Incidence, Mortality and Survival Trends in Breast Cancers Coincident with Introduction of Mammography in the Nordic Countries
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
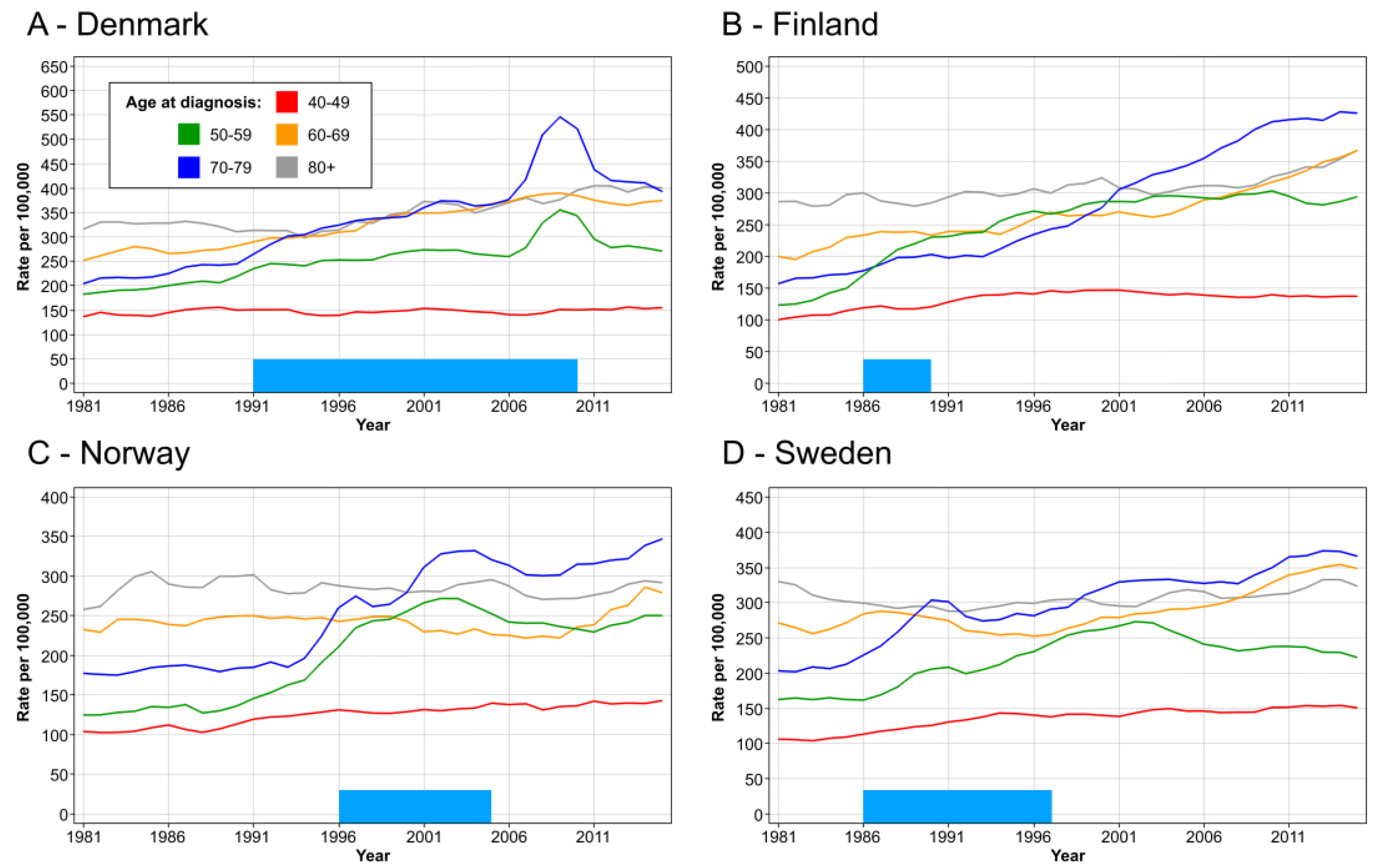
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC. Breast Cancer Screening; IARC Press: Lyon, France, 2016; 469p. [Google Scholar]
- The Independet UK Panel. The benefits and harms of breast cancer screening: An independent review. Lancet 2012, 380, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Coldman, A.; Phillips, N.; Wilson, C.; Decker, K.; Chiarelli, A.M.; Brisson, J.; Zhang, B.; Payne, J.; Doyle, G.; Ahmad, R. Pan-Canadian study of mammography screening and mortality from breast cancer. J. Natl. Cancer Inst. 2014, 106, dju261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Møller, B.; Weedon-Fekjaer, H.; Hakulinen, T.; Tryggvadottir, L.; Storm, H.; Talbäck, M.; Haldorsen, T. The influence of mammographic screening on national trends in breast cancer incidence. Eur. J. Cancer Prev. 2005, 14, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Koechlin, A.; Smans, M.; Vatten, L.; Boniol, M. Mammography screening and breast cancer mortality in Sweden. J. Natl. Cancer Inst. 2012, 104, 1080–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beau, A.B.; Lynge, E.; Njor, S.H.; Vejborg, I.; Lophaven, S.N. Benefit-to-harm ratio of the Danish breast cancer screening programme. Int. J. Cancer 2017, 141, 512–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njor, S.H.; Schwartz, W.; Blichert-Toft, M.; Lynge, E. Decline in breast cancer mortality: How much is attributable to screening? J. Med. Screen 2015, 22, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Sebuødegård, S.; Botteri, E.; Hofvind, S. Breast Cancer Mortality After Implementation of Organized Population-Based Breast Cancer Screening in Norway. J. Natl. Cancer Inst. 2020, 112, 839–846. [Google Scholar] [CrossRef]
- Zahl, P.H. RE: Breast Cancer Mortality After Implementation of Organized Population-Based Screening in Norway. J. Natl. Cancer Inst. 2020, 112, 1174. [Google Scholar] [CrossRef]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Jognson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995-2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef] [Green Version]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, H.; Ardanaz, E.; et al. Cancer survival in Europe 1999-2007 by country and age: Results of EUROCARE--5-a population-based study. Lancet Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.; Carioli, G.; Bertuccio, P.; Malvezzi, M.; Pastorino, U.; Boffetta, P.; Negri, E.; Bosetti, C.; La Vecchia, C. Progress in cancer mortality, incidence, and survival: A global overview. Eur. J. Cancer Prev. 2020, 29, 367–381. [Google Scholar] [CrossRef]
- Hemminki, J.; Försti, A.; Hemminki, A.; Hemminki, K. Survival trends in solid cancers in the Nordic countries through 50 years. Eur. J. Cancer 2022, in press. [CrossRef]
