T-Cell Infiltration and Clonality May Identify Distinct Survival Groups in Colorectal Cancer: Development and Validation of a Prognostic Model Based on The Cancer Genome Atlas (TCGA) and Clinical Proteomic Tumor Analysis Consortium (CPTAC)
Abstract
Simple summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. T-Cell Receptor Analysis
2.3. Statistical Analyses
3. Results
3.1. Study Population
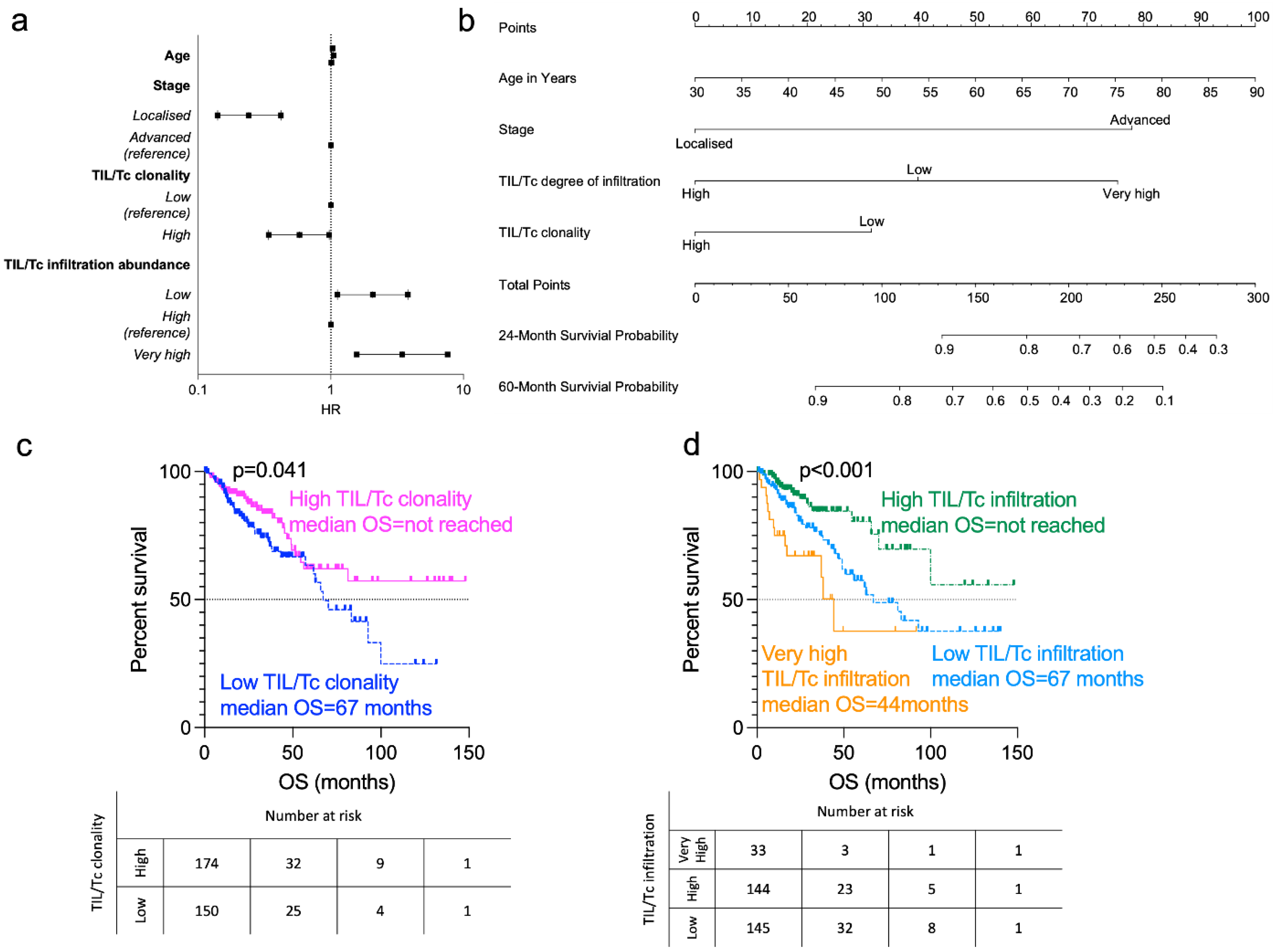
3.2. Prognostic Analysis
3.3. Survival Analysis
3.4. TIL/Tc Degree of Infiltration According to Clinical and Molecular Characteristics
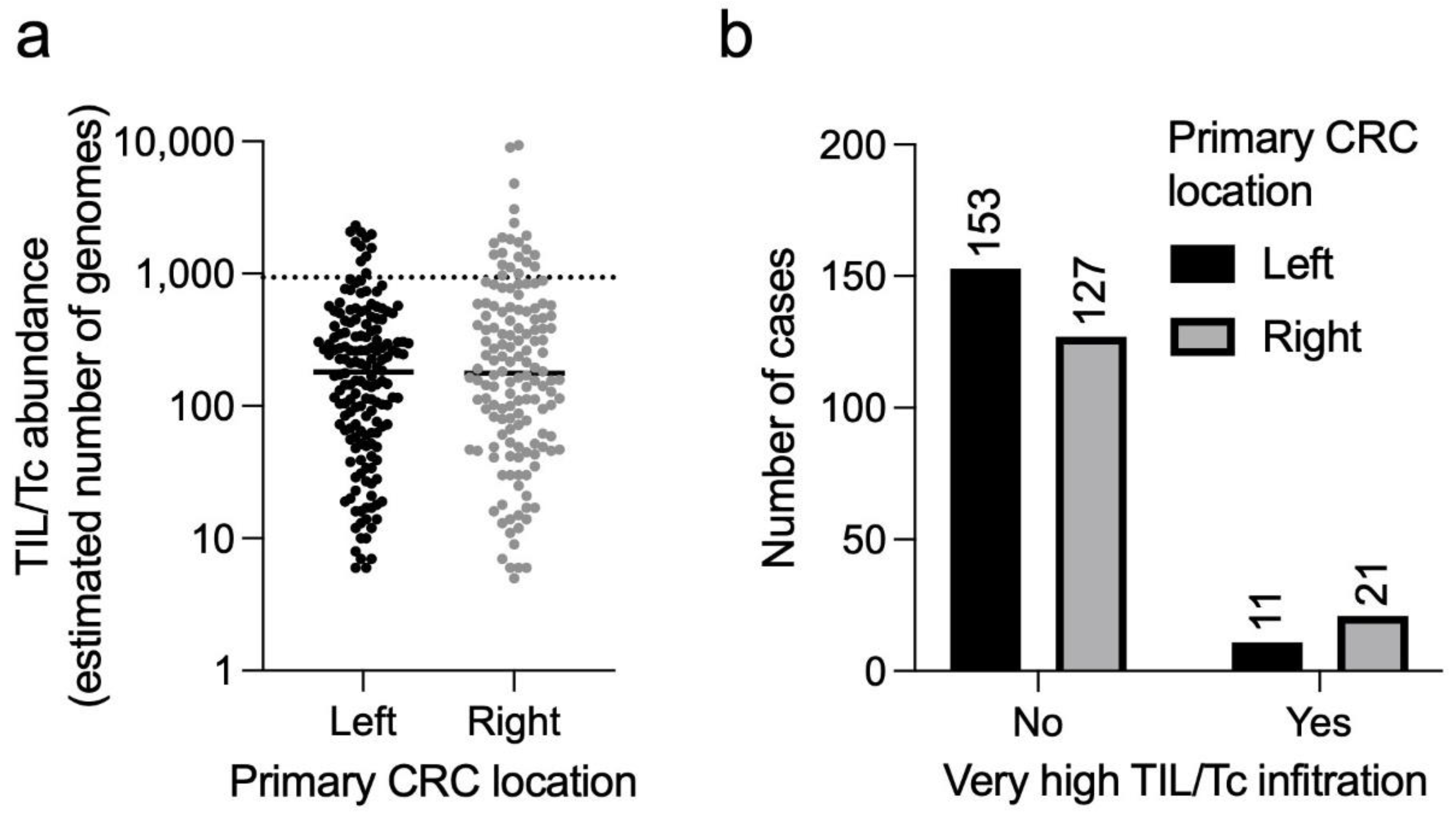
3.4.1. Disease Stage
3.4.2. CRC Sidedness
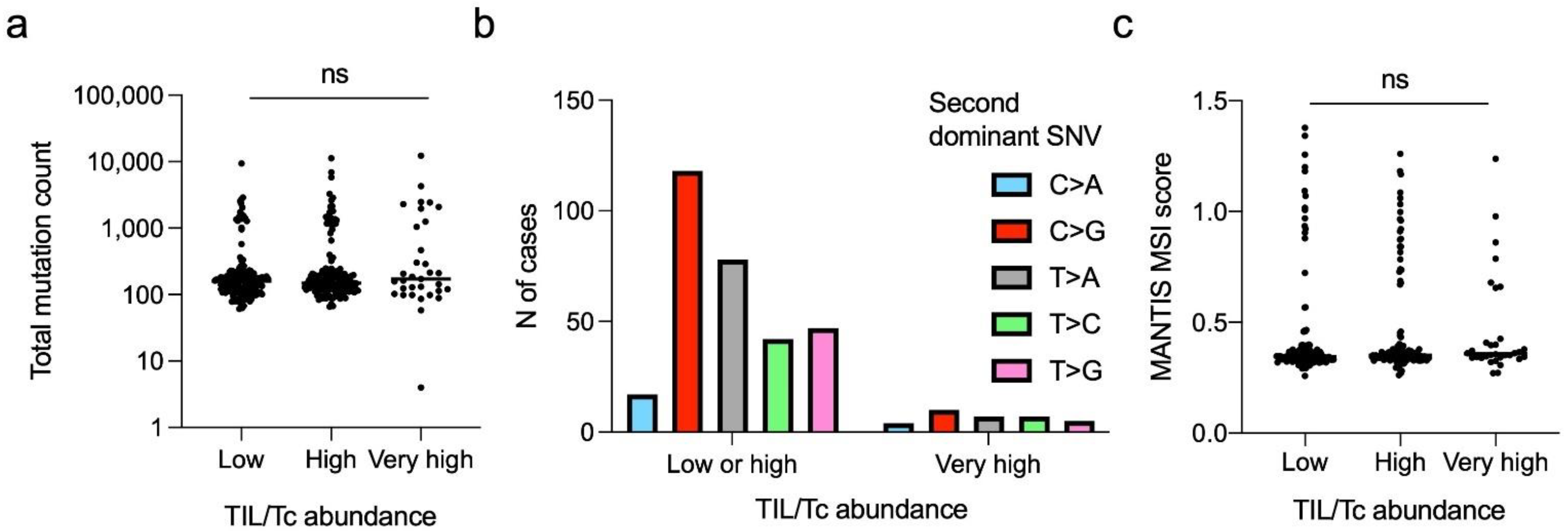
3.4.3. SNV Pattern
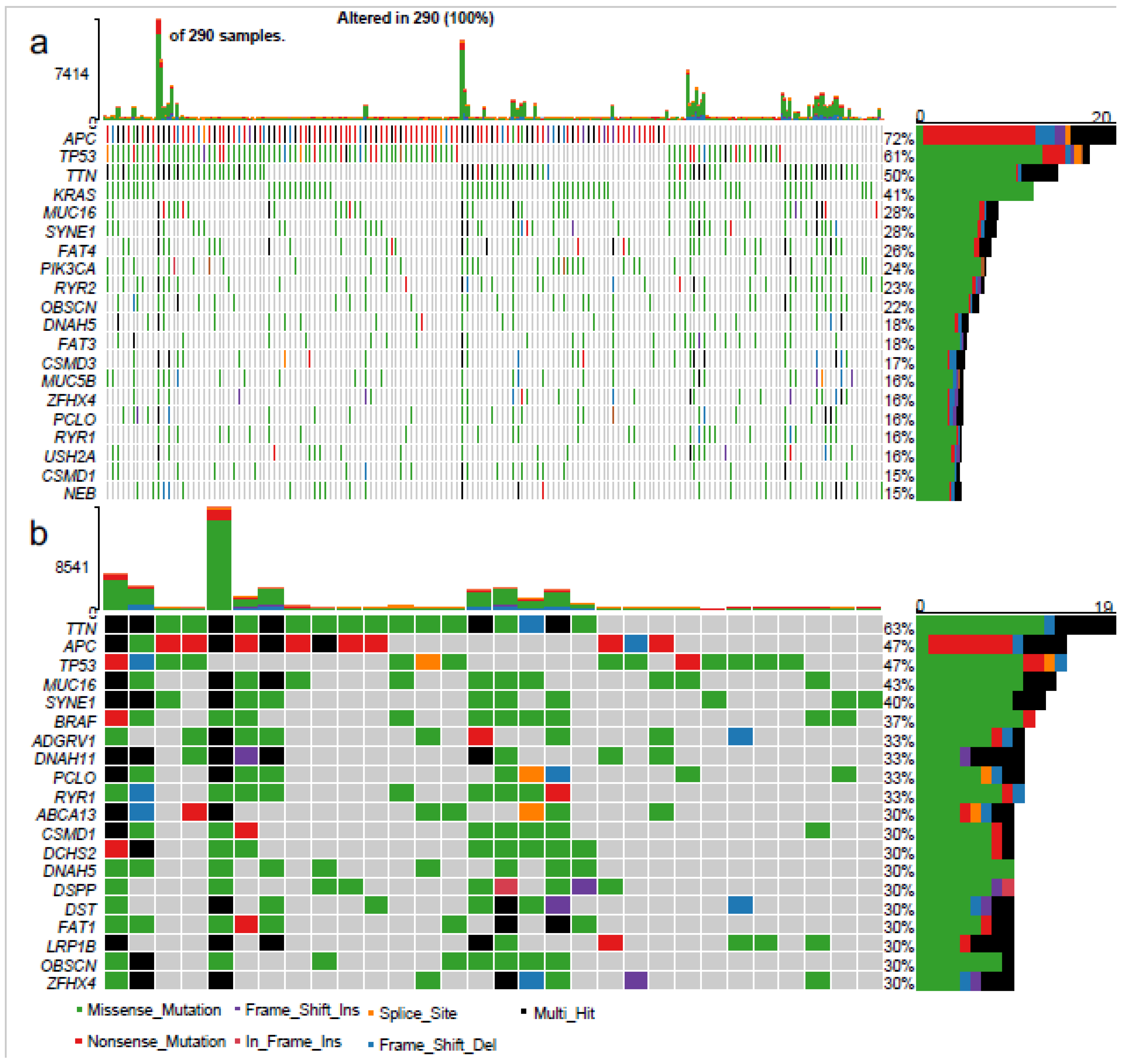
3.5. Characterisation of the “Very Highly” Infiltrated CRCs
Mutational Profile
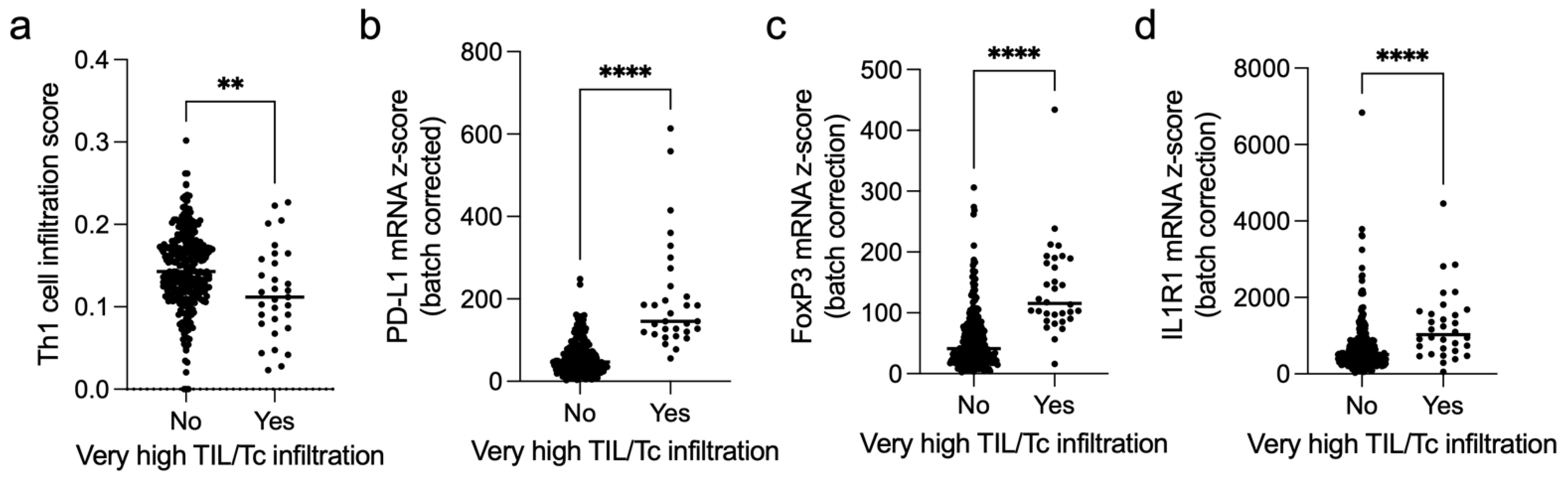
3.6. Immune Microenvironment
4. Discussion
4.1. Prognostic Role of TIL/Tc Clonality
4.2. Prognostic Role of TIL/Tc Infiltration
4.3. Immunologic Features of the “Highly Infiltrated” CRCs
4.4. Clinical Features of the “Highly Infiltrated” CRCs
4.5. Genetic Features of the “Highly Infiltrated” CRCs
4.6. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| AIC | Akaike information criterion |
| BRAF | B-rapidly accelerated fibrosarcoma |
| CI | Confidence interval |
| CDR3 | Complementarity determining region 3 |
| CPTAC | Clinical Proteomic Tumor Analysis Consortium |
| CRC | Colorectal cancer |
| EGFR | Epidermal growth factor receptor |
| HR | Hazard ratio |
| ICIs | Immune checkpoint inhibitors |
| MANTIS | Microsatellite analysis for normal-tumor instability |
| MMRd | Mismatch repair-deficient |
| MMRp | Mismatch repair-proficient |
| MSI | Microsatellite instable |
| MSS | Microsatellite stable |
| OS | Overall survival |
| PD-L1 | Programmed death ligand 1 |
| REMARK | REporting recommendations for tumour MARKer prognostic studies |
| SBS | Single-base substitution |
| SNV | Single-nucleotide variation |
| TCGA | The Cancer Genome Atlas |
| TCR | T-cell receptor |
| TILs | Tumour-infiltrating lymphocytes |
| TIL/Tc | Tumour-infiltrating lymphocyte T cells |
| TMB | Tumour mutational burden |
| TNM | Tumour–Node–Metastasis |
| UICC | Union for International Cancer Control |
| VELIPI | Vascular emboli, lymphatic invasion, perineural invasion |
References
