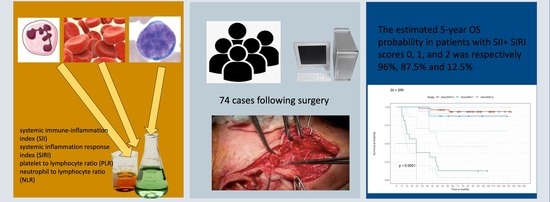
The Combination of Inflammatory Biomarkers as Prognostic Indicator in Salivary Gland Malignancy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- -
- Previous salivary gland surgery.
- -
- Post-operative histopathology-confirmed salivary gland malignant tumor.
- -
- No active infection, chronic inflammation, autoimmune disease, or other malignancy at the time of admission.
- -
- A mean follow-up period of 5 years.
- -
- Serious complications or death occurring within 15 days of the surgery.
2.2. Data Collection
2.3. Follow-Up Investigation
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
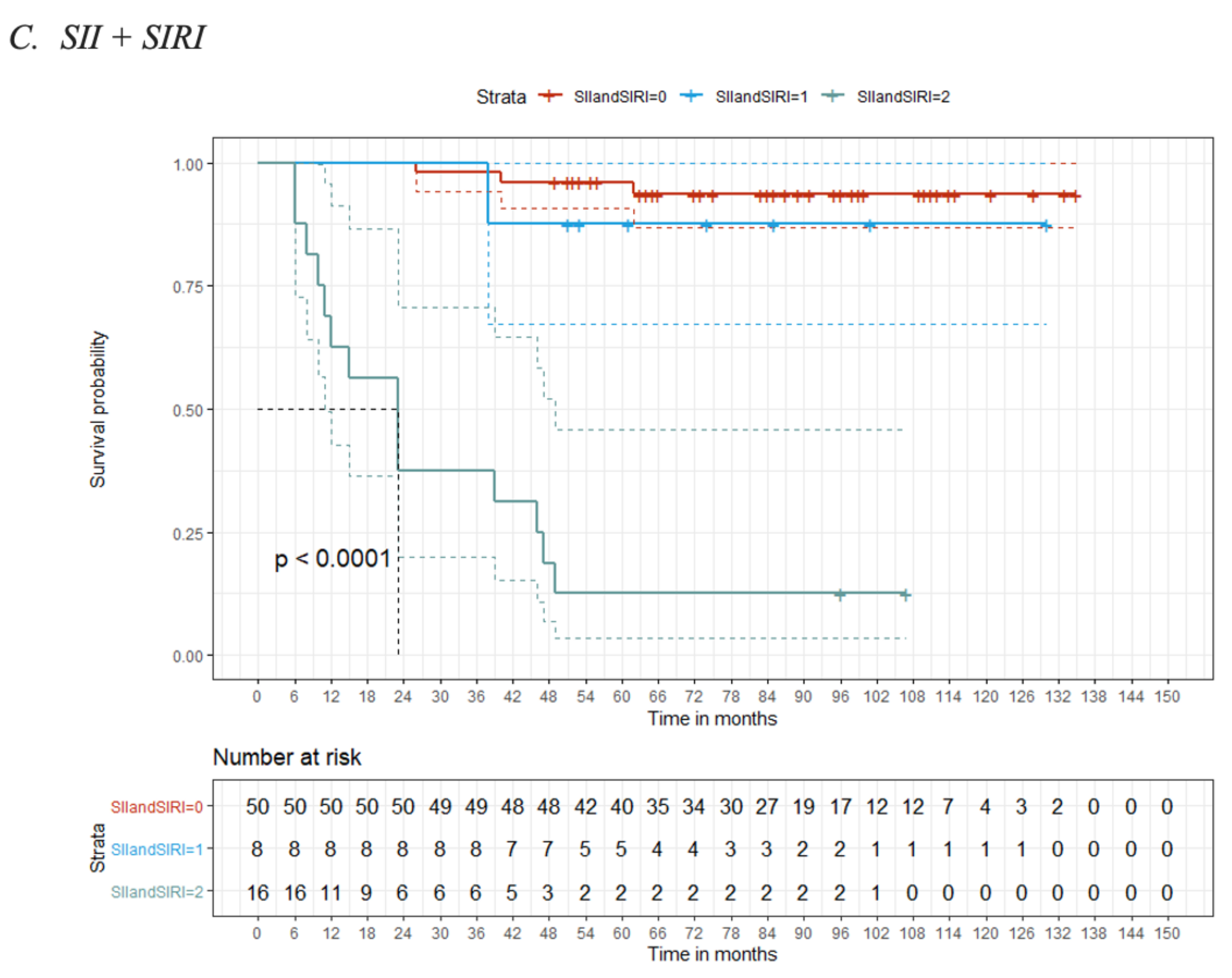
3.2. Optimal Cut-Off Values for the Biomarkers (NLR, PLR, SII and SIRI)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jegadeesh, N.; Liu, Y.; Prabhu, R.S.; Magliocca, K.R.; Marcus, D.M.; Higgins, K.A.; Vainshtein, J.M.; Wadsworth, J.T.; Beitler, J.J. Outcomes and prognostic factors in modern era management of major salivary gland cancer. Oral Oncol. 2015, 51, 770–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhusseiny, K.M.; Abd-Elhay FA, E.; Kamel, M.G.; Abd El Hamid Hassan, H.H.; El Tanany, H.H.M.; Hieu, T.H.; Huy, N.T. Examined and positive lymph nodes counts and lymph nodes ratio are associated with survival in major salivary gland cancer. Head Neck 2019, 41, 2625–2635. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, R. Malignant salivary gland tumors: A short review. Oral Maxillofac. Res. Dent Oral Maxillofac Res. 2021, 7, 1–5. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis JRTakata, T.S.P. WHO Classification of Head and Neck Tumours, 4th ed.; IARC Publication: Lyon, France, 2017; pp. 159–202. [Google Scholar]
- Young, A.; Okuyemi, O.T. Malignant Salivary Gland Tumors. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 1743–1747. [Google Scholar]
- American Joint Committee on Cancer. Major Salivary Glands. In AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017; pp. 69–75. [Google Scholar]
- Soffer, J.M.; Nassif, S.J.; Plato, M.V.; Chisholm, J.; O’Leary, M.A. Survival and prognostic factors of salivary gland malignant mixed tumor-not otherwise specified: A population-based analysis. Am. J. Otolaryngol. 2021, 42, 103135. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, S.; Fathehi, K.; Nair, D.; Deshmukh, A.; Pantvaidya, G.; Chaukar, D.A.; D’Cruz, A.K. Surgical morbidities and outcomes of major salivary gland neoplasms treated at a tertiary cancer center. Indian J. Cancer 2018, 55, 33. