Magnetic Resonance Spectroscopy Metabolites as Biomarkers of Disease Status in Pediatric Diffuse Intrinsic Pontine Gliomas (DIPG) Treated with Glioma-Associated Antigen Peptide Vaccines
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and MR Spectroscopy
2.2. Statistical Analysis
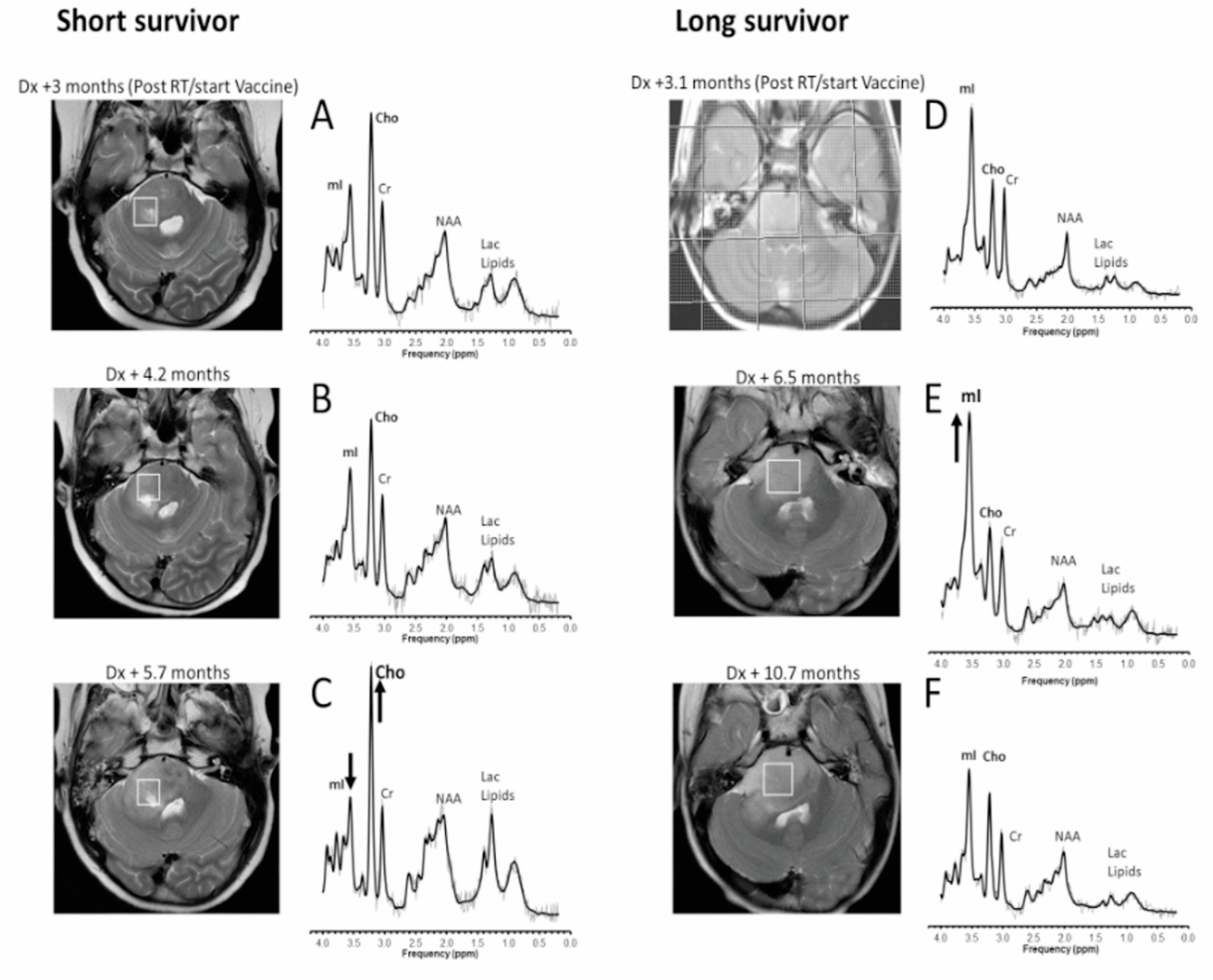
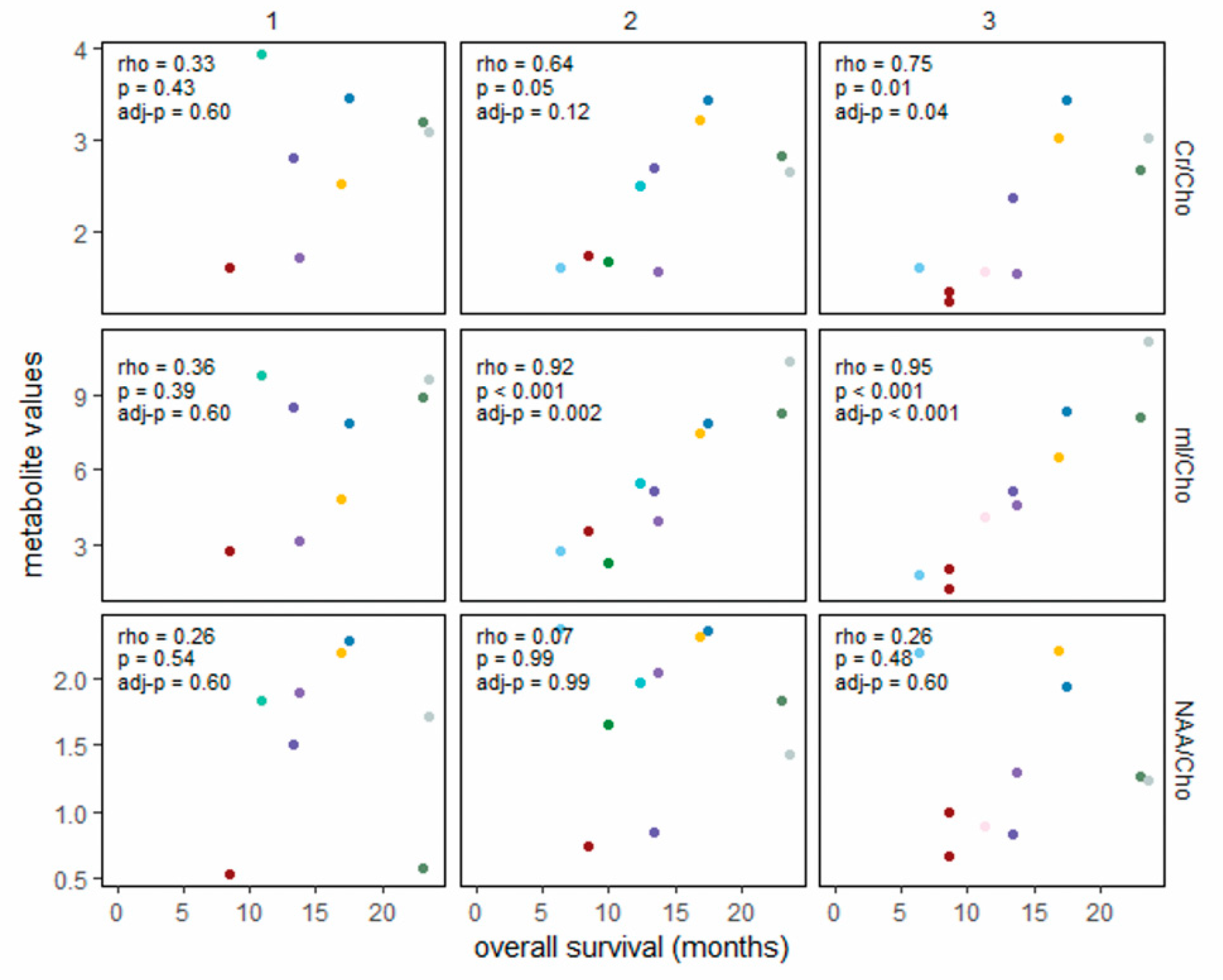
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clymer, J.; Kieran, M.W. The Integration of Biology Into the Treatment of Diffuse Intrinsic Pontine Glioma: A Review of the North American Clinical Trial Perspective. Front. Oncol. 2018, 8, 169. [Google Scholar] [CrossRef]
- Hargrave, D.; Ute, B.; Eric, B. Diffuse brainstem glioma in children: Critical review of clinical trials. Lancet. Oncol. 2006, 7, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Khuong-Quang, D.-A.; Buczkowicz, P.; Rakopoulos, P.; Liu, X.-Y.; Fontebasso, A.M.; Bouffet, E.; Bartels, U.; Albrecht, S.; Schwartzentruber, J.; Letourneau, L. K27 M mutation in histone H3. 3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012, 124, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Castel, D.; Philippe, C.; Calmon, R.; Le Dret, L.; Truffaux, N.; Boddaert, N.; Taylor, K.R.; Saulnier, P.; Lacroix, L.; Mackay, A. Histone H3 F3 A and HIST1 H3 B K27 M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015, 130, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Laprie, A.; Pirzkall, A.; Haas-Kogan, D.A.; Cha, S.; Banerjee, A.; Le, T.P.; Lu, Y.; Nelson, S.; McKnight, T.R. Longitudinal multivoxel MR spectroscopy study of pediatric diffuse brainstem gliomas treated with radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 20–31. [Google Scholar] [CrossRef]
- Panigrahy, A.; Nelson, M.D., Jr.; Finlay, J.L.; Sposto, R.; Krieger, M.D.; Gilles, F.H.; Bluml, S. Metabolism of diffuse intrinsic brainstem gliomas in children. Neuro. Oncol. 2008, 10, 32–44. [Google Scholar] [CrossRef]
- Negendank, W.G. Studies of human tumors by MRS: A review. NMR Biomed. 1992, 5, 303–324. [Google Scholar] [CrossRef]
- Ross, B.D.; Bluml, S. Neurospectroscopy. In Neuroimaging Second Edition; A Companion to Adams and Victor’s Principles of Neurology; Greenberg, J.O., Ed.; McGraw Hill: New York, NY, USA, 1999; pp. 727–773. [Google Scholar]
