Anoctamins and Calcium Signalling: An Obstacle to EGFR Targeted Therapy in Glioblastoma?
Abstract
Simple Summary
Abstract
1. Introduction
2. EGFR Signalling and Variant Expression in GBM
3. EGFR Inhibition in GBM–Clinical Trials and Limitations
3.1. EGFR Small Molecule Inhibitors
3.2. Anti-EGFR Antibodies and Antibody-Drug Conjugates
3.3. Other EGFR-Targeted Agents
4. The Role of EGFR in Cancer Cell Growth, Invasion, and Survival in GBM
EGFR and Cell Migration and Invasion: Extracellular Matrix Remodelling and Intracellular Signalling
5. ANOs in Cancer
5.1. ANOs and Cell Migration: Regulators of Cell Volume
5.2. ANO1 in Glioma
5.3. Other ANOs in Cancer
6. ANO-Targeted Therapy in Cancer
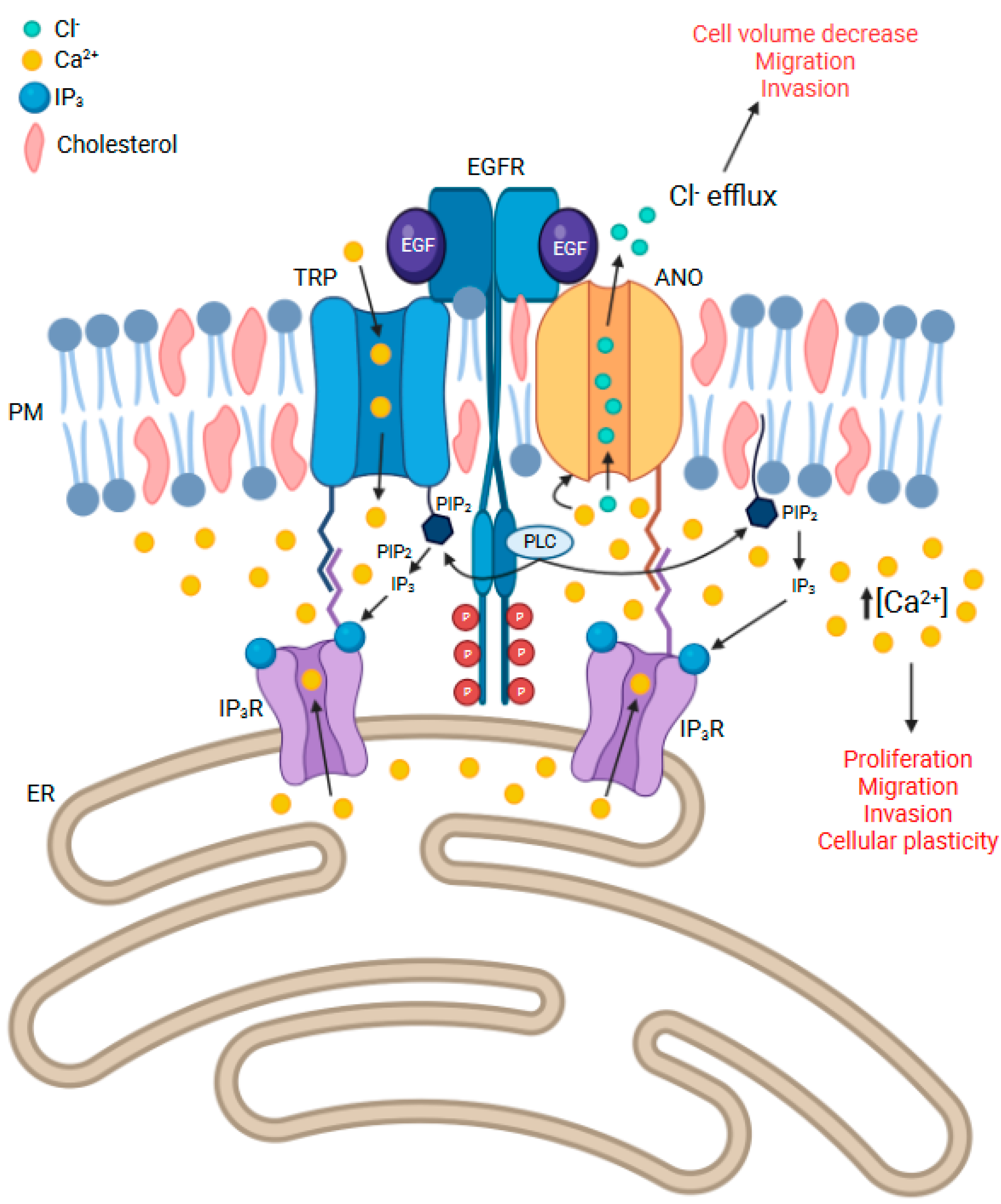
7. Calcium Channels and Their Relevance to ANO and EGFR-Related Signalling
7.1. IP3R
7.2. TRP Channels
7.3. Lipid Rafts Containing EGFR, ANOs, and Other Calcium Channels May Be Key to Cell Invasion, Cellular Plasticity, and Drug Resistance in GBM
8. Future Directions and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| ANO | anoctamin |
| CAR | Chimeric antigen receptor |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| ER | endoplasmic reticulum |
| FASN | fatty acid synthase |
| GBM | glioblastoma multiforme |
| Grb2 | growth factor receptor-bound protein 2 |
| HNSCC | head and neck squamous cell carcinoma |
| IDH | isocitrate dehydrogenase |
| IP3R | inositol 1,4,5-trisphosphate |
| JAK | janus kinase |
| mTOR | mammalian target of rapamycin (mTOR) |
| MMP | matrix metalloproteinase |
| NFA | niflumic acid |
| NF-κB | nuclear factor-κB |
| NPPB | 5-nitro-2-(3 phenylpropylalanine) benzoate |
| NSCLC | non-small cell lung cancer |
| PI3K | phosphoinositide 3-kinase |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PIP3 | phosphatidylinositol-3,4,5-triphosphate |
| PLC | phosphoinositide phospholipase C |
| RVD | regulatory volume decrease |
| SGLT1 | sodium/glucose cotransporter 1 |
| Sos | son of sevenless |
| TRP | transient receptor potential |
| TRPC | TRP canonical |
| TRPV | TRP vanilloid |
References
- Leece, R.; Xu, J.; Ostrom, Q.T.; Chen, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. Global incidence of malignant brain and other central nervous system tumors by histology, 2003–2007. Neuro Oncol. 2017, 19, 1553–1564. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Ron, B.; Asna, N.; Pamela, S.; Nicole, F.; Moshe, S. Glioblastoma Multiforme, Diagnosis and Treatment; Recent Literature Review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Dobes, M.; Khurana, V.G.; Shadbolt, B.; Jain, S.; Smith, S.F.; Smee, R.; Dexter, M.; Cook, R. Increasing incidence of glioblastoma multiforme and meningioma, and decreasing incidence of Schwannoma (2000–2008): Findings of a multicenter Australian study. Surg. Neurol. Int. 2011, 2, 176. [Google Scholar] [CrossRef]
- Rock, K.; McArdle, O.; Forde, P.; Dunne, M.; Fitzpatrick, D.; O’Neill, B.; Faul, C. A clinical review of treatment outcomes in glioblastoma multiforme--the validation in a non-trial population of the results of a randomised Phase III clinical trial: Has a more radical approach improved survival? Br. J. Radiol. 2012, 85, e729–e733. [Google Scholar] [CrossRef]
- Illic, R.; Somma, T.; Savic, D.; Frio, F.; Milicevic, M.; Solari, D.; Nikitovic, M.; Lavrnic, S.; Raicevic, S.; Milosevic, S.; et al. A Survival Analysis with Identification of Prognostic Factors in a Series of 110 Patients with Newly Diagnosed Glioblastoma Before and After Introduction of the Stupp Regimen: A Single-Center Observational Study. World Neurosurg. 2017, 104, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Witthayanuwat, S.; Pesee, M.; Supaadirek, C.; Supakalin, N.; Thamronganantasakul, K.; Krusun, S. Survival Analysis of Glioblastoma Multiforme. Asian Pac. J. Cancer Prev. 2018, 19, 2613–2617. [Google Scholar] [CrossRef] [PubMed]
- Topkan, E.; Selek, U.; Ozdemir, Y.; Yildirim, B.A.; Guler, O.C.; Ciner, F.; Mertsoylu, H.; Tufan, K. Prognostic value of the Glasgow Prognostic Score for glioblastoma multiforme patients treated with radiotherapy and temozolomide. J. Neuro-Oncol. 2018, 139, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Darefsky, A.S.; King, J.T., Jr.; Dubrow, R. Adult glioblastoma multiforme survival in the temozolomide era: A population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer 2012, 118, 2163–2172. [Google Scholar] [CrossRef]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Cohen, M.H.; Johnson, J.R.; Pazdur, R. Food and Drug Administration Drug approval summary: Temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin. Cancer Res. 2005, 11, 6767–6771. [Google Scholar] [CrossRef]
- Barbagallo, G.M.; Paratore, S.; Caltabiano, R.; Palmucci, S.; Parra, H.S.; Privitera, G.; Motta, F.; Lanzafame, S.; Scaglione, G.; Longo, A.; et al. Long-term therapy with temozolomide is a feasible option for newly diagnosed glioblastoma: A single-institution experience with as many as 101 temozolomide cycles. Neurosurg. Focus 2014, 37, E4. [Google Scholar] [CrossRef] [PubMed]
- Jalali, R.; Basu, A.; Gupta, T.; Munshi, A.; Menon, H.; Sarin, R.; Goel, A. Encouraging experience of concomitant Temozolomide with radiotherapy followed by adjuvant Temozolomide in newly diagnosed glioblastoma multiforme: Single institution experience. Br. J. Neurosurg. 2007, 21, 583–587. [Google Scholar] [CrossRef]
- Wong, E.T.; Lok, E.; Swanson, K.D. Alternating Electric Fields Therapy for Malignant Gliomas: From Bench Observation to Clinical Reality. Prog. Neurol. Surg. 2018, 32, 180–195. [Google Scholar] [PubMed]
- Ornelas, A.S.; Porter, A.B.; Sharma, A.; Knox, M.G.; Marks, L.A.; Wingerchuk, D.M.; O’Carroll, C.B. What is the Role of Tumor-treating Fields in Newly Diagnosed Glioblastoma? Neurologist 2019, 24, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef]
- Lu, V.M.; Goyal, A.; Graffeo, C.S.; Perry, A.; Burns, T.C.; Parney, I.F.; Quinones-Hinojosa, A.; Chaichana, K.L. Survival Benefit of Maximal Resection for Glioblastoma Reoperation in the Temozolomide Era: A Meta-Analysis. World Neurosurg. 2019, 127, 31–37. [Google Scholar] [CrossRef]
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef] [PubMed]
- González-Tablas, M.; Arandia, D.; Jara-Acevedo, M.; Otero, Á.; Vital, A.L.; Prieto, C.; González-Garcia, N.; Nieto-Librero, A.B.; Tao, H.; Pascual, D.; et al. Heterogeneous EGFR, CDK4, MDM4, and PDGFRA Gene Expression Profiles in Primary GBM: No Association with Patient Survival. Cancers 2020, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Day, S.E.; Waziri, A. Clinical trials of small molecule inhibitors in high-grade glioma. Neurosurg. Clin. N. Am. 2012, 23, 407–416. [Google Scholar] [CrossRef]
- Wilson, T.A.; Karajannis, M.A.; Harter, D.H. Glioblastoma multiforme: State of the art and future therapeutics. Surg. Neurol. Int. 2014, 5, 64. [Google Scholar] [CrossRef]
- Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2000, 103, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Olayioye, M.A.; Neve, R.M.; Lane, H.A.; Hynes, N.E. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000, 19, 3159–3167. [Google Scholar] [CrossRef] [PubMed]
- Micallef, J.; Taccone, M.; Mukherjee, J.; Croul, S.; Busby, J.; Moran, M.F.; Guha, A. Epidermal Growth Factor Receptor Variant III–Induced Glioma Invasion Is Mediated through Myristoylated Alanine-Rich Protein Kinase C Substrate Overexpression. Cancer Res. 2009, 69, 7548–7556. [Google Scholar] [CrossRef] [PubMed]
- McCoy, E.S.; Haas, B.R.; Sontheimer, H. Water permeability through aquaporin-4 is regulated by protein kinase C and becomes rate-limiting for glioma invasion. Neuroscience 2010, 168, 971–981. [Google Scholar] [CrossRef]
- Cho, K.-K.; Mikkelsen, T.; Lee, Y.J.; Jiang, F.; Chopp, M.; Rosenblum, M.L. The role of protein kinase Cα in U-87 glioma invasion. Int. J. Dev. Neurosci. 1999, 17, 447–461. [Google Scholar] [CrossRef]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.W.; Weiss, W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef]
- Yarden, Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 2001, 37 (Suppl 4), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Emdad, L.; Das, S.K.; Fisher, P.B. EGFR: An essential receptor tyrosine kinase-regulator of cancer stem cells. Adv. Cancer Res. 2020, 147, 161–188. [Google Scholar] [CrossRef] [PubMed]
- Furnari, F.B.; Cloughesy, T.F.; Cavenee, W.K.; Mischel, P.S. Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat. Rev. Cancer 2015, 15, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Frederick, L.; Wang, X.Y.; Eley, G.; James, C.D. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000, 60, 1383–1387. [Google Scholar] [PubMed]
- Nishikawa, R.; Sugiyama, T.; Narita, Y.; Furnari, F.; Cavenee, W.K.; Matsutani, M. Immunohistochemical analysis of the mutant epidermal growth factor, deltaEGFR, in glioblastoma. Brain Tumor Pathol. 2004, 21, 53–56. [Google Scholar] [CrossRef]
- Aldape, K.D.; Ballman, K.; Furth, A.; Buckner, J.C.; Giannini, C.; Burger, P.C.; Scheithauer, B.W.; Jenkins, R.B.; James, C.D. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J. Neuropathol. Exp. Neurol. 2004, 63, 700–707. [Google Scholar] [CrossRef]
- Saikali, S.; Avril, T.; Collet, B.; Hamlat, A.; Bansard, J.Y.; Drenou, B.; Guegan, Y.; Quillien, V. Expression of nine tumour antigens in a series of human glioblastoma multiforme: Interest of EGFRvIII, IL-13Ralpha2, gp100 and TRP-2 for immunotherapy. J. Neurooncol. 2007, 81, 139–148. [Google Scholar] [CrossRef]
- Biernat, W.; Huang, H.; Yokoo, H.; Kleihues, P.; Ohgaki, H. Predominant expression of mutant EGFR (EGFRvIII) is rare in primary glioblastomas. Brain Pathol. 2004, 14, 131–136. [Google Scholar] [CrossRef]