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-J.; Qian, L.-Q.; Ding, Z.-P.; Luo, Q.-Q.; Zhao, H.; Xia, W.-Y.; Fu, Y.-Y.; Feng, W.; Zhang, Q.; Yu, W.; et al. Prognostic Value of Inflammatory Biomarkers in Patients with Stage I Lung Adenocarcinoma Treated with Surgical Dissection. Front. Oncol. 2021, 11, 347. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.W.; Kwon, S.; Kim, H.J.; Cha, I.H.; Nam, W. Prognostic value of systemic inflammatory markers for oral cancer patients based on the 8th edition of AJCC staging system. Sci. Rep. 2020, 10, 12111. [Google Scholar] [CrossRef]
- Engqvist, H.; Parris, T.Z.; Kovács, A.; Rönnerman, E.W.; Sundfeldt, K.; Karlsson, P.; Helou, K. Validation of Novel Prognostic Biomarkers for Early-Stage Clear-Cell, Endometrioid and Mucinous Ovarian Carcinomas Using Immunohistochemistry. Front. Oncol. 2020, 10, 162. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yan, Z.P.; Hou, Z.H.; Huang, P.; Yang, M.J.; Zhang, S.; Zhang, S.; Zhang, S.H.; Zhu, X.L.; Ni, C.F.; et al. Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as Predictors of Outcomes in Patients With Unresectable Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization Plus Sorafenib. Front. Mol. Biosci. 2021, 8, 624366. [Google Scholar] [CrossRef]
- Geiger, J.L.; Ismaila, N.; Beadle, B.; Caudell, J.J.; Chau, N.; Deschler, D.; Glastonbury, C.; Kaufman, M.; Lamarre, E.; Lau, H.Y.; et al. Management of Salivary Gland Malignancy: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1909–1941. [Google Scholar] [CrossRef]
- Byrd, S.; Morris, L.G.T. Neck dissection for salivary gland malignancies. Oper. Tech. Otolayngol. Head Neck Surg. 2018, 29, 157–161. [Google Scholar] [CrossRef]
- Jacobson, J.J.; Epstein, J.B.; Eichmiller, F.C.; Gibson, T.B.; Carls, G.S.; Vogtmann, E.; Wang, S.; Murphy, B. The cost burden of oral, oral pharyngeal, and salivary gland cancers in three groups: Commercial insurance, Medicare, and Medicaid. Head Neck Oncol. 2012, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Sano, Y.; Kogashiwa, Y.; Araki, R.; Enoki, Y.; Ikeda, T.; Yoda, T.; Nakahira, M.; Sugasawa, M. Correlation of Inflammatory Markers, Survival, and COX2 Expression in Oral Cancer and Implications for Prognosis. Otolaryngol. Head Neck Surg. 2018, 158, 667–676. [Google Scholar] [CrossRef]
- Mizuno, R.; Kawada, K.; Itatani, Y.; Ogawa, R.; Kiyasu, Y.; Sakai, Y. The role of tumor-associated neutrophils in colorectal cancer. Int. J. Mol. Sci. 2019, 20, 529. [Google Scholar] [CrossRef] [Green Version]