- Mahar, A.L.; Compton, C.; Halabi, S.; Hess, K.R.; Weiser, M.R.; Groome, P.A. Personalizing Prognosis in Colorectal Cancer: A Systematic Review of the Quality and Nature of Clinical Prognostic Tools for Survival Outcomes. J. Surg. Oncol. 2017, 116, 969–982. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Grothey, A.; Yaeger, R.; van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Tsikitis, V.L.; Larson, D.W.; Huebner, M.; Lohse, C.M.; Thompson, P.A. Predictors of Recurrence Free Survival for Patients with Stage II and III Colon Cancer. BMC Cancer 2014, 14, 336. [Google Scholar] [CrossRef]
- Sobrero, A.; Grothey, A.; Iveson, T.; Labianca, R.; Yoshino, T.; Taieb, J.; Maughan, T.; Buyse, M.; André, T.; Meyerhardt, J.; et al. The Hard Road to Data Interpretation: 3 or 6 Months of Adjuvant Chemotherapy for Patients with Stage III Colon Cancer? Ann. Oncol. 2018, 29, 1099–1107. [Google Scholar] [CrossRef]
- Koch, M.; Beckhove, P.; Winkel, J.O.D.; Autenrieth, D.; Wagner, P.; Nummer, D.; Specht, S.; Antolovic, D.; Galindo, L.; Schmitz-Winnenthal, F.H.; et al. Tumor Infiltrating T Lymphocytes in Colorectal Cancer: Tumor-Selective Activation and Cytotoxic Activity in Situ. Ann. Surg. 2006, 244, 986–992. [Google Scholar] [CrossRef]
- Pagès, F.; Berger, A.; Camus, M.; Sanchez-Cabo, F.; Costes, A.; Molidor, R.; Mlecnik, B.; Kirilovsky, A.; Nilsson, M.; Damotte, D.; et al. Effector Memory T Cells, Early Metastasis, and Survival in Colorectal Cancer. N. Engl. J. Med. 2005, 353, 2654–2666. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Mlecnik, B.; Bifulco, C.; Bindea, G.; Marliot, F.; Lugli, A.; Jack Lee, J.; Zlobec, I.; Rau, T.T.; Berger, M.D.; Nagtegaal, I.D.; et al. Multicenter International Society for Immunotherapy of Cancer Study of the Consensus Immunoscore for the Prediction of Survival and Response to Chemotherapy in Stage III Colon Cancer. J. Clin. Oncol. 2020, 38, 3638–3651. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Shi, Q.; Catteau, A.; Poage, G.M.; Zemla, T.J.; Mlecnik, B.; Benson, A.B.; Gill, S.; Goldberg, R.M.; Kahlenberg, M.S.; et al. Immunoscore Is Prognostic in Low-Risk and High-Risk Stage III Colon Carcinomas Treated With Adjuvant Infusional Fluorouracil, Leucovorin, and Oxaliplatin in a Phase III Trial. JCO Precis. Oncol. 2022, 6, e2200010. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor Mutational Load Predicts Survival after Immunotherapy across Multiple Cancer Types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M. Immunotherapy Sensitivity of Mismatch Repair-Deficient Cancer: Mutation Load Is Not Enough. Cancer Cell 2021, 39, 16–18. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High Tumor Mutation Burden Fails to Predict Immune Checkpoint Blockade Response across All Cancer Types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, M.; Janikovits, J.; von Knebel Doeberitz, M.; Kloor, M. Complex Pattern of Immune Evasion in MSI Colorectal Cancer. Oncoimmunology 2018, 7, 1–10. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant Immunotherapy Leads to Pathological Responses in MMR-Proficient and MMR-Deficient Early-Stage Colon Cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef]
- Lumish, M.A.; Cercek, A. Immunotherapy for the Treatment of Colorectal Cancer. J. Surg. Oncol. 2021, 123, 760–774. [Google Scholar] [CrossRef]
- Valpione, S.; Mundra, P.A.; Galvani, E.; Campana, L.G.; Lorigan, P.; de Rosa, F.; Gupta, A.; Weightman, J.; Mills, S.; Dhomen, N.; et al. The T Cell Receptor Repertoire of Tumor Infiltrating T Cells Is Predictive and Prognostic for Cancer Survival. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef]
- Han, A.; Glanville, J.; Hansmann, L.; Davis, M.M. Linking T-Cell Receptor Sequence to Functional Phenotype at the Single-Cell Level. Nat. Biotechnol. 2014, 32, 684–692. [Google Scholar] [CrossRef]
- Sherwood, A.M.; Emerson, R.O.; Scherer, D.; Habermann, N.; Buck, K.; Staffa, J.; Desmarais, C.; Halama, N.; Jaeger, D.; Schirmacher, P.; et al. Tumor-Infiltrating Lymphocytes in Colorectal Tumors Display a Diversity of T Cell Receptor Sequences That Differ from the T Cells in Adjacent Mucosal Tissue. Cancer Immunol. Immunother. 2013, 62, 1453–1461. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, X.; Zheng, L.; Zhang, Y.; Li, Y.; Fang, Q.; Gao, R.; Kang, B.; Zhang, Q.; Huang, J.Y.; et al. Lineage Tracking Reveals Dynamic Relationships of T Cells in Colorectal Cancer. Nature 2018, 564, 268–272. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Zhang, L.; Hu, X.; Ren, X.; Zhang, Z. Deep Single-Cell RNA Sequencing Data of Individual T Cells from Treatment-Naïve Colorectal Cancer Patients. Sci. Data 2019, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Hazama, S.; Yamaguchi, R.; Imoto, S.; Takenouchi, H.; Inoue, Y.; Kanekiyo, S.; Shindo, Y.; Miyano, S.; Nakamura, Y.; et al. Characterization of the T Cell Repertoire by Deep T Cell Receptor Sequencing in Tissues and Blood from Patients with Advanced Colorectal Cancer. Oncol. Lett. 2016, 11, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Liao, W.J.; Huang, Y.T.; Shi, M.; Zhang, Y.; Wen, Q.; Zhou, M.Q.; Ma, L. Normalization of T Cell Receptor Repertoire Diversity in Patients with Advanced Colorectal Cancer Who Responded to Chemotherapy. Cancer Sci. 2011, 102, 706–712. [Google Scholar] [CrossRef]