- Ekstrand, A.J.; Sugawa, N.; James, C.D.; Collins, V.P. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proc. Natl. Acad. Sci. USA 1992, 89, 4309–4313. [Google Scholar] [CrossRef]
- Heimberger, A.B.; Hlatky, R.; Suki, D.; Yang, D.; Weinberg, J.; Gilbert, M.; Sawaya, R.; Aldape, K. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin. Cancer Res. 2005, 11, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.K.; Cvrljevic, A.N.; Johns, T.G. The epidermal growth factor receptor variant III (EGFRvIII): Where wild things are altered. FEBS J. 2013, 280, 5350–5370. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.M.; Zhang, C.Z.; Maire, C.L.; Jung, J.; Manzo, V.E.; Adalsteinsson, V.A.; Homer, H.; Haidar, S.; Blumenstiel, B.; Pedamallu, C.S.; et al. EGFR variant heterogeneity in glioblastoma resolved through single-nucleus sequencing. Cancer Discov. 2014, 4, 956–971. [Google Scholar] [CrossRef] [PubMed]
- Inda, M.M.; Bonavia, R.; Mukasa, A.; Narita, Y.; Sah, D.W.; Vandenberg, S.; Brennan, C.; Johns, T.G.; Bachoo, R.; Hadwiger, P.; et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010, 24, 1731–1745. [Google Scholar] [CrossRef]
- Del Vecchio, C.A.; Giacomini, C.P.; Vogel, H.; Jensen, K.C.; Florio, T.; Merlo, A.; Pollack, J.R.; Wong, A.J. EGFRvIII gene rearrangement is an early event in glioblastoma tumorigenesis and expression defines a hierarchy modulated by epigenetic mechanisms. Oncogene 2013, 32, 2670–2681. [Google Scholar] [CrossRef]
- van den Bent, M.J.; Gao, Y.; Kerkhof, M.; Kros, J.M.; Gorlia, T.; van Zwieten, K.; Prince, J.; van Duinen, S.; Sillevis Smitt, P.A.; Taphoorn, M.; et al. Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro-Oncology 2015, 17, 935–941. [Google Scholar] [CrossRef]
- Vogt, N.; Lefèvre, S.H.; Apiou, F.; Dutrillaux, A.M.; Cör, A.; Leuraud, P.; Poupon, M.F.; Dutrillaux, B.; Debatisse, M.; Malfoy, B. Molecular structure of double-minute chromosomes bearing amplified copies of the epidermal growth factor receptor gene in gliomas. Proc. Natl. Acad. Sci. USA 2004, 101, 11368–11373. [Google Scholar] [CrossRef]
- Sanborn, J.Z.; Salama, S.R.; Grifford, M.; Brennan, C.W.; Mikkelsen, T.; Jhanwar, S.; Katzman, S.; Chin, L.; Haussler, D. Double minute chromosomes in glioblastoma multiforme are revealed by precise reconstruction of oncogenic amplicons. Cancer Res. 2013, 73, 6036–6045. [Google Scholar] [CrossRef]
- Bolcaen, J.; Nair, S.; Driver, C.H.S.; Boshomane, T.M.G.; Ebenhan, T.; Vandevoorde, C. Novel Receptor Tyrosine Kinase Pathway Inhibitors for Targeted Radionuclide Therapy of Glioblastoma. Pharmaceuticals 2021, 14, 626. [Google Scholar] [CrossRef]
- Uhm, J.H.; Ballman, K.V.; Wu, W.; Giannini, C.; Krauss, J.C.; Buckner, J.C.; James, C.D.; Scheithauer, B.W.; Behrens, R.J.; Flynn, P.J.; et al. Phase II evaluation of gefitinib in patients with newly diagnosed Grade 4 astrocytoma: Mayo/North Central Cancer Treatment Group Study N0074. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 347–353. [Google Scholar] [CrossRef]
- Rich, J.N.; Reardon, D.A.; Peery, T.; Dowell, J.M.; Quinn, J.A.; Penne, K.L.; Wikstrand, C.J.; Van Duyn, L.B.; Dancey, J.E.; McLendon, R.E.; et al. Phase II Trial of Gefitinib in Recurrent Glioblastoma. J. Clin. Oncol. 2004, 22, 133–142. [Google Scholar] [CrossRef]
- Franceschi, E.; Cavallo, G.; Lonardi, S.; Magrini, E.; Tosoni, A.; Grosso, D.; Scopece, L.; Blatt, V.; Urbini, B.; Pession, A.; et al. Gefitinib in patients with progressive high-grade gliomas: A multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br. J. Cancer 2007, 96, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, A.; Wang, M.; Robins, H.I.; Lautenschlaeger, T.; Curran, W.J.; Brachman, D.G.; Schultz, C.J.; Choucair, A.; Dolled-Filhart, M.; Christiansen, J.; et al. RTOG 0211: A phase 1/2 study of radiation therapy with concurrent gefitinib for newly diagnosed glioblastoma patients. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Peereboom, D.M.; Shepard, D.R.; Ahluwalia, M.S.; Brewer, C.J.; Agarwal, N.; Stevens, G.H.; Suh, J.H.; Toms, S.A.; Vogelbaum, M.A.; Weil, R.J.; et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J. Neurooncol. 2010, 98, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Krishnan, S.; Sarkaria, J.N.; Wu, W.; Jaeckle, K.A.; Uhm, J.H.; Geoffroy, F.J.; Arusell, R.; Kitange, G.; Jenkins, R.B.; et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J. Clin. Oncol. 2008, 26, 5603–5609. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Brandes, A.A.; Rampling, R.; Kouwenhoven, M.C.; Kros, J.M.; Carpentier, A.F.; Clement, P.M.; Frenay, M.; Campone, M.; Baurain, J.F.; et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J. Clin. Oncol. 2009, 27, 1268–1274. [Google Scholar] [CrossRef]
- Wen, P.Y.; Chang, S.M.; Lamborn, K.R.; Kuhn, J.G.; Norden, A.D.; Cloughesy, T.F.; Robins, H.I.; Lieberman, F.S.; Gilbert, M.R.; Mehta, M.P.; et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04–02. Neuro-Oncology 2014, 16, 567–578. [Google Scholar] [CrossRef]
- Reardon, D.A.; Groves, M.D.; Wen, P.Y.; Nabors, L.; Mikkelsen, T.; Rosenfeld, S.; Raizer, J.; Barriuso, J.; McLendon, R.E.; Suttle, A.B.; et al. A phase I/II trial of pazopanib in combination with lapatinib in adult patients with relapsed malignant glioma. Clin. Cancer Res. 2013, 19, 900–908. [Google Scholar] [CrossRef]
- Yung, W.K.; Vredenburgh, J.J.; Cloughesy, T.F.; Nghiemphu, P.; Klencke, B.; Gilbert, M.R.; Reardon, D.A.; Prados, M.D. Safety and efficacy of erlotinib in first-relapse glioblastoma: A phase II open-label study. Neuro Oncol. 2010, 12, 1061–1070. [Google Scholar] [CrossRef]
- Krishnan, S.; Brown, P.D.; Ballman, K.V.; Fiveash, J.B.; Uhm, J.H.; Giannini, C.; Jaeckle, K.A.; Geoffroy, F.J.; Nabors, L.B.; Buckner, J.C. Phase I trial of erlotinib with radiation therapy in patients with glioblastoma multiforme: Results of North Central Cancer Treatment Group protocol N0177. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1192–1199. [Google Scholar] [CrossRef]
- Kesavabhotla, K.; Schlaff, C.D.; Shin, B.; Mubita, L.; Kaplan, R.; Tsiouris, A.J.; Pannullo, S.C.; Christos, P.; Lavi, E.; Scheff, R.; et al. Phase I/II study of oral erlotinib for treatment of relapsed/refractory glioblastoma multiforme and anaplastic astrocytoma. J. Exp. Ther. Oncol. 2012, 10, 71–81. [Google Scholar] [PubMed]
- Raizer, J.J.; Abrey, L.E.; Lassman, A.B.; Chang, S.M.; Lamborn, K.R.; Kuhn, J.G.; Yung, W.K.; Gilbert, M.R.; Aldape, K.A.; Wen, P.Y.; et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 2010, 12, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.L.; Molinaro, A.M.; Phillips, J.J.; Butowski, N.A.; Chang, S.M.; Perry, A.; Costello, J.F.; DeSilva, A.A.; Rabbitt, J.E.; Prados, M.D. A single-institution phase II trial of radiation, temozolomide, erlotinib, and bevacizumab for initial treatment of glioblastoma. Neuro Oncol. 2014, 16, 984–990. [Google Scholar] [CrossRef]
- Barkovich, K.J.; Hariono, S.; Garske, A.L.; Zhang, J.; Blair, J.A.; Fan, Q.W.; Shokat, K.M.; Nicolaides, T.; Weiss, W.A. Kinetics of inhibitor cycling underlie therapeutic disparities between EGFR-driven lung and brain cancers. Cancer Discov. 2012, 2, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Nabors, L.B.; Mason, W.P.; Perry, J.R.; Shapiro, W.; Kavan, P.; Mathieu, D.; Phuphanich, S.; Cseh, A.; Fu, Y.; et al. Phase I/randomized phase II study of afatinib, an irreversible ErbB family blocker, with or without protracted temozolomide in adults with recurrent glioblastoma. Neuro Oncol. 2015, 17, 430–439. [Google Scholar] [CrossRef]
- Sepúlveda-Sánchez, J.M.; Vaz, M.; Balañá, C.; Gil-Gil, M.; Reynés, G.; Gallego, Ó.; Martínez-García, M.; Vicente, E.; Quindós, M.; Luque, R.; et al. Phase II trial of dacomitinib, a pan-human EGFR tyrosine kinase inhibitor, in recurrent glioblastoma patients with EGFR amplification. Neuro Oncol. 2017, 19, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.S.; Cahill, D.P.; Reardon, D.A.; Wen, P.Y.; Mikkelsen, T.; Peereboom, D.M.; Wong, E.T.; Gerstner, E.R.; Dietrich, J.; Plotkin, S.R.; et al. Exploring Predictors of Response to Dacomitinib in EGFR-Amplified Recurrent Glioblastoma. JCO Precis. Oncol. 2020, 4, 593–613. [Google Scholar] [CrossRef]
- Cross, D.A.E.; Ashton, S.E.; Ghiorghiu, S.; Eberlein, C.; Nebhan, C.A.; Spitzler, P.J.; Orme, J.P.; Finlay, M.R.V.; Ward, R.A.; Mellor, M.J.; et al. AZD9291, an Irreversible EGFR TKI, Overcomes T790M-Mediated Resistance to EGFR Inhibitors in Lung Cancer. Cancer Discov. 2014, 4, 1046. [Google Scholar] [CrossRef]
- Chagoya, G.; Kwatra, S.G.; Nanni, C.W.; Roberts, C.M.; Phillips, S.M.; Nullmeyergh, S.; Gilmore, S.P.; Spasojevic, I.; Corcoran, D.L.; Young, C.C.; et al. Efficacy of osimertinib against EGFRvIII+ glioblastoma. Oncotarget 2020, 11, 2074–2082. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, C.D.; Wu, H.; Wang, Z.W.; Wang, L.; Jiang, Z.R.; Wang, A.L.; Hu, C.; Dong, Y.F.; Niu, W.X.; et al. Osimertinib successfully combats EGFR-negative glioblastoma cells by inhibiting the MAPK pathway. Acta Pharm. Sin. 2021, 42, 108–114. [Google Scholar] [CrossRef]
- Makhlin, I.; Salinas, R.D.; Zhang, D.; Jacob, F.; Ming, G.L.; Song, H.; Saxena, D.; Dorsey, J.F.; Nasrallah, M.P.; Morrissette, J.J.; et al. Clinical activity of the EGFR tyrosine kinase inhibitor osimertinib in EGFR-mutant glioblastoma. CNS Oncol. 2019, 8, Cns43. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, B.; Lassen, U.; Hansen, S.; Holmberg, M.; Sørensen, M.; Kosteljanetz, M.; Broholm, H.; Stockhausen, M.T.; Poulsen, H.S. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: A phase II trial. Neuro Oncol. 2010, 12, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Neyns, B.; Sadones, J.; Joosens, E.; Bouttens, F.; Verbeke, L.; Baurain, J.F.; D’Hondt, L.; Strauven, T.; Chaskis, C.; In’t Veld, P.; et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann. Oncol. 2009, 20, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Peng, Y.; Liao, Y.; Jiang, W.; Wei, R.; Huo, L.; Han, Z.; Duan, C.; Zhong, M. Nimotuzumab prolongs survival in patients with malignant gliomas: A phase I/II clinical study of concomitant radiochemotherapy with or without nimotuzumab. Exp. Ther. Med. 2012, 4, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, L.; Sheng, X.F.; Chen, S.; Dai, J.Z. Nimotuzumab, a humanized monoclonal antibody specific for the EGFR, in combination with temozolomide and radiation therapy for newly diagnosed glioblastoma multiforme: First results in Chinese patients. Asia Pac. J. Clin. Oncol. 2016, 12, e23–e29. [Google Scholar] [CrossRef]