- Quigley, D.A.; Kristensen, V. Predicting prognosis and therapeutic response from interactions between lymphocytes and tumor cells. Mol. Oncol. 2015, 9, 2054–2062. [Google Scholar] [CrossRef]
- Schlesinger, M. Role of Platelets and Platelet Receptors in Cancer Metastasis. J. Hematol. Oncol. 2018, 11, 125. [Google Scholar] [CrossRef] [Green Version]
- Palacios-Acedo, A.L.; Mege, D.; Crescence, L.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Platelets, Thrombo-Inflammation, and Cancer: Collaborating With the Enemy. Front. Immunol. 2019, 10, 1805. [Google Scholar] [CrossRef] [Green Version]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Zhu, D.; Wu, C.; Wu, J.; Wang, Q.; Li, R.; Jiang, J.; Wu, C. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. Int. Immunopharmacol. 2018, 65, 503–510. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Ono, T.; Kawahara, A.; Matsuo, K.; Kondo, R.; Sato, K.; Akiba, J.; Kawaguchi, T.; Kakuma, T.; Chitose, S.; et al. Prognostic Value of Tumor Proportion Score in Salivary Gland Carcinoma. Laryngoscope 2021, 131, E1481–E1488. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Lou, N.; Jin, C.; Hu, C.; Arvine, C.; Zhu, G.; Pang, W.; Lou, N.; Jin, C.; Hu, C.; et al. Combination of preoperative platelet/lymphocyte and neutrophil/lymphocyte rates and tumor-related factors to predict lymph node metastasis in patients with gastric cancer. Eur. J. Gastroenterol. Hepatol. 2016, 28, 493–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.G.; Gao, K.; Jia, R.; Li, J.; Wang, C. Prognostic significance of the combination of preoperative fibrinogen and the neutrophil-lymphocyte ratio in patients with non-small cell lung cancer following surgical resection. Oncol. Lett. 2019, 17, 1435–1444. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Chen, Y.; Chen, J.; Chen, W.; Pan, Y.; Bao, L.; Gao, X. Combination of systemic inflammation response index and platelet-to-lymphocyte ratio as a novel prognostic marker of upper tract urothelial carcinoma after radical nephroureterectomy. Front. Oncol. 2019, 9, 914. [Google Scholar] [CrossRef]
- Kordzinska-Cisek, I.; Cisek, P.; Grzybowska-Szatkowska, L. The role of prognostic factors in salivary gland tumors treated by surgery and adjuvant radio-or chemoradiotherapy–A Single Institution Experience. Cancer Manag. Res. 2020, 12, 1047. [Google Scholar] [CrossRef]



| Variables | Total Cases 74 |
|---|---|
| Age (years) | |
| ≤60 | 45 (61%) |
| >60 | 29 (39%) |
| Gender | |
| Female | 36 (48%) |
| Male | 38 (52%) |
| Tumor location | |
| Major salivary glands | 59 (80%) |
| Minor salivary glands | 15 (20%) |