- Curless, R.G.; Bowen, B.C.; Pattany, P.M.; Gonik, R.; Kramer, D.L. Magnetic resonance spectroscopy in childhood brainstem tumors. Pediatr Neurol. 2002, 26, 374–378. [Google Scholar] [CrossRef]
- Astrakas, L.G.; Zurakowski, D.; Tzika, A.A.; Zarifi, M.K.; Anthony, D.C.; De Girolami, U.; Tarbell, N.J.; Black, P.M. Noninvasive magnetic resonance spectroscopic imaging biomarkers to predict the clinical grade of pediatric brain tumors. Clin. Cancer Res. 2004, 10, 8220–8228. [Google Scholar] [CrossRef]
- Thakur, S.B.; Karimi, S.; Dunkel, I.J.; Koutcher, J.A.; Huang, W. Longitudinal MR spectroscopic imaging of pediatric diffuse pontine tumors to assess tumor aggression and progression. AJNR Am. J. Neuroradiol. 2006, 27, 806–809. [Google Scholar]
- Lobel, U.; Hwang, S.; Edwards, A.; Li, Y.; Li, X.; Broniscer, A.; Patay, Z. Discrepant longitudinal volumetric and metabolic evolution of diffuse intrinsic Pontine gliomas during treatment: Implications for current response assessment strategies. Neuroradiology 2016, 58, 1027–1034. [Google Scholar] [CrossRef]
- Pollack, I.F.; Jakacki, R.I.; Butterfield, L.H.; Hamilton, R.L.; Panigrahy, A.; Potter, D.M.; Connelly, A.K.; Dibridge, S.A.; Whiteside, T.L.; Okada, H. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J. Clin. Oncol. 2014, 32, 2050–2058. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Negendank, W.G.; Sauter, R.; Brown, T.R.; Evelhoch, J.L.; Falini, A.; Gotsis, E.D.; Heerschap, A.; Kamada, K.; Lee, B.C.; Mengeot, M.M.; et al. Proton magnetic resonance spectroscopy in patients with glial tumors: A multicenter study. J. Neurosurg. 1996, 84, 449–458. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Sayour, E.; Grippin, A.; Mendez-Gomez, H.; De Leon, G.; Mitchell, D. Immu-63. Overcoming glioblastoma resistance to immune checkpoint blockade via RNA-loaded nanoparticles. Neuro.-Oncol. 2017, 19, vi126. [Google Scholar] [CrossRef][Green Version]
- Tedeschi, G.; Lundbom, N.; Raman, R.; Bonavita, S.; Duyn, J.H.; Alger, J.R.; Di Chiro, G. Increased choline signal coinciding with malignant degeneration of cerebral gliomas: A serial proton magnetic resonance spectroscopy imaging study. J. Neurosurg. 1997, 87, 516–524. [Google Scholar] [CrossRef]