- Pukkala, E.; Engholm, G.; Schmidt, L.K.H.; Storm, H.; Khan, S.; Lambe, M.; Pettersson, D.; Ólafsdóttir, E.; Tryggvadóttir, L.; Hakanen, T.; et al. Nordic Cancer Registries—An overview of their procedures and data comparability. Acta Oncol. 2018, 57, 440–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utada, M.; Ohno, Y.; Shimizu, S.; Hori, M.; Soda, M. Comparison between overall, cause-specific, and relative survival rates based on data from a population-based cancer registry. Asian Pac. J. Cancer Prev. 2012, 13, 5681–5685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engholm, G.; Ferlay, J.; Christensen, N.; Bray, F.; Gjerstorff, M.L.; Klint, A.; Køtlum, J.E.; Ólafsdóttir, E.; Pukkala, E.; Storm, H.H.; et al. NORDCAN—A Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010, 49, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Storm, H.H.; Engholm, G.; Hakulinen, T.; Tryggvadóttir, L.; Klint, A.; Gislum, M.; Kejs, A.M.T.; Bray, F. Survival of patients diagnosed with cancer in the Nordic countries up to 1999-2003 followed to the end of 2006. A critical overview of the results. Acta Oncol. 2010, 49, 532–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, F.E.; Andersson, T.M.-L.; Lambe, M.; Engholm, G.; Mørch, L.S.; Johannesen, T.B.; Virtanen, A.; Pettersson, D.; Ólafsdóttir, E.J.; Birgisson, H.; et al. Trends in cancer survival in the Nordic countries 1990-2016: The NORDCAN survival studies. Acta Oncol. 2020, 59, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Arndt, V.; Gefeller, O.; Hakulinen, T. An alternative approach to age adjustment of cancer survival rates. Eur. J. Cancer 2004, 40, 2317–2322. [Google Scholar] [CrossRef]
- Giordano, L.; Von Karsa, L.; Tomatis, M.; Majek, O.; De Wolf, C.; Lancucki, L.; Hofvind, S.; Nystrom, L.; Segnan, N.; Ponti, A. Mammographic screening programmes in Europe: Organization, coverage and participation. J. Med. Screen 2012, 19 (Suppl. 1), 72–82. [Google Scholar] [CrossRef]
- Duffy, S.W.; Tabár, L.; Chen, H.-H.; Holmqvist, M.; Yen, M.-F.; Abdsalah, S.; Epstein, B.; Frodis, E.; Ljungberg, E.; Hedborg-Melander, C.; et al. The impact of organized mammography service screening on breast carcinoma mortality in seven Swedish counties. Cancer 2002, 95, 458–469. [Google Scholar] [CrossRef]
- Chaltiel, D.; Hill, C. Estimations of overdiagnosis in breast cancer screening vary between 0% and over 50%: Why? BMJ Open 2021, 11, e046353. [Google Scholar] [CrossRef]
- Ellis, L.; Woods, L.M.; Estève, J.; Eloranta, S.; Coleman, M.P.; Rachet, B. Cancer incidence, survival and mortality: Explaining the concepts. Int. J. Cancer 2014, 135, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Riihimaki, M.; Thomsen, H.; Brandt, A.; Sundquist, J.; Hemminki, K. Death causes in breast cancer patients. Ann. Oncol. 2012, 23, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Nordenskjöld, A.E.; Fohlin, H.; Arnesson, L.G.; Einbeigi, Z.; Holmberg, E.; Albertsson, P.; Karlsson, P. Breast cancer survival trends in different stages and age groups—A population-based study 1989–2013. Acta Oncol. 2019, 58, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Costa, A.; Senkus, E.; Aapro, M.; Andre, F.; Barrios, C.; Bergh, J.; Bhattacharyya, G.; Biganzoli, L.; Cardoso, M.J.; et al. 3rd ESO-ESMO international consensus guidelines for Advanced Breast Cancer (ABC 3). Breast 2017, 31, 244–259. [Google Scholar] [CrossRef] [PubMed]