- Li, B.; Li, T.; Pignon, J.C.; Wang, B.; Wang, J.; Shukla, S.A.; Dou, R.; Chen, Q.; Hodi, F.S.; Choueiri, T.K.; et al. Landscape of Tumor-Infiltrating T Cell Repertoire of Human Cancers. Nat. Genet. 2016, 48, 725–732. [Google Scholar] [CrossRef]
- Sanz-Pamplona, R.; Melas, M.; Maoz, A.; Schmit, S.L.; Rennert, H.; Lejbkowicz, F.; Greenson, J.K.; Sanjuan, X.; Lopez-Zambrano, M.; Alonso, M.H.; et al. Lymphocytic Infiltration in Stage II Microsatellite Stable Colorectal Tumors: A Retrospective Prognosis Biomarker Analysis. PLoS Med. 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Ling, A.; Lundberg, I.V.; Eklöf, V.; Wikberg, M.L.; Öberg, Å.; Edin, S.; Palmqvist, R. The Infiltration, and Prognostic Importance, of Th1 Lymphocytes Vary in Molecular Subgroups of Colorectal Cancer. J. Pathol. Clin. Res. 2016, 2, 21–31. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, B.; Wang, Y.; Li, M.; Liu, X.; Cao, J.; Li, C.; Hu, J. Clinicopathological and Prognostic Significance of PD-L1 Expression in Colorectal Cancer: A Meta-Analysis. Int. J. Color. Dis. 2021, 36, 117–130. [Google Scholar] [CrossRef]
- Salama, P.; Phillips, M.; Grieu, F.; Morris, M.; Zeps, N.; Joseph, D.; Platell, C.; Iacopetta, B. Tumor-Infiltrating FOXP3+ T Regulatory Cells Show Strong Prognostic Significance in Colorectal Cancer. J. Clin. Oncol. 2009, 27, 186–192. [Google Scholar] [CrossRef]
- Mair, F.; Erickson, J.R.; Frutoso, M.; Konecny, A.J.; Greene, E.; Voillet, V.; Maurice, N.J.; Rongvaux, A.; Dixon, D.; Barber, B.; et al. Extricating Human Tumour Immune Alterations from Tissue Inflammation. Nature 2022, 605, 728–735. [Google Scholar] [CrossRef]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Kautto, E.A.; Bonneville, R.; Miya, J.; Yu, L.; Krook, M.A.; Reeser, J.W.; Roychowdhury, S. Performance Evaluation for Rapid Detection of Pan-Cancer Microsatellite Instability with MANTIS. Oncotarget 2017, 8, 7452. [Google Scholar] [CrossRef] [PubMed]
- Aran, D.; Hu, Z.; Butte, A.J. XCell: Digitally Portraying the Tissue Cellular Heterogeneity Landscape. Genome Biol. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.; Huang, C.; Wang, X.; Petyuk, V.A.; Savage, S.R.; Wen, B.; Dou, Y.; Zhang, Y.; Shi, Z.; Arshad, O.A.; et al. Proteogenomic Analysis of Human Colon Cancer Reveals New Therapeutic Opportunities. Cell 2019, 177, 1035–1049.e19. [Google Scholar] [CrossRef]
