3 | −36 ± 5 * | −29 ± 8 * |
| contralateral | D1 | 22 ± 5 | 26 ± 4 | 33 ± 4 | −22 ± 5 * | −27 ± 7 * |
| D50 | 11 ± 2 | 15 ± 3 | 18 ± 3 | −40 ± 6 * | −20 ± 10 | |
| ID | 15 ± 3 | 20 ± 4 | 27 ± 5 | −36 ± 5 * | −29 ± 8 * | |
| Pituitary | Dmean | 9 ± 4 | 12 ± 4 | 15 ± 3 | −39 ± 7 * | −48 ± 16 * |
| D1 | 14 ± 5 | 16 ± 5 | 19 ± 4 | −33 ± 8 * | −41 ± 13 * | |
| D50 | 9 ± 4 | 12 ± 4 | 15 ± 3 | −40 ± 7 * | −48 ± 17 * | |
| ID | 1.5 ± 0.5 | 1.9 ± 0.6 | 2.9 ± 0.5 | −39 ± 7 * | −48 ± 16 * | |
| Inner ear | Dmean | 25 ± 4 | 29 ± 4 | 34 ± 3 | −24 ± 6 * | −17 ± 9 * |
| ipsilateral | D1 | 38 ± 5 | 41 ± 4 | 43 ± 4 | −14 ± 6 | −6 ± 10 |
| D50 | 23 ± 4 | 29 ± 4 | 34 ± 3 | −26 ± 6 * | −17 ± 10 * | |
| ID | 27 ± 5 | 32 ± 5 | 36 ± 4 | −24 ± 6 * | −17 ± 9 * | |
| Inner ear | Dmean | 12 ± 3 | 18 ± 3 | 21 ± 3 | −38 ± 6 * | −19 ± 12 |
| contralateral | D1 | 23 ± 4 | 25 ± 5 | 29 ± 3 | −18 ± 6 * | −18 ± 12 |
| D50 | 11 ± 2 | 17 ± 3 | 20 ± 2 | −42 ± 6 * | −18 ± 12 | |
| ID | 12 ± 3 | 19 ± 3 | 21 ± 3 | −38 ± 6 * | −19 ± 12 | |
| Brainstem | Dmean | 39 ± 3 | 41 ± 3 | 44 ± 2 | −5 ± 2 * | −9 ± 4 * |
| D1 | 54.5 ± 0.3 | 54.3 ± 0.3 | 55.1 ± 0.3 | 0.3 ± 0.4 | −1.4 ± 0.6 * | |
| D50 | 44 ± 4 | 44 ± 3 | 46 ± 3 | −5 ± 4 | −9 ± 6 | |
| ID | 752 ± 55 | 776 ± 51 | 843 ± 48 | −5 ± 2 * | −9 ± 4 * | |
| Brain | Dmean | 6.4 ± 0.6 | 7.5 ± 0.7 | 10.1 ± 0.7 | −15 ± 1 * | −27 ± 2 * |
| D1 | 54.19 ± 0.07 | 54.4 ± 0.1 | 54.6 ± 0.1 | −0.3 ± 0.1 * | −0.4 ± 0.1 * | |
| D50 | 0.14 ± 0.05 | 0.5 ± 0.3 | 1.9 ± 0.4 | nan ± nan | −74 ± 10 * | |
| ID | 8294 ± 614 | 9712 ± 716 | 13,325 ± 855 | −15 ± 1 * | −27 ± 2 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wickert, R.; Tessonnier, T.; Deng, M.; Adeberg, S.; Seidensaal, K.; Hoeltgen, L.; Debus, J.; Herfarth, K.; Harrabi, S.B. Radiotherapy with Helium Ions Has the Potential to Improve Both Endocrine and Neurocognitive Outcome in Pediatric Patients with Ependymoma. Cancers 2022, 14, 5865. https://doi.org/10.3390/cancers14235865
Wickert R, Tessonnier T, Deng M, Adeberg S, Seidensaal K, Hoeltgen L, Debus J, Herfarth K, Harrabi SB. Radiotherapy with Helium Ions Has the Potential to Improve Both Endocrine and Neurocognitive Outcome in Pediatric Patients with Ependymoma. Cancers. 2022; 14(23):5865. https://doi.org/10.3390/cancers14235865
Chicago/Turabian StyleWickert, Ricarda, Thomas Tessonnier, Maximilian Deng, Sebastian Adeberg, Katharina Seidensaal, Line Hoeltgen, Jürgen Debus, Klaus Herfarth, and Semi B. Harrabi. 2022. "Radiotherapy with Helium Ions Has the Potential to Improve Both Endocrine and Neurocognitive Outcome in Pediatric Patients with Ependymoma" Cancers 14, no. 23: 5865. https://doi.org/10.3390/cancers14235865