- Westphal, M.; Heese, O.; Steinbach, J.P.; Schnell, O.; Schackert, G.; Mehdorn, M.; Schulz, D.; Simon, M.; Schlegel, U.; Senft, C.; et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur. J. Cancer 2015, 51, 522–532. [Google Scholar] [CrossRef]
- Gan, H.K.; Reardon, D.A.; Lassman, A.B.; Merrell, R.; van den Bent, M.; Butowski, N.; Lwin, Z.; Wheeler, H.; Fichtel, L.; Scott, A.M.; et al. Safety, pharmacokinetics, and antitumor response of depatuxizumab mafodotin as monotherapy or in combination with temozolomide in patients with glioblastoma. Neuro Oncol. 2018, 20, 838–847. [Google Scholar] [CrossRef]
- Van Den Bent, M.; Eoli, M.; Sepulveda, J.M.; Smits, M.; Walenkamp, A.; Frenel, J.S.; Franceschi, E.; Clement, P.M.; Chinot, O.; De Vos, F.; et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma. Neuro Oncol. 2020, 22, 684–693. [Google Scholar] [CrossRef]
- Padovan, M.; Eoli, M.; Pellerino, A.; Rizzato, S.; Caserta, C.; Simonelli, M.; Michiara, M.; Caccese, M.; Anghileri, E.; Cerretti, G.; et al. Depatuxizumab Mafodotin (Depatux-M) Plus Temozolomide in Recurrent Glioblastoma Patients: Real-World Experience from a Multicenter Study of Italian Association of Neuro-Oncology (AINO). Cancers 2021, 13, 2773. [Google Scholar] [CrossRef]
- Schuster, J.; Lai, R.K.; Recht, L.D.; Reardon, D.A.; Paleologos, N.A.; Groves, M.D.; Mrugala, M.M.; Jensen, R.; Baehring, J.M.; Sloan, A.; et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: The ACT III study. Neuro Oncol. 2015, 17, 854–861. [Google Scholar] [CrossRef]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.L.; Morgan, R.A.; Yang, J.C.; Sherry, R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.C.; Lu, L.; Zheng, Z.; et al. Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor-transduced T Cells Targeting EGFRvIII in Patients With Glioblastoma. J. Immunother. 2019, 42, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.-Q.; Zeng, L.-S.; Wang, L.-F.; Wang, Y.-Y.; Cheng, J.-T.; Zhang, Y.; Han, Z.-W.; Zhou, Y.; Huang, S.-L.; Wang, X.-W.; et al. The Latest Battles Between EGFR Monoclonal Antibodies and Resistant Tumor Cells. Front. Oncol. 2020, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
- Dreier, A.; Barth, S.; Goswami, A.; Weis, J. Cetuximab induces mitochondrial translocalization of EGFRvIII, but not EGFR: Involvement of mitochondria in tumor drug resistance? Tumour Biol. 2012, 33, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Susam-Sen, H.; Varan, A.; Bajin, İ.; Göçmen, R.; Aydın, B.; Yalcin, B.; Kurucu, N.; Kutluk, T.; Bayhan, T.; Akyuz, C. Nimotuzumab therapy in the treatment of pediatric central nervous system tumors: Single-center experience. Naunyn Schmiedebergs Arch. Pharm. 2021, 394, 1769–1777. [Google Scholar] [CrossRef]
- Cabanas, R.; Saurez, G.; Rios, M.; Alert, J.; Reyes, A.; Valdes, J.; Gonzalez, M.C.; Pedrayes, J.L.; Avila, M.; Herrera, R.; et al. Treatment of children with high grade glioma with nimotuzumab: A 5-year institutional experience. MAbs 2013, 5, 202–207. [Google Scholar] [CrossRef]
- Greenall, S.A.; McKenzie, M.; Seminova, E.; Dolezal, O.; Pearce, L.; Bentley, J.; Kuchibhotla, M.; Chen, S.C.; McDonald, K.L.; Kornblum, H.I.; et al. Most clinical anti-EGFR antibodies do not neutralize both wtEGFR and EGFRvIII activation in glioma. Neuro Oncol. 2019, 21, 1016–1027. [Google Scholar] [CrossRef]
- Jungbluth, A.A.; Stockert, E.; Huang, H.J.S.; Collins, V.P.; Coplan, K.; Iversen, K.; Kolb, D.; Johns, T.J.; Scott, A.M.; Gullick, W.J.; et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 639. [Google Scholar] [CrossRef]
- Heimberger, A.B.; Crotty, L.E.; Archer, G.E.; Hess, K.R.; Wikstrand, C.J.; Friedman, A.H.; Friedman, H.S.; Bigner, D.D.; Sampson, J.H. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin. Cancer Res. 2003, 9, 4247–4254. [Google Scholar]
- Choi, B.D.; Suryadevara, C.M.; Gedeon, P.C.; Herndon, J.E., 2nd; Sanchez-Perez, L.; Bigner, D.D.; Sampson, J.H. Intracerebral delivery of a third generation EGFRvIII-specific chimeric antigen receptor is efficacious against human glioma. J. Clin. Neurosci. 2014, 21, 189–190. [Google Scholar] [CrossRef]
- Morgan, R.A.; Johnson, L.A.; Davis, J.L.; Zheng, Z.; Woolard, K.D.; Reap, E.A.; Feldman, S.A.; Chinnasamy, N.; Kuan, C.T.; Song, H.; et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum. Gene Ther. 2012, 23, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Yabo, Y.A.; Niclou, S.P.; Golebiewska, A. Cancer cell heterogeneity and plasticity: A paradigm shift in glioblastoma. Neuro Oncol. 2021, 24, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Neftel, C.; Laffy, J.; Filbin, M.G.; Hara, T.; Shore, M.E.; Rahme, G.J.; Richman, A.R.; Silverbush, D.; Shaw, M.L.; Hebert, C.M.; et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 2019, 178, 835–849.e821. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Legate, K.R.; Wickström, S.A.; Fässler, R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009, 23, 397–418. [Google Scholar] [CrossRef]
- Spiering, D.; Hodgson, L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adhes. Migr. 2011, 5, 170–180. [Google Scholar] [CrossRef]
- Vengoji, R.; Macha, M.A.; Nimmakayala, R.K.; Rachagani, S.; Siddiqui, J.A.; Mallya, K.; Gorantla, S.; Jain, M.; Ponnusamy, M.P.; Batra, S.K.; et al. Afatinib and Temozolomide combination inhibits tumorigenesis by targeting EGFRvIII-cMet signaling in glioblastoma cells. J. Exp. Clin. Cancer Res. 2019, 38, 266. [Google Scholar] [CrossRef]
- Greenall, S.A.; Donoghue, J.F.; Van Sinderen, M.; Dubljevic, V.; Budiman, S.; Devlin, M.; Street, I.; Adams, T.E.; Johns, T.G. EGFRvIII-mediated transactivation of receptor tyrosine kinases in glioma: Mechanism and therapeutic implications. Oncogene 2015, 34, 5277–5287. [Google Scholar] [CrossRef]
- Zheng, Q.; Han, L.; Dong, Y.; Tian, J.; Huang, W.; Liu, Z.; Jia, X.; Jiang, T.; Zhang, J.; Li, X.; et al. JAK2/STAT3 targeted therapy suppresses tumor invasion via disruption of the EGFRvIII/JAK2/STAT3 axis and associated focal adhesion in EGFRvIII-expressing glioblastoma. Neuro-Oncology 2014, 16, 1229–1243. [Google Scholar] [CrossRef]
- Long, W.; Yi, P.; Amazit, L.; LaMarca, H.L.; Ashcroft, F.; Kumar, R.; Mancini, M.A.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. SRC-3Delta4 mediates the interaction of EGFR with FAK to promote cell migration. Mol. Cell 2010, 37, 321–332. [Google Scholar] [CrossRef]
- Sieg, D.J.; Hauck, C.R.; Ilic, D.; Klingbeil, C.K.; Schaefer, E.; Damsky, C.H.; Schlaepfer, D.D. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000, 2, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ellerbroek, S.M.; Halbleib, J.M.; Benavidez, M.; Warmka, J.K.; Wattenberg, E.V.; Stack, M.S.; Hudson, L.G. Phosphatidylinositol 3-kinase activity in epidermal growth factor-stimulated matrix metalloproteinase-9 production and cell surface association. Cancer Res. 2001, 61, 1855–1861. [Google Scholar] [PubMed]
- Senft, C.; Priester, M.; Polacin, M.; Schröder, K.; Seifert, V.; Kögel, D.; Weissenberger, J. Inhibition of the JAK-2/STAT3 signaling pathway impedes the migratory and invasive potential of human glioblastoma cells. J. Neuro-Oncol. 2011, 101, 393–403. [Google Scholar] [CrossRef]
- Kwiatkowska, A.; Kijewska, M.; Lipko, M.; Hibner, U.; Kaminska, B. Downregulation of Akt and FAK phosphorylation reduces invasion of glioblastoma cells by impairment of MT1-MMP shuttling to lamellipodia and downregulates MMPs expression. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2011, 1813, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Schmidt, M.H.H. EGFR and EGFRvIII Promote Angiogenesis and Cell Invasion in Glioblastoma: Combination Therapies for an Effective Treatment. Int. J. Mol. Sci. 2017, 18, 1295. [Google Scholar] [CrossRef]
- Raithatha, S.A.; Muzik, H.; Muzik, H.; Rewcastle, N.B.; Johnston, R.N.; Edwards, D.R.; Forsyth, P.A. Localization of gelatinase-A and gelatinase-B mRNA and protein in human gliomas. Neuro Oncol. 2000, 2, 145–150. [Google Scholar] [CrossRef]
- Forsyth, P.A.; Wong, H.; Laing, T.D.; Rewcastle, N.B.; Morris, D.G.; Muzik, H.; Leco, K.J.; Johnston, R.N.; Brasher, P.M.; Sutherland, G.; et al. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br. J. Cancer 1999, 79, 1828–1835. [Google Scholar] [CrossRef]
- Lampert, K.; Machein, U.; Machein, M.R.; Conca, W.; Peter, H.H.; Volk, B. Expression of matrix metalloproteinases and their tissue inhibitors in human brain tumors. Am. J. Pathol. 1998, 153, 429–437. [Google Scholar] [CrossRef][Green Version]
- Ramachandran, R.K.; Sørensen, M.D.; Aaberg-Jessen, C.; Hermansen, S.K.; Kristensen, B.W. Expression and prognostic impact of matrix metalloproteinase-2 (MMP-2) in astrocytomas. PLoS ONE 2017, 12, e0172234. [Google Scholar] [CrossRef]
- Choe, G.; Park, J.K.; Jouben-Steele, L.; Kremen, T.J.; Liau, L.M.; Vinters, H.V.; Cloughesy, T.F.; Mischel, P.S. Active matrix metalloproteinase 9 expression is associated with primary glioblastoma subtype. Clin. Cancer Res. 2002, 8, 2894–2901. [Google Scholar]