- Bolotin, D.A.; Poslavsky, S.; Mitrophanov, I.; Shugay, M.; Mamedov, I.Z.; Putintseva, E.V.; Chudakov, D.M. MiXCR: Software for comprehensive adaptive immunity profiling. Nat. Methods 2015, 12, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Spreafico, R.; Rossetti, M.; van Loosdregt, J.; Wallace, C.A.; Massa, M.; Magni-Manzoni, S.; Gattorno, M.; Martini, A.; Lovell, D.J.; Albani, S. A Circulating Reservoir of Pathogenic-like CD4+ T Cells Shares a Genetic and Phenotypic Signature with the Inflamed Synovial Micro-Environment. Ann. Rheum. Dis. 2016, 75, 459–465. [Google Scholar] [CrossRef]
- Valpione, S.; Galvani, E.; Tweedy, J.; Mundra, P.A.; Banyard, A.; Middlehurst, P.; Barry, J.; Mills, S.; Salih, Z.; Weightman, J.; et al. Immune Awakening Revealed by Peripheral T Cell Dynamics after One Cycle of Immunotherapy. Nat. Cancer 2020, 1, 210–221. [Google Scholar] [CrossRef]
- Salih, Z.; Banyard, A.; Tweedy, J.; Galvani, E.; Middlehurst, P.; Mills, S.; Weightman, J.; Gupta, A.; Lorigan, P.C.; Zhou, C.; et al. T Cell Immune Awakening in Response to Immunotherapy Is Age-Dependent. Eur. J. Cancer 2022, 162, 11–21. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting Recommendations for Tumour MARKer Prognostic Studies (REMARK). Eur. J. Cancer 2005, 41, 1690–1696. [Google Scholar] [CrossRef]
- Lausen, B.; Schumacher, M. Maximally Selected Rank Statistics. Biometrics 1992, 48, 73–85. [Google Scholar] [CrossRef]
- López-Ratón, M.; Rodríguez-Álvarez, M.X.; Cadarso-Suárez, C.; Gude, F. OptimalCutpoints: An R Package for Selecting Optimal Cutpoints in Diagnostic Tests. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef]
- Valpione, S.; Campana, L.G.; Weightmand, J.; Salih, Z.; Galvani, E.; Mundra, P.A.; De Rosa, F.; Gupta, A.; Serra-Bellver, P.; Lorigan, P.; et al. Tumour Infiltrating B Cells Discriminate Checkpoint Blockade-Induced Responses. Eur. J. Cancer 2022, 177, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.; Lueza, B.; Douillard, J.Y.; Peeters, M.; Lenz, H.J.; Venook, A.; Heinemann, V.; van Cutsem, E.; Pignon, J.P.; Tabernero, J.; et al. Prognostic and Predictive Value of Primary Tumour Side in Patients with RAS Wild-Type Metastatic Colorectal Cancer Treated with Chemotherapy and EGFR Directed Antibodies in Six Randomized Trials. Ann. Oncol. 2017, 28, 1713–1729. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Goel, A. Microsatellite Instability in Colorectal Cancer. Gastroenterology 2010, 138. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Kim, J.; Haradhvala, N.J.; Huang, M.N.; Tian Ng, A.W.; Wu, Y.; Boot, A.; Covington, K.R.; Gordenin, D.A.; Bergstrom, E.N.; et al. The Repertoire of Mutational Signatures in Human Cancer. Nature 2020, 578, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.H.; Harrison, T.A.; Phipps, A.I.; Steinfelder, R.; Trinh, Q.M.; Qu, C.; Banbury, B.L.; Georgeson, P.; Grasso, C.S.; Giannakis, M.; et al. Landscape of Somatic Single Nucleotide Variants and Indels in Colorectal Cancer and Impact on Survival. Nat. Commun. 2020, 11, 3644. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.N.; Hu, C.Y.; Snyder, R.A.; Cuddy, A.; You, Y.N.; Lowenstein, L.M.; Volk, R.J.; Chang, G.J. Predicting Risk of Recurrence After Colorectal Cancer Surgery in the United States: An Analysis of a Special Commission on Cancer National Study. Ann. Surg. Oncol. 2020, 27, 2740–2749. [Google Scholar] [CrossRef] [PubMed]
- Quasar Collaborative Group; Gray, R.; Barnwell, J.; McConkey, C.; Hills, R.K.; Williams, N.S.; Kerr, D.J. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007, 370, 2020–2029. [Google Scholar] [CrossRef]
- Kuebler, J.P.; Wieand, H.S.; O’Connell, M.J.; Smith, R.E.; Colangelo, L.H.; Yothers, G.; Petrelli, N.J.; Findlay, M.P.; Seay, T.E.; Atkins, J.N.; et al. Oxaliplatin Combined with Weekly Bolus Fluorouracil and Leucovorin as Surgical Adjuvant Chemotherapy for Stage II and III Colon Cancer: Results from NSABP C-07. J. Clin. Oncol. 2007, 25, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Bylesjo, M.; Kerr, P.; Davison, T.; Black, J.M.; Kay, E.W.; Holt, R.J.; Proutski, V.; Ahdesmaki, M.; Farztdinov, V.; et al. Development and Independent Validation of a Prognostic Assay for Stage Ii Colon Cancer Using Formalin-Fixed Paraffin-Embedded Tissue. J. Clin. Oncol. 2011, 29, 4620–4626. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tibiche, C.; Zou, J.; Zaman, N.; Trifiro, M.; O’Connor-McCourt, M.; Wang, E. Identification and Construction of Combinatory Cancer Hallmark-Based Gene Signature Sets to Predict Recurrence and Chemotherapy Benefit in Stage II Colorectal Cancer. JAMA Oncol. 2016, 2, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bortolomeazzi, M.; Keddar, M.R.; Montorsi, L.; Acha-Sagredo, A.; Benedetti, L.; Temelkovski, D.; Choi, S.; Petrov, N.; Todd, K.; Wai, P.; et al. Immunogenomics of Colorectal Cancer Response to Checkpoint Blockade: Analysis of the KEYNOTE 177 Trial and Validation Cohorts. Gastroenterology 2021, 161, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Idos, G.E.; Kwok, J.; Bonthala, N.; Kysh, L.; Gruber, S.B.; Qu, C. The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 3360. [Google Scholar] [CrossRef] [PubMed]