| Period | 5-Year Survival | 10-Year Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Denmark | Finland | Norway | Sweden | Denmark | Finland | Norway | Sweden | |
| 1971–1975 | 61.2 [59.8–62.7] | 59.3 [57.3–61.3] | 65.0 [63.3–66.8] | 67.4 [66.4–68.4] | 45.8 [43.8–47.9] | 41.6 [39.3–44.0] | 51.1 [48.6–53.8] | 52.6 [51.1–54.2] |
| 1976–1980 | 66.1 [64.8–67.3] * | 64.0 [62.5–65.5] * | 70.2 [68.6–71.7] * | 74.9 [74.0–75.8] * | 50.5 [48.7–52.4] * | 46.8 [44.8–48.9] * | 55.4 [53.0–57.8] | 61.6 [60.1–63.1] * |
| 1981–1985 | 66.6 [65.5–67.8] | 71.4 [70.0–72.8] * | 73.2 [71.8–74.6] * | 76.6 [75.7–77.5] | 52.0 [50.3–53.8] | 58.7 [56.5–60.8] * | 59.2 [57.1–61.4] | 63.5 [62.1–64.9] |
| 1986–1990 | 70.4 [69.3–71.5] * | 77.8 [76.5–79.1] * | 75.2 [73.9–76.5] | 80.0 [79.2–80.7] * | 57.5 [55.8–59.3] * | 67.6 [65.5–69.7] * | 62.2 [60.2–64.3] | 69.5 [68.3–70.8] * |
| 1991–1995 | 74.5 [73.4–75.5] * | 80.5 [79.3–81.7] * | 76.8 [75.6–78.1] | 82.8 [82.1–83.6] * | 63.8 [62.1–65.6] * | 70.2 [68.2–72.2] | 65.7 [63.8–67.7] | 74.4 [73.2–75.7] * |
| 1996–2000 | 78.8 [77.8–79.8] * | 84.3 [83.3–85.3] * | 84.0 [82.9–85.1] * | 85.5 [84.8–86.2] * | 71.1 [69.4–72.8] * | 75.0 [73.1–76.9] * | 75.2 [73.4–77.1] * | 77.6 [76.4–78.7] * |
| 2001–2005 | 82.5 [81.6–83.4] * | 86.4 [85.5–87.4] * | 85.2 [84.2–86.3] | 86.7 [86.1–87.4] | 75.7 [74.1–77.3] * | 79.8 [78.2–81.4] * | 76.2 [74.5–78.0] | 80.6 [79.5–81.7] * |
| 2006–2010 | 86.2 [85.4–87.0] * | 88.4 [87.6–89.2] * | 86.5 [85.5–87.6] | 89.6 [89.0–90.2] * | 81.5 [80.0–83.0] * | 83.8 [82.4–85.3] * | 79.6 [77.9–81.3] | 83.9 [82.9–85.0] * |
| 2011–2015 | 89.6 [88.9–90.4] * | 90.2 [89.5–90.9] * | 89.9 [89.0–90.8] * | 91.7 [91.1–92.2] * | 86.3 [85.0–87.7] * | 86.1 [84.8–87.4] | 84.0 [82.3–85.8] * | 87.2 [86.1–88.2] * |
| 2016–2020 | 90.2 [89.5–90.9] | 90.8 [90.2–91.5] | 90.8 [90.0–91.7] | 92.3 [91.7–92.8] | 86.9 [85.7–88.2] | 86.6 [85.5–87.8] | 84.9 [83.1–86.7] | 87.8 [86.8–88.8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemminki, K.; Försti, A. Incidence, Mortality and Survival Trends in Breast Cancers Coincident with Introduction of Mammography in the Nordic Countries. Cancers 2022, 14, 5907. https://doi.org/10.3390/cancers14235907
Hemminki K, Försti A. Incidence, Mortality and Survival Trends in Breast Cancers Coincident with Introduction of Mammography in the Nordic Countries. Cancers. 2022; 14(23):5907. https://doi.org/10.3390/cancers14235907
Chicago/Turabian StyleHemminki, Kari, and Asta Försti. 2022. "Incidence, Mortality and Survival Trends in Breast Cancers Coincident with Introduction of Mammography in the Nordic Countries" Cancers 14, no. 23: 5907. https://doi.org/10.3390/cancers14235907