- Lakka, S.S.; Jasti, S.L.; Kyritsis, A.P.; Yung, W.K.A.; Ali-Osman, F.; Nicolson, G.L.; Rao, J.S. Regulation of MMP-9 (type IV collagenase) production and invasiveness in gliomas by the extracellular signal-regulated kinase and jun amino-terminal kinase signaling cascades. Clin. Exp. Metastasis 2000, 18, 245–252. [Google Scholar] [CrossRef]
- Hauck, C.R.; Sieg, D.J.; Hsia, D.A.; Loftus, J.C.; Gaarde, W.A.; Monia, B.P.; Schlaepfer, D.D. Inhibition of Focal Adhesion Kinase Expression or Activity Disrupts Epidermal Growth Factor-stimulated Signaling Promoting the Migration of Invasive Human Carcinoma Cells1. Cancer Res. 2001, 61, 7079–7090. [Google Scholar]
- Kolli-Bouhafs, K.; Boukhari, A.; Abusnina, A.; Velot, E.; Gies, J.-P.; Lugnier, C.; Rondé, P. Thymoquinone reduces migration and invasion of human glioblastoma cells associated with FAK, MMP-2 and MMP-9 down-regulation. Investig. New Drugs 2012, 30, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Xu, Q.; Wang, C.; Lin, X.; Zhang, Q.; Wu, N. A tropomyosin-like Meretrix meretrix Linnaeus polypeptide inhibits the proliferation and metastasis of glioma cells via microtubule polymerization and FAK/Akt/MMPs signaling. Int. J. Biol. Macromol. 2020, 145, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, C.; Hackmann, O.; Delic, S.; Schmidt, N.; Reifenberger, G.; Riemenschneider, M.J. SOCS3 promoter methylation is mutually exclusive to EGFR amplification in gliomas and promotes glioma cell invasion through STAT3 and FAK activation. Acta Neuropathol. 2011, 122, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.C.; Wei, X.T.; Guan, J.H.; Shu, H.; Chen, D. EGF stimulates glioblastoma metastasis by induction of matrix metalloproteinase-9 in an EGFR-dependent mechanism. Oncotarget 2017, 8, 65969–65982. [Google Scholar] [CrossRef]
- Lorimer, I.A.J.; Lavictoire, S.J. Activation of extracellular-regulated kinases by normal and mutant EGF receptors. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2001, 1538, 1–9. [Google Scholar] [CrossRef]
- Leirdal, M.; Sioud, M. Protein kinase Calpha isoform regulates the activation of the MAP kinase ERK1/2 in human glioma cells: Involvement in cell survival and gene expression. Mol. Cell Biol. Res. Commun. 2000, 4, 106–110. [Google Scholar] [CrossRef]
- Hu, J.G.; Wang, X.F.; Zhou, J.S.; Wang, F.C.; Li, X.W.; Lü, H.Z. Activation of PKC-alpha is required for migration of C6 glioma cells. Acta Neurobiol. Exp. (Wars) 2010, 70, 239–245. [Google Scholar]
- Sangar, V.; Funk, C.C.; Kusebauch, U.; Campbell, D.S.; Moritz, R.L.; Price, N.D. Quantitative Proteomic Analysis Reveals Effects of Epidermal Growth Factor Receptor (EGFR) on Invasion-promoting Proteins Secreted by Glioblastoma Cells *. Mol. Cell. Proteom. 2014, 13, 2618–2631. [Google Scholar] [CrossRef]
- Thomas, R.; Weihua, Z. Rethink of EGFR in Cancer With Its Kinase Independent Function on Board. Front Oncol. 2019, 9, 800. [Google Scholar] [CrossRef]
- Weihua, Z.; Tsan, R.; Huang, W.C.; Wu, Q.; Chiu, C.H.; Fidler, I.J.; Hung, M.C. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell 2008, 13, 385–393. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Simon, O.J.; Müntefering, T.; Grauer, O.M.; Meuth, S.G. The role of ion channels in malignant brain tumors. J. Neuro-Oncol. 2015, 125, 225–235. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010, 16, 107–121. [Google Scholar] [CrossRef]
- Bazargani, N.; Attwell, D. Astrocyte calcium signaling: The third wave. Nat. Neurosci. 2016, 19, 182–189. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Ousingsawat, J.; Benedetto, R.; Cabrita, I.; Schreiber, R. Contribution of Anoctamins to Cell Survival and Cell Death. Cancers 2019, 11, 382. [Google Scholar] [CrossRef]
- Picollo, A.; Malvezzi, M.; Accardi, A. TMEM16 proteins: Unknown structure and confusing functions. J. Mol. Biol. 2015, 427, 94–105. [Google Scholar] [CrossRef]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef]
- Pifferi, S.; Dibattista, M.; Menini, A. TMEM16B induces chloride currents activated by calcium in mammalian cells. Pflug. Arch. 2009, 458, 1023–1038. [Google Scholar] [CrossRef]
- Schroeder, B.C.; Cheng, T.; Jan, Y.N.; Jan, L.Y. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 2008, 134, 1019–1029. [Google Scholar] [CrossRef]
- Yang, Y.D.; Cho, H.; Koo, J.Y.; Tak, M.H.; Cho, Y.; Shim, W.S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.M.; et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef]
- Wang, H.; Zou, L.; Ma, K.; Yu, J.; Wu, H.; Wei, M.; Xiao, Q. Cell-specific mechanisms of TMEM16A Ca(2+)-activated chloride channel in cancer. Mol. Cancer 2017, 16, 152. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Zhang, Z.; Fan, Y.; Zhang, L.; Gao, K.; Luo, S.; Xiao, Q.; Sun, C. Simvastatin inhibits oral squamous cell carcinoma by targeting TMEM16A Ca(2+)-activated chloride channel. J. Cancer Res. Clin. Oncol. 2021, 147, 1699–1711. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, C.; Ma, Z.; Wang, H.; Tuo, B.; Cheng, X.; Liu, X.; Li, T. Pathophysiological role of ion channels and transporters in HER2-positive breast cancer. Cancer Gene Ther. 2022, 29, 1097–1104. [Google Scholar] [CrossRef]
- Britschgi, A.; Bill, A.; Brinkhaus, H.; Rothwell, C.; Clay, I.; Duss, S.; Rebhan, M.; Raman, P.; Guy, C.T.; Wetzel, K.; et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E1026–E1034. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Ren, Y.; Kang, L.; Zhang, L. Transmembrane protein with unknown function 16A overexpression promotes glioma formation through the nuclear factor-κB signaling pathway. Mol. Med. Rep. 2014, 9, 1068–1074. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Yang, F.; Gao, K.; Luo, S.; Bai, L.; Ma, K.; Liu, M.; Wu, S.; Wang, H.; et al. Honokiol inhibits proliferation of colorectal cancer cells by targeting anoctamin 1/TMEM16A Ca(2+) -activated Cl(-) channels. Br. J. Pharm. 2021, 178, 4137–4154. [Google Scholar] [CrossRef]
- Bill, A.; Gutierrez, A.; Kulkarni, S.; Kemp, C.; Bonenfant, D.; Voshol, H.; Duvvuri, U.; Gaither, L.A. ANO1/TMEM16A interacts with EGFR and correlates with sensitivity to EGFR-targeting therapy in head and neck cancer. Oncotarget 2015, 6, 9173–9188. [Google Scholar] [CrossRef]
- Fröbom, R.; Sellberg, F.; Xu, C.; Zhao, A.; Larsson, C.; Lui, W.O.; Nilsson, I.L.; Berglund, E.; Bränström, R. Biochemical Inhibition of DOG1/TMEM16A Achieves Antitumoral Effects in Human Gastrointestinal Stromal Tumor Cells In Vitro. Anticancer Res. 2019, 39, 3433–3442. [Google Scholar] [CrossRef]
- Shiwarski, D.J.; Shao, C.; Bill, A.; Kim, J.; Xiao, D.; Bertrand, C.A.; Seethala, R.S.; Sano, D.; Myers, J.N.; Ha, P.; et al. To “grow” or “go”: TMEM16A expression as a switch between tumor growth and metastasis in SCCHN. Clin. Cancer Res. 2014, 20, 4673–4688. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, J.-Y.; Jung, C.-W.; Lee, Y.-S.; An, J.-Y.; Kim, E.H.; Kim, K.-H.; Lee, S.P.; Park, J.-Y.; Park, M.-J. ANO1 regulates the maintenance of stemness in glioblastoma stem cells by stabilizing EGFRvIII. Oncogene 2021, 40, 1490–1502. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-Y.; Zhou, L.; Hou, X.-F.; Lian, H. Anoctamin 5 regulates cell proliferation and migration in pancreatic cancer. Int. J. Clin. Exp. Pathol. 2019, 12, 4263–4270. [Google Scholar] [PubMed]
- Chang, Z.; Cai, C.; Han, D.; Gao, Y.; Li, Q.; Feng, L.; Zhang, W.; Zheng, J.; Jin, J.; Zhang, H.; et al. Anoctamin5 regulates cell migration and invasion in thyroid cancer. Int. J. Oncol. 2017, 51, 1311–1319. [Google Scholar] [CrossRef]
- Pan, R.; Lu, Q.; Ren, C.; Li, H.; Zeng, F.; Tian, X.; Chen, H. Anoctamin 5 promotes osteosarcoma development by increasing degradation of Nel-like proteins 1 and 2. Aging 2021, 13, 17316–17327. [Google Scholar] [CrossRef]
- Xuan, Z.B.; Wang, Y.J.; Xie, J. ANO6 promotes cell proliferation and invasion in glioma through regulating the ERK signaling pathway. OncoTargets Ther. 2019, 12, 6721–6731. [Google Scholar] [CrossRef]
- Marx, A.; Koopmann, L.; Höflmayer, D.; Büscheck, F.; Hube-Magg, C.; Steurer, S.; Eichenauer, T.; Clauditz, T.S.; Wilczak, W.; Simon, R.; et al. Reduced anoctamin 7 (ANO7) expression is a strong and independent predictor of poor prognosis in prostate cancer. Cancer Biol. Med. 2021, 18, 245–255. [Google Scholar] [CrossRef]
- Jun, I.; Park, H.S.; Piao, H.; Han, J.W.; An, M.J.; Yun, B.G.; Zhang, X.; Cha, Y.H.; Shin, Y.K.; Yook, J.I.; et al. ANO9/TMEM16J promotes tumourigenesis via EGFR and is a novel therapeutic target for pancreatic cancer. Br. J. Cancer 2017, 117, 1798–1809. [Google Scholar] [CrossRef]
- Li, C.; Cai, S.; Wang, X.; Jiang, Z. Identification and characterization of ANO9 in stage II and III colorectal carcinoma. Oncotarget 2015, 6, 29324–29334. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Tian, Y.; Martins, J.R.; Faria, D.; Kongsuphol, P.; Ousingsawat, J.; Thevenod, F.; Roussa, E.; Rock, J.; Schreiber, R. Anoctamins. Pflügers Arch. Eur. J. Physiol. 2011, 462, 195–208. [Google Scholar] [CrossRef]
- Almaça, J.; Tian, Y.; Aldehni, F.; Ousingsawat, J.; Kongsuphol, P.; Rock, J.R.; Harfe, B.D.; Schreiber, R.; Kunzelmann, K. TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A. J. Biol. Chem. 2009, 284, 28571–28578. [Google Scholar] [CrossRef] [PubMed]
- Cuddapah, V.A.; Sontheimer, H. Ion channels and transporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration. Am. J. Physiol. Cell Physiol. 2011, 301, C541–C549. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.W.; Wang, L.W.; Jacob, T.; Sun, X.R.; Li, H.; Zhu, L.Y.; Li, P.; Zhong, P.; Nie, S.H.; Chen, L.X. Involvement of regulatory volume decrease in the migration of nasopharyngeal carcinoma cells. Cell Res. 2005, 15, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef]