- Chifman, J.; Pullikuth, A.; Chou, J.W.; Bedognetti, D.; Miller, L.D. Conservation of Immune Gene Signatures in Solid Tumors and Prognostic Implications. BMC Cancer 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Abraham, H.G.; Xia, Y.; Mukherjee, B.; Merajver, S.D. Incidence and Survival of Inflammatory Breast Cancer between 1973 and 2015 in the SEER Database. Breast Cancer Res. Treat. 2021, 185, 229–238. [Google Scholar] [CrossRef]
- Bruni, D.; Angell, H.K.; Galon, J. The Immune Contexture and Immunoscore in Cancer Prognosis and Therapeutic Efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Parmiani, G. Tumor-Infiltrating T Cells—Friend or Foe of Neoplastic Cells? N. Engl. J. Med. 2005, 353, 2640–2641. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Solomon, I.; Amann, M.; Goubier, A.; Arce Vargas, F.; Zervas, D.; Qing, C.; Henry, J.Y.; Ghorani, E.; Akarca, A.U.; Marafioti, T.; et al. CD25-Treg-Depleting Antibodies Preserving IL-2 Signaling on Effector T Cells Enhance Effector Activation and Antitumor Immunity. Nat. Cancer 2020, 1, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Tejpar, S.; Stintzing, S.; Ciardiello, F.; Tabernero, J.; van Cutsem, E.; Beier, F.; Esser, R.; Lenz, H.J.; Heinemann, V. Prognostic and Predictive Relevance of Primary Tumor Location in Patients with Ras Wild-Type Metastatic Colorectal Cancer Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2017, 3, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Barras, D.; Missiaglia, E.; Wirapati, P.; Sieber, O.M.; Jorissen, R.N.; Love, C.; Molloy, P.L.; Jones, I.T.; McLaughlin, S.; Gibbs, P.; et al. BRAF V600E Mutant Colorectal Cancer Subtypes Based on Gene Expression. Clin. Cancer Res. 2017, 23, 104–115. [Google Scholar] [CrossRef]
- Flaherty, D.C.; Jalas, J.R.; Sim, M.S.; Stojadinovic, A.; Protic, M.; Lee, D.J.; Bilchik, A.J. The Negative Impact of Body Mass Index on the Tumor Microenvironment in Colon Cancer: Results of a Prospective Trial. Ann. Surg. Oncol. 2018, 25, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
| Variables | N. or Median (%/Range) |
|---|---|
| Age at diagnosis | 67 (33–90) |
| Sex | |
| Female | 154 (47) |