- Li, X.; Jin, H.; Lu, Y.; Oh, J.; Chang, S.; Nelson, S.J. Identification of MRI and 1 H MRSI parameters that may predict survival for patients with malignant gliomas. NMR Biomed. 2004, 17, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, A.; Gruber, S.; Nimsky, C.; Fahlbusch, R.; Hammen, T.; Buslei, R.; Tomandl, B.; Moser, E.; Ganslandt, O. Preoperative grading of gliomas by using metabolite quantification with high-spatial-resolution proton MR spectroscopic imaging. Radiology 2006, 238, 958–969. [Google Scholar] [CrossRef]
- Steffen-Smith, E.A.; Shih, J.H.; Hipp, S.J.; Bent, R.; Warren, K.E. Proton magnetic resonance spectroscopy predicts survival in children with diffuse intrinsic pontine glioma. J. Neurooncol. 2011, 105, 365–373. [Google Scholar] [CrossRef]
- Yamasaki, F.; Kurisu, K.; Kajiwara, Y.; Watanabe, Y.; Takayasu, T.; Akiyama, Y.; Saito, T.; Hanaya, R.; Sugiyama, K. Magnetic resonance spectroscopic detection of lactate is predictive of a poor prognosis in patients with diffuse intrinsic pontine glioma. Neuro.-Oncol. 2011, 13, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Petanjek, Z.; Kostovic, I. Epigenetic regulation of fetal brain development and neurocognitive outcome. Proc. Natl. Acad. Sci. USA 2012, 109, 11062–11063. [Google Scholar] [CrossRef] [PubMed]
- Poussaint, T.Y. MRI as a central component of clinical trials analysis in brainstem glioma: A report from the Pediatric Brain Tumor Consortium (PBTC). Neuro.-Ldots. 2011, 13, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Negendank, W.; Sauter, R. Intratumoral lipids in 1 H MRS in vivo in brain tumors: Experience of the Siemens cooperative clinical trial. Anticancer. Res. 1996, 16, 1533–1538. [Google Scholar] [PubMed]
- Fernando, K.; McLean, M.; Chard, D.; MacManus, D.; Dalton, C.; Miszkiel, K.; Gordon, R.; Plant, G.; Thompson, A.; Miller, D. Elevated white matter myo-inositol in clinically isolated syndromes suggestive of multiple sclerosis. Brain 2004, 127, 1361–1369. [Google Scholar] [CrossRef]