- Ruiz, C.; Martins, J.R.; Rudin, F.; Schneider, S.; Dietsche, T.; Fischer, C.A.; Tornillo, L.; Terracciano, L.M.; Schreiber, R.; Bubendorf, L.; et al. Enhanced expression of ANO1 in head and neck squamous cell carcinoma causes cell migration and correlates with poor prognosis. PLoS ONE 2012, 7, e43265. [Google Scholar] [CrossRef]
- Morishita, K.; Watanabe, K.; Ichijo, H. Cell volume regulation in cancer cell migration driven by osmotic water flow. Cancer Sci. 2019, 110, 2337–2347. [Google Scholar] [CrossRef]
- Sauter, D.; Novak, I.; Pedersen, S.; Larsen, E.; Hoffmann, E. ANO1 (TMEM16A) in pancreatic ductal adenocarcinoma (PDAC). Pflügers Arch. Eur. J. Physiol. 2015, 467, 1495–1508. [Google Scholar] [CrossRef]
- Lee, Y.S.; Bae, Y.; Park, N.; Yoo, J.C.; Cho, C.H.; Ryoo, K.; Hwang, E.M.; Park, J.Y. Surface expression of the Anoctamin-1 (ANO1) channel is suppressed by protein-protein interactions with β-COP. Biochem. Biophys. Res. Commun. 2016, 475, 216–222. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, J.K.; Bae, Y.; Lee, B.S.; Kim, E.; Cho, C.H.; Ryoo, K.; Yoo, J.; Kim, C.H.; Yi, G.S.; et al. Suppression of 14-3-3γ-mediated surface expression of ANO1 inhibits cancer progression of glioblastoma cells. Sci. Rep. 2016, 6, 26413. [Google Scholar] [CrossRef]
- Stec, W.; Rosiak, K.; Treda, C.; Smolarz, M.; Peciak, J.; Pacholczyk, M.; Lenart, A.; Grzela, D.; Stoczynska-Fidelus, E.; Rieske, P. Cyclic trans-phosphorylation in a homodimer as the predominant mechanism of EGFRvIII action and regulation. Oncotarget 2018, 9, 8560–8572. [Google Scholar] [CrossRef][Green Version]
- Grandal, M.V.; Zandi, R.; Pedersen, M.W.; Willumsen, B.M.; van Deurs, B.; Poulsen, H.S. EGFRvIII escapes down-regulation due to impaired internalization and sorting to lysosomes. Carcinogenesis 2007, 28, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.S.; Nagane, M.; Klingbeil, C.K.; Lin, H.; Nishikawa, R.; Ji, X.-D.; Huang, C.-M.; Gill, G.N.; Wiley, H.S.; Cavenee, W.K. The Enhanced Tumorigenic Activity of a Mutant Epidermal Growth Factor Receptor Common in Human Cancers Is Mediated by Threshold Levels of Constitutive Tyrosine Phosphorylation and Unattenuated Signaling *. J. Biol. Chem. 1997, 272, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Scudieri, P.; Caci, E.; Venturini, A.; Sondo, E.; Pianigiani, G.; Marchetti, C.; Ravazzolo, R.; Pagani, F.; Galietta, L.J. Ion channel and lipid scramblase activity associated with expression of TMEM16F/ANO6 isoforms. J. Physiol. 2015, 593, 3829–3848. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Umeda, M.; Sims, P.J.; Nagata, S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature 2010, 468, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Juul, C.A.; Grubb, S.; Poulsen, K.A.; Kyed, T.; Hashem, N.; Lambert, I.H.; Larsen, E.H.; Hoffmann, E.K. Anoctamin 6 differs from VRAC and VSOAC but is involved in apoptosis and supports volume regulation in the presence of Ca2+. Pflug. Arch. 2014, 466, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Cernysiov, V.; Davidson, D.; Song, H.; Tang, J.; Luo, S.; Lu, Y.; Qian, J.; Gyurova, I.E.; Waggoner, S.N.; et al. Critical Role of Lipid Scramblase TMEM16F in Phosphatidylserine Exposure and Repair of Plasma Membrane after Pore Formation. Cell Rep. 2020, 30, 1129–1140.e1125. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, K.S.; Zeeberg, K.; Sauter, D.R.; Poulsen, K.A.; Hoffmann, E.K.; Schwab, A. The role of TMEM16A (ANO1) and TMEM16F (ANO6) in cell migration. Pflug. Arch. 2013, 465, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.K. Ion Channels Involved in Cell Volume Regulation: Effects on Migration, Proliferation, and Programmed Cell Death in Non Adherent EAT Cells and Adherent ELA Cells. Cell. Physiol. Biochem. 2011, 28, 1061–1078. [Google Scholar] [CrossRef]
- Soroceanu, L.; Manning, T.J., Jr.; Sontheimer, H. Modulation of glioma cell migration and invasion using Cl(-) and K(+) ion channel blockers. J. Neurosci. 1999, 19, 5942–5954. [Google Scholar] [CrossRef]
- Kulkarni, S.; Bill, A.; Godse, N.R.; Khan, N.I.; Kass, J.I.; Steehler, K.; Kemp, C.; Davis, K.; Bertrand, C.A.; Vyas, A.R.; et al. TMEM16A/ANO1 suppression improves response to antibody-mediated targeted therapy of EGFR and HER2/ERBB2. Genes Chromosom. Cancer 2017, 56, 460–471. [Google Scholar] [CrossRef]
- Seo, Y.; Jeong, S.B.; Woo, J.H.; Kwon, O.-B.; Lee, S.; Oh, H.I.; Jo, S.; Park, S.J.; Namkung, W.; Moon, U.Y.; et al. Diethylstilbestrol, a Novel ANO1 Inhibitor, Exerts an Anticancer Effect on Non-Small Cell Lung Cancer via Inhibition of ANO1. Int. J. Mol. Sci. 2021, 22, 7100. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Song, Y.; Gao, J.; Gao, J.; Wang, K. Inhibition of calcium-activated chloride channel ANO1 suppresses proliferation and induces apoptosis of epithelium originated cancer cells. Oncotarget 2016, 7, 78619–78630. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Choi, J.; Lee, J.H.; Kim, T.G.; Park, S.-h.; Han, G.; Namkung, W.; Kim, I. Diversity-oriented generation and biological evaluation of new chemical scaffolds bearing a 2,2-dimethyl-2H-chromene unit: Discovery of novel potent ANO1 inhibitors. Bioorg. Chem. 2020, 101, 104000. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Ryu, S.; Sim, K.; Song, C.; Shin, I.; Kim, S.S.; Lee, Y.S.; Park, J.Y.; Sim, T. Anti-glioma effects of 2-aminothiophene-3-carboxamide derivatives, ANO1 channel blockers. Eur. J. Med. Chem. 2020, 208, 112688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, G.; Zhao, Z.; Xiu, R.; Jia, J.; Chen, P.; Liu, Y.; Wang, Y.; Yi, J. Cepharanthine, a novel selective ANO1 inhibitor with potential for lung adenocarcinoma therapy. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2021, 1868, 119132. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Tao, L.; Cao, Y.X.; Dong, L.; Hu, Y.Z.; Yang, A.G.; Zhou, S.S. Effects of niflumic acid on the proliferation of human hepatoma cells. Sheng Li Xue Bao 2003, 55, 160–164. [Google Scholar] [PubMed]
- Luo, S.; Huang, G.; Wang, Z.; Wan, Z.; Chen, H.; Liao, D.; Chen, C.; Li, H.; Li, B.; Chen, L.; et al. Niflumic acid exhibits anti-tumor activity in nasopharyngeal carcinoma cells through affecting the expression of ERK1/2 and the activity of MMP2 and MMP9. Int. J. Clin. Exp. Pathol. 2015, 8, 9990–10001. [Google Scholar]
- Park, M.; Song, C.; Yoon, H.; Choi, K.H. Double Blockade of Glioma Cell Proliferation and Migration by Temozolomide Conjugated with NPPB, a Chloride Channel Blocker. ACS Chem. Neurosci. 2016, 7, 275–285. [Google Scholar] [CrossRef]
- Kei, K.; Yoshie, S.; Tsuji, S.; Murono, S.; Hazama, A. Dysfunction of Cl(-) channels promotes epithelial to mesenchymal transition in oral squamous cell carcinoma via activation of Wnt/β-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2021, 555, 95–101. [Google Scholar] [CrossRef]
- Wieland, A.; Trageser, D.; Gogolok, S.; Reinartz, R.; Höfer, H.; Keller, M.; Leinhaas, A.; Schelle, R.; Normann, S.; Klaas, L.; et al. Anticancer Effects of Niclosamide in Human Glioblastoma. Clin. Cancer Res. 2013, 19, 4124–4136. [Google Scholar] [CrossRef]
- Oh, H.-C.; Shim, J.-K.; Park, J.; Lee, J.-H.; Choi, R.J.; Kim, N.H.; Kim, H.S.; Moon, J.H.; Kim, E.H.; Chang, J.H.; et al. Combined effects of niclosamide and temozolomide against human glioblastoma tumorspheres. J. Cancer Res. Clin. Oncol. 2020, 146, 2817–2828. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Morales, L.D.; Zhang, Y.; Mito, S.; Tsin, A. Niclosamide induces protein ubiquitination and inhibits multiple pro-survival signaling pathways in the human glioblastoma U-87 MG cell line. PLoS ONE 2017, 12, e0184324. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, N.A.; Nik Salleh, N.N.; Tan, S.C. Gallotannin-Enriched Fraction from Quercus infectoria Galls as an Antioxidant and Inhibitory Agent against Human Glioblastoma Multiforme. Plants 2021, 10, 2581. [Google Scholar] [CrossRef] [PubMed]
- Al-Halabi, R.; Bou Chedid, M.; Abou Merhi, R.; El-Hajj, H.; Zahr, H.; Schneider-Stock, R.; Bazarbachi, A.; Gali-Muhtasib, H. Gallotannin inhibits NFĸB signaling and growth of human colon cancer xenografts. Cancer Biol. Ther. 2011, 12, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.G.; Han, Y.H.; Jeon, H.D.; Yoon, D.H.; Lee, Y.G.; Hong, S.H.; Kee, J.Y. Inhibitory Effect of Gallotannin on Lung Metastasis of Metastatic Colorectal Cancer Cells by Inducing Apoptosis, Cell Cycle Arrest and Autophagy. Am. J. Chin. Med. 2021, 49, 1535–1555. [Google Scholar] [CrossRef]
- Al-Halabi, R.; Abou Merhi, R.; Chakilam, S.; El-Baba, C.; Hamade, E.; Di Fazio, P.; Ocker, M.; Schneider-Stock, R.; Gali-Muhtasib, H. Gallotannin is a DNA damaging compound that induces senescence independently of p53 and p21 in human colon cancer cells. Mol. Carcinog. 2015, 54, 1037–1050. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, Q.; del Rincon, S.V.; Lovato, A.; Marques, M.; Witcher, M. Gallotannin imposes S phase arrest in breast cancer cells and suppresses the growth of triple-negative tumors in vivo. PLoS ONE 2014, 9, e92853. [Google Scholar] [CrossRef]
- Park, E.; Kwon, H.Y.; Jung, J.H.; Jung, D.B.; Jeong, A.; Cheon, J.; Kim, B.; Kim, S.H. Inhibition of Myeloid Cell Leukemia 1 and Activation of Caspases Are Critically Involved in Gallotannin-induced Apoptosis in Prostate Cancer Cells. Phytother. Res. 2015, 29, 1225–1236. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Kim, J.H.; Kim, B.; Srivastava, S.K.; Kim, S.H. Regulation of SIRT1/AMPK axis is critically involved in gallotannin-induced senescence and impaired autophagy leading to cell death in hepatocellular carcinoma cells. Arch. Toxicol. 2018, 92, 241–257. [Google Scholar] [CrossRef]
- Namkung, W.; Thiagarajah, J.R.; Phuan, P.-W.; Verkman, A.S. Inhibition of Ca2+-activated Cl- channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J. 2010, 24, 4178–4186. [Google Scholar] [CrossRef]