| Male | 175 (53) |
| n.a. | 8 |
| Disease stage | |
| Localised | 168 (54) |
| I | 52 (17) |
| II | 116 (37) |
| Advanced | 143 (46) |
| III | 100 (32) |
| IV | 43 (14) |
| n.a. | 26 |
| Primary tumour side | |
| right | 148 (47) |
| left | 166 (53) |
| n.a. | 23 |
| Neoadjuvant therapy | |
| no | 328 (99.7) |
| yes | 1 (0.3) |
| n.a. | 8 |
| Total TIL/Tc genomes a | 182 (5–9336) |
| TIL/Tc clonality | 0.21 (0.03–0.76) |
| TIL/Tc diversity | 2.7 (0.2–5.3) |
| Total SNVs | 155 (4–12338) |
| MANTIS–MSI score | 0.35 (0.26–1.38) |
| IFNγ mRNA z-score | −0.24 (−1.76–3.34) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campana, L.G.; Mansoor, W.; Hill, J.; Macutkiewicz, C.; Curran, F.; Donnelly, D.; Hornung, B.; Charleston, P.; Bristow, R.; Lord, G.M.; et al. T-Cell Infiltration and Clonality May Identify Distinct Survival Groups in Colorectal Cancer: Development and Validation of a Prognostic Model Based on The Cancer Genome Atlas (TCGA) and Clinical Proteomic Tumor Analysis Consortium (CPTAC). Cancers 2022, 14, 5883. https://doi.org/10.3390/cancers14235883
Campana LG, Mansoor W, Hill J, Macutkiewicz C, Curran F, Donnelly D, Hornung B, Charleston P, Bristow R, Lord GM, et al. T-Cell Infiltration and Clonality May Identify Distinct Survival Groups in Colorectal Cancer: Development and Validation of a Prognostic Model Based on The Cancer Genome Atlas (TCGA) and Clinical Proteomic Tumor Analysis Consortium (CPTAC). Cancers. 2022; 14(23):5883. https://doi.org/10.3390/cancers14235883
Chicago/Turabian StyleCampana, Luca G., Wasat Mansoor, James Hill, Christian Macutkiewicz, Finlay Curran, David Donnelly, Ben Hornung, Peter Charleston, Robert Bristow, Graham M. Lord, and et al. 2022. "T-Cell Infiltration and Clonality May Identify Distinct Survival Groups in Colorectal Cancer: Development and Validation of a Prognostic Model Based on The Cancer Genome Atlas (TCGA) and Clinical Proteomic Tumor Analysis Consortium (CPTAC)" Cancers 14, no. 23: 5883. https://doi.org/10.3390/cancers14235883
APA StyleCampana, L. G., Mansoor, W., Hill, J., Macutkiewicz, C., Curran, F., Donnelly, D., Hornung, B., Charleston, P., Bristow, R., Lord, G. M., & Valpione, S. (2022). T-Cell Infiltration and Clonality May Identify Distinct Survival Groups in Colorectal Cancer: Development and Validation of a Prognostic Model Based on The Cancer Genome Atlas (TCGA) and Clinical Proteomic Tumor Analysis Consortium (CPTAC). Cancers, 14(23), 5883. https://doi.org/10.3390/cancers14235883