| cTstatus | |
| T1 | 22 (30%) |
| T2 | 41 (55%) |
| T3 | 11 (15%) |
| cNstatus | |
| cN− | 53 (72%) |
| cN+ | 21 (28%) |
| pTstatus | |
| T1 | 22 (30%) |
| T2 | 41 (55%) |
| T3 | 11 (15%) |
| pNstatus (on 24 cases) | |
| N0 | 3 (12.5%) |
| N1 | 18 (75%) |
| N2a | 2 (8.5%) |
| N2b | 1 (4%) |
| AJCC TNMstage | |
| I | 20 (27%) |
| II | 28 (38%) |
| III | 23 (31%) |
| IV | 3 (3%) |
| Tumor Localization | N° Cases | Histopathological Types |
|---|---|---|
| Parotid Gland | 49 | Mucoepidermoid Cancer 9 (18.4%) |
| Adenocarcinoma 8 (16.3%) | ||
| Squamous Cell Carcinoma 6 (12.2%) | ||
| Adenoid Cystic Carcinoma 5 (10.2%) | ||
| Myoepithelioma 5 (10.5%) | ||
| Carcinoma ex pleomorphic adenoma 5 (10.5%) | ||
| Mammary analog secretory carcinoma 3 (6.1%) | ||
| Acinar Adenocarcinoma 2 (4.1%) | ||
| Microcystic adnexal carcinoma 1 (2%) | ||
| Intraductal Carcinoma 1 (2%) | ||
| Undifferentiated carcinoma 1 (2%) | ||
| Sarcoma 1 (2%) | ||
| Lymphoepithelial Carcinoma 1 (2%) | ||
| B-cell lymphoma 1 (2%) | ||
| Submandibular Gland | 10 | Adenoid Cystic Carcinoma 3 (30%) |
| Carcinoma ex pleomorphic adenoma 2 (20%) | ||
| Squamous Cell Carcinoma 2 (20%) | ||
| Carcinosarcoma 1 (10%) | ||
| Sarcoma 1 (10%) | ||
| Adenocarcinoma 1 (10%) | ||
| Minor salivary Glands | 15 | Adenocarcinoma 4 (27%) |
| Adenoid Cystic Carcinoma 4 (27%) | ||
| Mucoepidermoid Cancer 4 (27%) | ||
| Carcinoma ex pleomorphic adenoma 1 (6.7%) | ||
| Myoepithelioma 1 (6.7%) | ||
| Clear-cell carcinoma 1 (6.7%) | ||
| TOTAL | 74 | Adenocarcinoma 13 (17.6%) |
| Mucoepidermoid Cancer 13 (17.6%) | ||
| Adenoid Cystic Carcinoma 12 (16%) | ||
| Carcinoma ex pleomorphic adenoma 8 (10.8%) | ||
| Squamous Cell Carcinoma 8 (10.8%) | ||
| Myoepithelioma 6 (8%) | ||
| Mammary analog secretory carcinoma 3 (4%) | ||
| Acinar Adenocarcinoma 2 (2.7%) | ||
| Sarcoma 2 (2.7%) | ||
| Microcystic adnexal carcinoma 1 (1.4%) | ||
| Intraductal Carcinoma 1 (1.4%) | ||
| Undifferentiated carcinoma 1 (1.4%) | ||
| Lymphoepithelial Carcinoma 1 (1.4%) | ||
| B-cell lymphoma 1 (1.4%) | ||
| Clear-cell carcinoma 1 (1.4%) | ||
| Carcinosarcoma 1 (1.4%) |
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | HR | 95% CI | p-Value | aHR | 95% CI | p-Value |
| Sex | 74 | 0.053 | |||||
| F | — | — | |||||
| M | 2.62 | 0.93, 7.34 | |||||
| Age | 74 | 0.029 | 0.917 | ||||
| ≤60 | — | — | — | — | |||
| >60 | 2.83 | 1.09, 7.31 | 0.94 | 0.30, 2.92 | |||
| AJCC TNM_Stage | 74 | <0.001 | |||||
| I | — | — | — | — | |||
| II | 0.86 | 0.12, 6.12 | 0.78 | 0.10, 5.97 | 0.808 | ||