- Hwang, S.J.; Basma, N.; Sanders, K.M.; Ward, S.M. Effects of new-generation inhibitors of the calcium-activated chloride channel anoctamin 1 on slow waves in the gastrointestinal tract. Br. J. Pharm. 2016, 173, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Centeio, R.; Cabrita, I.; Benedetto, R.; Talbi, K.; Ousingsawat, J.; Schreiber, R.; Sullivan, J.K.; Kunzelmann, K. Pharmacological Inhibition and Activation of the Ca(2+) Activated Cl(-) Channel TMEM16A. Int. J. Mol. Sci. 2020, 21, 2557. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, K.; Vink, J.Y.; Fu, X.W.; Wakita, H.; Danielsson, J.; Wapner, R.; Gallos, G. Calcium-activated chloride channels anoctamin 1 and 2 promote murine uterine smooth muscle contractility. Am. J. Obstet. Gynecol. 2014, 211, 688.e1–688.e10. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.R.; Faria, D.; Kongsuphol, P.; Reisch, B.; Schreiber, R.; Kunzelmann, K. Anoctamin 6 is an essential component of the outwardly rectifying chloride channel. Proc. Natl. Acad. Sci. USA 2011, 108, 18168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Huang, D.; Qi, J.; Xu, J.; Gao, H.; Du, X.; Gamper, N.; Zhang, H. Characterization of the effects of Cl− channel modulators on TMEM16A and bestrophin-1 Ca2+ activated Cl− channels. Pflügers Arch. Eur. J. Physiol. 2015, 467, 1417–1430. [Google Scholar] [CrossRef]
- Zhou, S.-S.; Zhang, L.-B.; Sun, W.-P.; Xiao, F.-C.; Zhou, Y.-M.; Li, Y.-J.; Li, D.-L. Effects of monocarboxylic acid-derived Cl− channel blockers on depolarization-activated potassium currents in rat ventricular myocytes. Exp. Physiol. 2007, 92, 549–559. [Google Scholar] [CrossRef]
- Zhou, S.S.; Yang, J.; Li, Y.Q.; Zhao, L.Y.; Xu, M.; Ding, Y.F. Effect of Cl- channel blockers on aconitine-induced arrhythmias in rat heart. Exp. Physiol. 2005, 90, 865–872. [Google Scholar] [CrossRef]
- Zhou, S.S.; Gao, Z.; Dong, L.; Ding, Y.F.; Zhang, X.D.; Wang, Y.M.; Pei, J.M.; Gao, F.; Ma, X.L. Anion channels influence ECC by modulating L-type Ca(2+) channel in ventricular myocytes. J. Appl. Physiol. (1985) 2002, 93, 1660–1668. [Google Scholar] [CrossRef]
- Szteyn, K.; Schmid, E.; Nurbaeva, M.K.; Yang, W.; Münzer, P.; Kunzelmann, K.; Lang, F.; Shumilina, E. Expression and functional significance of the Ca(2+)-activated Cl(-) channel ANO6 in dendritic cells. Cell. Physiol. Biochem. 2012, 30, 1319–1332. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Lin, K.-N.; Jhang, L.-M.; Huang, C.-H.; Lee, Y.-C.; Chang, L.-S. Gallic acid abolishes the EGFR/Src/Akt/Erk-mediated expression of matrix metalloproteinase-9 in MCF-7 breast cancer cells. Chem. Biol. Interact. 2016, 252, 131–140. [Google Scholar] [CrossRef]
- Cabrita, I.; Benedetto, R.; Schreiber, R.; Kunzelmann, K. Niclosamide repurposed for the treatment of inflammatory airway disease. JCI Insight 2019, 4, e128414. [Google Scholar] [CrossRef] [PubMed]
- Miner, K.; Labitzke, K.; Liu, B.; Wang, P.; Henckels, K.; Gaida, K.; Elliott, R.; Chen, J.J.; Liu, L.; Leith, A.; et al. Drug Repurposing: The Anthelmintics Niclosamide and Nitazoxanide Are Potent TMEM16A Antagonists That Fully Bronchodilate Airways. Front. Pharm. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.D.; Hewlett, E.L. Niclosamide therapy for tapeworm infections. Ann. Intern. Med. 1985, 102, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Chen, Y.-K.; Hsu, Y.-J.; Lin, B.-R. Niclosamide inhibits the cell proliferation and enhances the responsiveness of esophageal cancer cells to chemotherapeutic agents. Oncol. Rep. 2020, 43, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Haugk, K.; McKiernan, J.S.; Gulati, R.; Cheng, H.H.; Maes, J.L.; Dumpit, R.F.; Nelson, P.S.; Montgomery, B.; McCune, J.S.; et al. A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PLoS ONE 2018, 13, e0198389. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Liu, C.; Wu, C.-Y.; Evans, C.P.; Dall’Era, M.; Robles, D.; Lara, P.N.; Agarwal, N.; Gao, A.C.; Pan, C.-X. Phase Ib trial of reformulated niclosamide with abiraterone/prednisone in men with castration-resistant prostate cancer. Sci. Rep. 2021, 11, 6377. [Google Scholar] [CrossRef] [PubMed]
- Burock, S.; Daum, S.; Tröger, H.; Kim, T.D.; Krüger, S.; Rieke, D.T.; Ochsenreither, S.; Welter, K.; Herrmann, P.; Sleegers, A.; et al. Niclosamide a new chemotherapy agent? Pharmacokinetics of the potential anticancer drug in a patient cohort of the NIKOLO trial. J. Clin. Oncol. 2018, 36, e14536. [Google Scholar] [CrossRef]
- Bredel, M.; Scholtens, D.M.; Yadav, A.K.; Alvarez, A.A.; Renfrow, J.J.; Chandler, J.P.; Yu, I.L.Y.; Carro, M.S.; Dai, F.; Tagge, M.J.; et al. NFKBIA deletion in glioblastomas. N. Engl. J. Med. 2011, 364, 627–637. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, S.H.; Yang, S.H.; Kim, M.S. Restricting extracellular Ca2+ on gefitinib-resistant non-small cell lung cancer cells reverses altered epidermal growth factor-mediated Ca2+ response, which consequently enhances gefitinib sensitivity. PLoS ONE 2020, 15, e0238155. [Google Scholar] [CrossRef]
- Sharma, A.; Ramena, G.T.; Elble, R.C. Advances in Intracellular Calcium Signaling Reveal Untapped Targets for Cancer Therapy. Biomedicines 2021, 9, 1077. [Google Scholar] [CrossRef]
- Berridge, M.J. Neuronal calcium signaling. Neuron 1998, 21, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.K.M.; Galione, A. The endoplasmic reticulum and junctional membrane communication during calcium signaling. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 2542–2559. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; Pozzan, T. Microdomains of Intracellular Ca2+: Molecular Determinants and Functional Consequences. Physiol. Rev. 2006, 86, 369–408. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Shah, S.; Liu, Y.; Zhang, H.; Lees, M.; Fu, Z.; Lippiat, J.D.; Beech, D.J.; Sivaprasadarao, A.; Baldwin, S.A.; et al. Activation of the Cl- channel ANO1 by localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci. Signal. 2013, 6, ra73. [Google Scholar] [CrossRef]
- Guéguinou, M.; Gambade, A.; Félix, R.; Chantôme, A.; Fourbon, Y.; Bougnoux, P.; Weber, G.; Potier-Cartereau, M.; Vandier, C. Lipid rafts, KCa/ClCa/Ca2+ channel complexes and EGFR signaling: Novel targets to reduce tumor development by lipids? Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 2603–2620. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bai, L.; Luo, S.; Wang, T.; Yang, F.; Xia, J.; Wang, H.; Ma, K.; Liu, M.; Wu, S.; et al. TMEM16A Ca2+-activated Cl− channel inhibition ameliorates acute pancreatitis via the IP3R/Ca2+/NFκB/IL-6 signaling pathway. J. Adv. Res. 2020, 23, 25–35. [Google Scholar] [CrossRef]
- Cabrita, I.; Benedetto, R.; Fonseca, A.; Wanitchakool, P.; Sirianant, L.; Skryabin, B.V.; Schenk, L.K.; Pavenstädt, H.; Schreiber, R.; Kunzelmann, K. Differential effects of anoctamins on intracellular calcium signals. FASEB J. 2017, 31, 2123–2134. [Google Scholar] [CrossRef]
- Chinigò, G.; Castel, H.; Chever, O.; Gkika, D. TRP Channels in Brain Tumors. Front. Cell Dev. Biol. 2021, 9, 617801. [Google Scholar] [CrossRef]
- Zhai, K.; Liskova, A.; Kubatka, P.; Büsselberg, D. Calcium Entry through TRPV1: A Potential Target for the Regulation of Proliferation and Apoptosis in Cancerous and Healthy Cells. Int. J. Mol. Sci. 2020, 21, 4177. [Google Scholar] [CrossRef]
- Bode, A.M.; Cho, Y.-Y.; Zheng, D.; Zhu, F.; Ericson, M.E.; Ma, W.-Y.; Yao, K.; Dong, Z. Transient receptor potential type vanilloid 1 suppresses skin carcinogenesis. Cancer Res. 2009, 69, 905–913. [Google Scholar] [CrossRef]
- Li, S.; Bode, A.M.; Zhu, F.; Liu, K.; Zhang, J.; Kim, M.O.; Reddy, K.; Zykova, T.; Ma, W.Y.; Carper, A.L.; et al. TRPV1-antagonist AMG9810 promotes mouse skin tumorigenesis through EGFR/Akt signaling. Carcinogenesis 2011, 32, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Z.; Capó-Aponte, J.E.; Zhang, F.; Pan, Z.; Reinach, P.S. Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells. Exp. Eye Res. 2010, 91, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, J.; Qiu, L. Transient receptor potential vanilloid 1 promotes EGFR ubiquitination and modulates EGFR/MAPK signalling in pancreatic cancer cells. Cell Biochem. Funct. 2020, 38, 401–408. [Google Scholar] [CrossRef] [PubMed]
- de Jong, P.R.; Takahashi, N.; Harris, A.R.; Lee, J.; Bertin, S.; Jeffries, J.; Jung, M.; Duong, J.; Triano, A.I.; Lee, J.; et al. Ion channel TRPV1-dependent activation of PTP1B suppresses EGFR-associated intestinal tumorigenesis. J. Clin. Investig. 2014, 124, 3793–3806. [Google Scholar] [CrossRef] [PubMed]
- Amantini, C.; Mosca, M.; Nabissi, M.; Lucciarini, R.; Caprodossi, S.; Arcella, A.; Giangaspero, F.; Santoni, G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J. Neurochem. 2007, 102, 977–990. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Arcella, A.; Cardinali, C.; Santoni, M.; Bernardini, G.; Santoni, A.; Santoni, G.; Amantini, C. Post-transcriptional regulation of 5’-untranslated regions of human Transient Receptor Potential Vanilloid type-1 (TRPV-1) channels: Role in the survival of glioma patients. Oncotarget 2016, 7, 81541–81554. [Google Scholar] [CrossRef]
- Lu, J.; Ju, Y.-T.; Li, C.; Hua, F.-Z.; Xu, G.-H.; Hu, Y.-H. Effect of TRPV1 combined with lidocaine on cell state and apoptosis of U87-MG glioma cell lines. Asian Pac. J. Trop. Med. 2016, 9, 288–292. [Google Scholar] [CrossRef]
- Stock, K.; Kumar, J.; Synowitz, M.; Petrosino, S.; Imperatore, R.; Smith, E.S.J.; Wend, P.; Purfürst, B.; Nuber, U.A.; Gurok, U.; et al. Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nat. Med. 2012, 18, 1232–1238. [Google Scholar] [CrossRef]
- Takayama, Y.; Uta, D.; Furue, H.; Tominaga, M. Pain-enhancing mechanism through interaction between TRPV1 and anoctamin 1 in sensory neurons. Proc. Natl. Acad. Sci. USA 2015, 112, 5213. [Google Scholar] [CrossRef]
- Takayama, Y.; Furue, H.; Tominaga, M. 4-isopropylcyclohexanol has potential analgesic effects through the inhibition of anoctamin 1, TRPV1 and TRPA1 channel activities. Sci. Rep. 2017, 7, 43132. [Google Scholar] [CrossRef]
- Ou-Yang, Q.; Li, B.; Xu, M.; Liang, H. TRPV4 promotes the migration and invasion of glioma cells via AKT/Rac1 signaling. Biochem. Biophys. Res. Commun. 2018, 503, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Xu, T.; Wang, Y.; Zhou, Y.; Yu, D.; Wang, Z.; He, L.; Chen, Z.; Zhang, Y.; Davidson, D.; et al. Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4. Autophagy 2021, 17, 3592–3606. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wu, P.-F.; Ma, J.-X.; Liao, M.-J.; Xu, L.-S.; Yi, L. TRPV4 activates the Cdc42/N-wasp pathway to promote glioblastoma invasion by altering cellular protrusions. Sci. Rep. 2020, 10, 14151. [Google Scholar] [CrossRef] [PubMed]
- Saifeddine, M.; El-Daly, M.; Mihara, K.; Bunnett, N.W.; McIntyre, P.; Altier, C.; Hollenberg, M.D.; Ramachandran, R. GPCR-mediated EGF receptor transactivation regulates TRPV4 action in the vasculature. Br. J. Pharm. 2015, 172, 2493–2506. [Google Scholar] [CrossRef]
- Zhang, Z.-R.; Chu, W.-F.; Song, B.; Gooz, M.; Zhang, J.-N.; Yu, C.-J.; Jiang, S.; Baldys, A.; Gooz, P.; Steele, S.; et al. TRPP2 and TRPV4 form an EGF-activated calcium permeable channel at the apical membrane of renal collecting duct cells. PLoS ONE 2013, 8, e73424. [Google Scholar] [CrossRef]
- Takayama, Y.; Shibasaki, K.; Suzuki, Y.; Yamanaka, A.; Tominaga, M. Modulation of water efflux through functional interaction between TRPV4 and TMEM16A/anoctamin 1. FASEB J. 2014, 28, 2238–2248. [Google Scholar] [CrossRef]
- Derouiche, S.; Takayama, Y.; Murakami, M.; Tominaga, M. TRPV4 heats up ANO1-dependent exocrine gland fluid secretion. FASEB J. 2018, 32, 1841–1854. [Google Scholar] [CrossRef]
- Wang, K.; Feng, X.; Zheng, L.; Chai, Z.; Yu, J.; You, X.; Li, X.; Cheng, X. TRPV4 is a Prognostic Biomarker that Correlates with the Immunosuppressive Microenvironment and Chemoresistance of Anti-Cancer Drugs. Front. Mol. Biosci. 2021, 8, 690500. [Google Scholar] [CrossRef] [PubMed]
- Alptekin, M.; Eroglu, S.; Tutar, E.; Sencan, S.; Geyik, M.A.; Ulasli, M.; Demiryurek, A.T.; Camci, C. Gene expressions of TRP channels in glioblastoma multiforme and relation with survival. Tumor Biol. 2015, 36, 9209–9213. [Google Scholar] [CrossRef]
- Bomben, V.C.; Sontheimer, H.W. Inhibition of transient receptor potential canonical channels impairs cytokinesis in human malignant gliomas. Cell Prolif. 2008, 41, 98–121. [Google Scholar] [CrossRef]
- Bomben, V.C.; Sontheimer, H. Disruption of transient receptor potential canonical channel 1 causes incomplete cytokinesis and slows the growth of human malignant gliomas. Glia 2010, 58, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Lepannetier, S.; Zanou, N.; Yerna, X.; Emeriau, N.; Dufour, I.; Masquelier, J.; Muccioli, G.; Tajeddine, N.; Gailly, P. Sphingosine-1-phosphate-activated TRPC1 channel controls chemotaxis of glioblastoma cells. Cell Calcium 2016, 60, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Cuddapah, V.A.; Turner, K.L.; Sontheimer, H. Calcium entry via TRPC1 channels activates chloride currents in human glioma cells. Cell Calcium 2013, 53, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Tajeddine, N.; Gailly, P. TRPC1 Protein Channel Is Major Regulator of Epidermal Growth Factor Receptor Signaling *. J. Biol. Chem. 2012, 287, 16146–16157. [Google Scholar] [CrossRef]
- Chen, W.L.; Barszczyk, A.; Turlova, E.; Deurloo, M.; Liu, B.; Yang, B.B.; Rutka, J.T.; Feng, Z.P.; Sun, H.S. Inhibition of TRPM7 by carvacrol suppresses glioblastoma cell proliferation, migration and invasion. Oncotarget 2015, 6, 16321–16340. [Google Scholar] [CrossRef]
- Bruce, J.I.E.; James, A.D. Targeting the Calcium Signalling Machinery in Cancer. Cancers 2020, 12, 2351. [Google Scholar] [CrossRef]
- Wong, R.; Turlova, E.; Feng, Z.P.; Rutka, J.T.; Sun, H.S. Activation of TRPM7 by naltriben enhances migration and invasion of glioblastoma cells. Oncotarget 2017, 8, 11239–11248. [Google Scholar] [CrossRef]
- Liu, M.; Inoue, K.; Leng, T.; Guo, S.; Xiong, Z.G. TRPM7 channels regulate glioma stem cell through STAT3 and Notch signaling pathways. Cell. Signal. 2014, 26, 2773–2781. [Google Scholar] [CrossRef]
- Deason-Towne, F.; Perraud, A.L.; Schmitz, C. Identification of Ser/Thr phosphorylation sites in the C2-domain of phospholipase C γ2 (PLCγ2) using TRPM7-kinase. Cell. Signal. 2012, 24, 2070–2075. [Google Scholar] [CrossRef]
- Zou, Z.G.; Rios, F.J.; Neves, K.B.; Alves-Lopes, R.; Ling, J.; Baillie, G.S.; Gao, X.; Fuller, W.; Camargo, L.L.; Gudermann, T.; et al. Epidermal growth factor signaling through transient receptor potential melastatin 7 cation channel regulates vascular smooth muscle cell function. Clin. Sci. 2020, 134, 2019–2035. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Lipid rafts as major platforms for signaling regulation in cancer. Adv. Biol. Regul. 2015, 57, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.E.; Mueller, K.L.; Bohin, N.; Ge, Y.; Boerner, J.L. Lipid raft localization of EGFR alters the response of cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J. Cell. Physiol. 2011, 226, 2316–2328. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Pan, Z.; Zhao, M.; Wang, Q.; Qiao, C.; Miao, L.; Ding, X. High cholesterol in lipid rafts reduces the sensitivity to EGFR-TKI therapy in non-small cell lung cancer. J. Cell. Physiol. 2018, 233, 6722–6732. [Google Scholar] [CrossRef] [PubMed]
- Abulrob, A.; Giuseppin, S.; Andrade, M.F.; McDermid, A.; Moreno, M.; Stanimirovic, D. Interactions of EGFR and caveolin-1 in human glioblastoma cells: Evidence that tyrosine phosphorylation regulates EGFR association with caveolae. Oncogene 2004, 23, 6967–6979. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Vind-Kezunovic, D.; Karvinen, S.; Gniadecki, R. Ligand-Independent Activation of the EGFR by Lipid Raft Disruption. J. Investig. Dermatol. 2006, 126, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Ringerike, T.; Blystad, F.D.; Levy, F.O.; Madshus, I.H.; Stang, E. Cholesterol is important in control of EGF receptor kinase activity but EGF receptors are not concentrated in caveolae. J. Cell Sci. 2002, 115, 1331–1340. [Google Scholar] [CrossRef]
- Parat, M.O.; Riggins, G.J. Caveolin-1, caveolae, and glioblastoma. Neuro Oncol. 2012, 14, 679–688. [Google Scholar] [CrossRef]
- Couet, J.; Sargiacomo, M.; Lisanti, M.P. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J. Biol. Chem. 1997, 272, 30429–30438. [Google Scholar] [CrossRef]
- Kazazic, M.; Roepstorff, K.; Johannessen, L.E.; Pedersen, N.M.; van Deurs, B.; Stang, E.; Madshus, I.H. EGF-induced activation of the EGF receptor does not trigger mobilization of caveolae. Traffic 2006, 7, 1518–1527. [Google Scholar] [CrossRef]
- Mineo, C.; James, G.L.; Smart, E.J.; Anderson, R.G. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J. Biol. Chem. 1996, 271, 11930–11935. [Google Scholar] [CrossRef]
- Mineo, C.; Gill, G.N.; Anderson, R.G. Regulated migration of epidermal growth factor receptor from caveolae. J. Biol. Chem. 1999, 274, 30636–30643. [Google Scholar] [CrossRef]
- Chen, X.; Resh, M.D. Cholesterol depletion from the plasma membrane triggers ligand-independent activation of the epidermal growth factor receptor. J. Biol. Chem. 2002, 277, 49631–49637. [Google Scholar] [CrossRef]
- Sones, W.R.; Davis, A.J.; Leblanc, N.; Greenwood, I.A. Cholesterol depletion alters amplitude and pharmacology of vascular calcium-activated chloride channels. Cardiovasc. Res. 2010, 87, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K.; Cabrita, I.; Wanitchakool, P.; Ousingsawat, J.; Sirianant, L.; Benedetto, R.; Schreiber, R. Modulating Ca2+ signals: A common theme for TMEM16, Ist2, and TMC. Pflügers Arch. Eur. J. Physiol. 2016, 468, 475–490. [Google Scholar] [CrossRef]
- Wei, C.; Wang, X.; Chen, M.; Ouyang, K.; Song, L.S.; Cheng, H. Calcium flickers steer cell migration. Nature 2009, 457, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.C.; Meyer, T. Ca2+ pulses control local cycles of lamellipodia retraction and adhesion along the front of migrating cells. Curr. Biol. 2012, 22, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Calcium in tumour metastasis: New roles for known actors. Nat. Rev. Cancer 2011, 11, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.K.; Olsen, M.L.; McFerrin, M.B.; Sontheimer, H. BK channels are linked to inositol 1,4,5-triphosphate receptors via lipid rafts: A novel mechanism for coupling [Ca2+](i) to ion channel activation. J. Biol. Chem. 2007, 282, 31558–31568. [Google Scholar] [CrossRef]
- Bong, A.H.L.; Monteith, G.R. Calcium signaling and the therapeutic targeting of cancer cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2018, 1865, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Mulder, C.; Prust, N.; van Doorn, S.; Reinecke, M.; Kuster, B.; van Bergen en Henegouwen, P.; Lemeer, S. Adaptive Resistance to EGFR-Targeted Therapy by Calcium Signaling in NSCLC Cells. Mol. Cancer Res. 2018, 16, 1773–1784. [Google Scholar] [CrossRef]
| Drug Class | Compound | Trial | Patients | Intended Therapy | Survival Outcomes | Other Comments | Reference |
|---|---|---|---|---|---|---|---|
| 1st generation EGFR SMI | Gefitinib | I/II | ND | Combination with RT | OS: 11.5 months; no OS benefit vs. RT alone. | Younger age correlates with better outcome; EGFR expression no prognostic value for treatment. | [53] |
| II | ND | Adjuvant (post-RT) | No difference in OS/PFS; only patients with AE demonstrated improved OS. | Clinical outcomes not affected by EGFR status. | [50] | ||