| III | 7.81 | 1.75, 35.0 | 2.35 | 0.27, 20.8 | 0.442 | ||
| IV | 15.4 | 2.16, 110 | 3.35 | 0.26, 43.5 | 0.355 | ||
| TUMOR_LOCATION | 74 | 0.290 | |||||
| major | — | — | |||||
| minor | 0.48 | 0.11, 2.11 | |||||
| TUMOR_SIZE | 74 | 0.001 | 0.021 | ||||
| ≤4 | — | — | — | — | |||
| >4 | 5.65 | 2.18, 14.6 | 3.53 | 1.21, 10.3 | |||
| Lymphnode_status | 74 | <0.001 | 0.038 | ||||
| N- | 53 | — | — | — | — | ||
| N+ | 21 | 8.68 | 3.08, 24.5 | 3.85 | 1.08, 13.8 | ||
| ADJUVANT_RADIOTHERAPY | 74 | <0.001 | 0.159 | ||||
| 0 | 41 | — | — | — | — | ||
| 1 | 33 | 5.58 | 1.83, 17.0 | 2.85 | 0.66, 12.2 | ||
| Smoke | 74 | 0.671 | |||||
| 0 | 54 | — | — | ||||
| 1 | 20 | 0.79 | 0.26, 2.40 | ||||
| BMI | 74 | 1.01 | 0.90, 1.13 | 0.843 | |||
| NLRevent | 74 | <0.001 | 0.661 | ||||
| ≤3.95 | _ | _ | _ | _ | |||
| >3.95 | 16.2 | 5.29, 49.7 | 0.57 | 0.05, 6.91 | |||
| PLRevent | 74 | <0.001 | 0.744 | ||||
| ≤187.6 | — | — | — | — | |||
| >187.6 | 7.92 | 3.10, 20.3 | 1.20 | 0.41, 3.51 | |||
| SIIevent | 74 | <0.001 | 0.007 | ||||
| ≤917.585 | — | — | — | — | |||
| >917.585 | 28.0 | 7.98, 98.0 | 15.7 | 2.11, 117 | |||
| SIRIevent | 74 | <0.001 | 0.164 | ||||
| ≤2.045 | — | — | — | — | |||
| >2.045 | 15.0 | 4.87, 46.2 | 4.19 | 0.56, 31.6 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbate, V.; Barone, S.; Troise, S.; Laface, C.; Bonavolontà, P.; Pacella, D.; Salzano, G.; Iaconetta, G.; Califano, L.; Dell’Aversana Orabona, G. The Combination of Inflammatory Biomarkers as Prognostic Indicator in Salivary Gland Malignancy. Cancers 2022, 14, 5934. https://doi.org/10.3390/cancers14235934
Abbate V, Barone S, Troise S, Laface C, Bonavolontà P, Pacella D, Salzano G, Iaconetta G, Califano L, Dell’Aversana Orabona G. The Combination of Inflammatory Biomarkers as Prognostic Indicator in Salivary Gland Malignancy. Cancers. 2022; 14(23):5934. https://doi.org/10.3390/cancers14235934
Chicago/Turabian StyleAbbate, Vincenzo, Simona Barone, Stefania Troise, Claudia Laface, Paola Bonavolontà, Daniela Pacella, Giovanni Salzano, Giorgio Iaconetta, Luigi Califano, and Giovanni Dell’Aversana Orabona. 2022. "The Combination of Inflammatory Biomarkers as Prognostic Indicator in Salivary Gland Malignancy" Cancers 14, no. 23: 5934. https://doi.org/10.3390/cancers14235934
APA StyleAbbate, V., Barone, S., Troise, S., Laface, C., Bonavolontà, P., Pacella, D., Salzano, G., Iaconetta, G., Califano, L., & Dell’Aversana Orabona, G. (2022). The Combination of Inflammatory Biomarkers as Prognostic Indicator in Salivary Gland Malignancy. Cancers, 14(23), 5934. https://doi.org/10.3390/cancers14235934