| II | Recurrent | Monotherapy | EFS: 17 weeks; OS: 39.4 weeks; 1-year survival probability: 35.6%. | Well tolerated; clinical outcomes not affected by EGFR status. | [51] | ||
| II | Recurrent | Monotherapy | 17.9% patients showed disease stabilisation; OS: 24.6 weeks; PFS(6): 14.3%; PFS(12): 7.1%. | Limited activity; EGFR status & p-Akt expression not predictive of drug activity. | [52] | ||
| Erlotinib | I | ND | Combination with RT | TTP: 26 weeks; OS: 55 weeks; progression in 84% of patients. | MTD not reached. | [60] | |
| I/II | ND | Combination with RT and TMZ | OS: 15.3 months; no benefit in OS vs. TMZ controls. | AE (grade 3/4); EGFR/PTEN/p53 status not predictive of survival. | [55] | ||
| I/II | Recurrent | Combination with temsirolimus (mTor inhibitor) | PFS6: 13%. | MTD: 15 mg temsirolium weekly + 150 mg erlotinib daily; MTD lower than expected due to increased toxicity; EGFR status not correlated with PFS. | [57] | ||
| I/II | Recurrent | Monotherapy | PFS: 1.9 months; OS: 6.9 months; all patients showed disease progression whilst receiving treatment | 90% patients with severe AE. | [61] | ||
| II | ND | Combination with RT and TMZ | PFS: 2.8 months; OS: 8.6 months; 4 (11.1%) treatment-related deaths. | Not efficacious with unacceptable toxicity; trial terminated after accrual of 27 patients. | [54] | ||
| II | ND | Combination with RT, TMZ, and bevacizumab (VEGF inhibitor) | OS: 19.8 months; PFS: 13.5 months. | Well tolerated; improved PFS but not OS. | [63] | ||
| II | Recurrent | Monotherapy | PFS(6): 11.4% (control 24%). | Well tolerated; EGFR and pAkt status not correlated with outcomes. | [56] | ||
| II | Recurrent | Monotherapy | OR: 6.3%, response duration: 7 months, 6PFS: 20%; OS: 9.7 months. | Outcomes not related to EGFR amplification/EIAED status. | [59] | ||
| II | Recurrent | Monotherapy | PFS(6): 3%; PFS: 2 months; OS at 12 months: 57%. | 1° toxicity dermatologic; no effect on EGFR signalling; rash development in cycle 1 correlated with survival. | [62] | ||
| Lapatinib | I/II | Recurrent | Combination with pazopanib (antiangiogenic) | MTR not reached; PFS6: 0%–PTEN/EGFRvIII-positive, 15%–PTEN/EGFRvIII-negative. | Early termination from poor survival; PK data determined exposure to lapatinib subtherapeutic. | [58] | |
| 2nd generation EGFR SMI | Afatinib | I/II | Recurrent | Monotherapy compared to combination with TMZ | PFS6: 3% MT, 10% with TMZ; 1 partial response with MT, 2 with TMZ. | MTD: 40mg/day; PFS longer in EGFRvIII-positive tumours vs. EGFRvIII-negative tumours. | [65] |
| Dacomitinib | II | Recurrent | Monotherapy | PFS6: 10.6%; PFS: 2.7 months; OS: 7.4 months; 1 complete response; 2 (4.1%) responses. | [66] | ||
| II | EGFR gene amplification | Monotherapy | PFS12: 8.9%; 14.3% of Px experienced clinical benefit. | EGFRvIII/EGFR ECD missense mutation not associated with clinical benefit. | [67] | ||
| 3rd generation EGFR SMI | Osimertinib | II | EGFR gene amplification | Monotherapy | No results published. | NCT03732352 | |
| Anti-EGFR antibodies | Cetuximab | II | Recurrent | Combination bevacizumab and irinotecan (chemotherapy) | PFS6: 30%; OS: 29 weeks; efficacy not superior versus bevacizumab/irinotecan alone. | RR: 2 Px complete, 9 partial; well tolerated except for skin toxicity. | [72] |
| II | Recurrent | Monotherapy | TTP: 19.9 months; PFS < 6 months; OS: 5 months. | No significant correlation between response, survival, EGFR amplification. | [73] | ||
| Nimotuzumab | I/II | Malignant gliomas | Combination with radiochemotherapy | OS: 10.4 months (control 10.5 months); 1 year survival rates: 81.3% (69.1%); RR: 7.0% (52.2%). | Differences not significant; trend towards improved treatment efficacy. | [74] | |
| II | ND | Combination with RT and TMZ | PFS: 10 months; OS: 15.9 months; PFS6: 69.2%. | No correlation between efficacy & EGFR expression; survival similar to historical data of standard therapy. | [75] | ||
| III | ND | Combination with radiochemotherapy | PFS12: 25.6% (control 20.3%); PFS; 5.6 months (4.0 months); OS: 19.5 months with residual tumour, 23.3 months without. | WT; EGFR amplification did not correlate with efficacy. | [76] | ||
| Depatux-m | I | ND; recurrent | Monotherapy compared to combination with TMZ | PFS6: 30.8%; OS: 10.7 months. | MTD: 1.5 mg/kg with TMZ, not reached as MT; RP2D: 1.25 mg/kg. | [77] | |
| II | Recurrent; EGFR amplification | Monotherapy compared to combination with TMZ | PFS: 1.9 months (Depatux-m) vs. 2.7 months (Depatux-m + TMZ); OS: 7.9 months vs. 9.6 months. | [78] | |||
| Observational | Recurrent | Combination with TMZ | OS: 9.04 months; OS(12): 37%. | MGMT methylation status only factor significantly associated with survival; moderate, manageable toxicity. | [79] | ||
| ABT-414 | II | ND | Combination with RT and TMZ | No results published. | NCT02573324 | ||
| EGFRvIII peptide vaccine | Rindopepimut | II | ND | Combination with TMZ | PFS(6): 66%; OS: 21.8 months; OS(36): 26%. | Well tolerated; EGFRvIII eliminated in 4/6 (67%) of tumour samples after >3 months therapy. | [80] |
| III | ND; EGFRvIII mutant | Combination with TMZ | No significant difference in OS; OS: 20.1 months vs. 20.0 months (control). | [81] | |||
| CAR T cell therapy | NA | I | Recurrent; EGFRvII mutant | Monotherapy | PFS: 1.3 months; OS: 6.9 months. | Persistence of CAR+ cells correlated with dose but no objective responses. | [82] |
| I | Recurrent | Monotherapy | No results published. | NCT03618381 |
| ANO | Role | Cancer Type | Comments/Mechanism | Reference |
|---|---|---|---|---|
| ANO1 | Proliferation | OSCC | OE; PS; promotes MVA pathway. | [134] |
| Breast | OE; upregulates EGFR/HER2 expression; activates EGFR and CAMK signalling. | [135,136] | ||
| Glioma | OE; upregulates NF-κB pathway. | [137] | ||
| Colorectal | OE. | [138] | ||
| HNSCC | OE; PS; binds and stabilises EGFR. | [139] | ||
| Metastasis | Gastrointestinal | OE; PS. | [140] | |
| HNSCC | UE; switch between proliferation and metastasis; dependent on promoter methylation status. | [141] | ||
| Invasion, migration | Glioblastoma | OE; binds and stabilises EGFRvIII; activates MAPK/PI3K signalling pathways. | [142] | |
| ANO5 | Proliferation | Pancreatic | OE; PS. | [143] |
| Migration | Thyroid | UE; activates JAK/STAT3 pathway. | [144] | |
| Proliferation, migration | Osteosarcoma | OE; promotes NELL1/2 degradation. | [145] | |
| ANO6 | Proliferation, invasion | Glioma | OE; PS; via ERK activation. | [146] |
| ANO7 | Differentiation | Prostate | UE; PS; interacts with PTEN/Akt pathway. | [147] |
| ANO9 | Proliferation | Pancreatic | OE; PS; activates ERK/EGFR signalling. | [148] |
| Colorectal carcinoma | UE; PS; inhibits ANO1 activity. | [149] |
| Compound | ANO Target | Condition | Effect of Inhibition | Reference |
|---|---|---|---|---|
| CaCCinh-AN01 | ANO1 ANO2 | Prostate cancer | Inhibited proliferation and induced apoptosis | [172] |
| Colon cancer | ||||
| GBM | Suppressed GSC activities, reduced expression of stemness-related factors, and reduced EGFRvII signalling | [142] | ||
| HNSCC | Reduced cell viability via reduced EGFR activity and sensitised cells to EGFR-targeted therapy | [139,170] | ||
| DES | ANO1 ANO2 | NSCLC | Inhibited cell viability and migration through reduced activation of EGFR | [171] |
| 3n | ANO1 ANO2 | Prostate cancer | Reduced cancer cell viability and induced apoptosis via caspase 3 activation and PARP cleavage | [173] |
| 2-aminothiophene-3-carboxamide | ANO1 | GBM | Suppressed proliferation, migration, and invasion of GBM cells | [174] |
| Cepharanthine | ANO1 ANO2 ANO6 | Lung adenocarcinoma | Inhibited cell proliferation and migration, induced apoptosis, and reduced tumour growth | [175] |
| NFA | ANO1 ANO2 ANO6 | Hepatoma | Blocked cell cycle progression and decreased intracellular Ca2+ | [176] |
| NPC | Inhibited proliferation, migration, and invasion through reduced ERK | [177] | ||
| NPPB | ANO1 ANO2 ANO6 | Glioma | NPPB conjugated to TMZ inhibited cell proliferation, migration, and invasion | [178] |
| OSCC | Induced EMT via Wnt/ß-catenin signalling | [179] | ||
| Niclosamide | ANO1 ANO6 | GBM | Reduced GBM malignancy via reduced Wnt, Notch, mTOR, and NF-κB signalling | [180,181,182] |
| Gallotannin | ANO1 ANO2 | GBM | Inhibited cell proliferation | [183] |
| Colorectal cancer | Inhibited lung metastasis; regulated PI3K/Akt/mTOR, AMPK signalling pathways; downregulated mesenchymal marker expression; induced senescence | [184,185,186] | ||
| Breast cancer | Inhibited proliferation via increased Chk2 phosphorylation | [187] | ||
| Prostate cancer | Induced apoptosis via reduced Mcl-1 signalling and activation of procaspase 9/3 expression | [188] | ||
| Liver cancer | Reduce cell viability via increased SIRT1, mTOR, and activated AMPK | [189] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewdney, B.; Ursich, L.; Fletcher, E.V.; Johns, T.G. Anoctamins and Calcium Signalling: An Obstacle to EGFR Targeted Therapy in Glioblastoma? Cancers 2022, 14, 5932. https://doi.org/10.3390/cancers14235932
Dewdney B, Ursich L, Fletcher EV, Johns TG. Anoctamins and Calcium Signalling: An Obstacle to EGFR Targeted Therapy in Glioblastoma? Cancers. 2022; 14(23):5932. https://doi.org/10.3390/cancers14235932
Chicago/Turabian StyleDewdney, Brittany, Lauren Ursich, Emily V. Fletcher, and Terrance G. Johns. 2022. "Anoctamins and Calcium Signalling: An Obstacle to EGFR Targeted Therapy in Glioblastoma?" Cancers 14, no. 23: 5932. https://doi.org/10.3390/cancers14235932
APA StyleDewdney, B., Ursich, L., Fletcher, E. V., & Johns, T. G. (2022). Anoctamins and Calcium Signalling: An Obstacle to EGFR Targeted Therapy in Glioblastoma? Cancers, 14(23), 5932. https://doi.org/10.3390/cancers14235932