- Kirov, I.I.; Patil, V.; Babb, J.S.; Rusinek, H.; Herbert, J.; Gonen, O. MR spectroscopy indicates diffuse multiple sclerosis activity during remission. J. Neurol. Neurosurg. Psychiatry. 2009, 80, 1330–1336. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sailasuta, N.; Hurd, R.; Nelson, S.; Pelletier, D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain 2005, 128, 1016–1025. [Google Scholar] [CrossRef]
- Hannoun, S.; Bagory, M.; Durand-Dubief, F.; Ibarrola, D.; Comte, J.-C.; Confavreux, C.; Cotton, F.; Sappey-Marinier, D. Correlation of diffusion and metabolic alterations in different clinical forms of multiple sclerosis. PLoS ONE 2012, 7, e32525. [Google Scholar] [CrossRef]
- Kirov, I.I.; Tal, A.; Babb, J.S.; Herbert, J.; Gonen, O. Serial proton MR spectroscopy of gray and white matter in relapsing-remitting MS. Neurology 2013, 80, 39–46. [Google Scholar] [CrossRef]
- Mayer, A.T.; Gambhir, S.S. The Immunoimaging Toolbox. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59, 1174–1182. [Google Scholar] [CrossRef]
- Nigam, S.; McCarl, L.; Kumar, R.; Edinger, R.S.; Kurland, B.F.; Anderson, C.J.; Panigrahy, A.; Kohanbash, G.; Edwards, W.B. Preclinical ImmunoPET Imaging of Glioblastoma-Infiltrating Myeloid Cells Using Zirconium-89 Labeled Anti-CD11 b Antibody. Mol. Imaging Biol. MIB Off. Publ. Acad. Mol. Imaging 2019, 22, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Ceschin, R.; Kurland, B.F.; Abberbock, S.R.; Ellingson, B.M.; Okada, H.; Jakacki, R.I.; Pollack, I.F.; Panigrahy, A. Parametric Response Mapping of Apparent Diffusion Coefficient as an Imaging Biomarker to Distinguish Pseudoprogression from True Tumor Progression in Peptide-Based Vaccine Therapy for Pediatric Diffuse Intrinsic Pontine Glioma. AJNR Am. J. Neuroradiol. 2015, 36, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Furtado, A.D.; Ceschin, R.; Blüml, S.; Mason, G.; Jakacki, R.I.; Okada, H.; Pollack, I.F.; Panigrahy, A. Neuroimaging of peptide-based vaccine therapy in pediatric brain tumors: Initial experience. Neuroimaging Clin. 2017, 27, 155–166. [Google Scholar] [CrossRef] [PubMed]

| Vaccine Cohort (n = 14) | Non-Vaccine Cohort (n = 32) | |
|---|---|---|
| Median (range) | Median (range) | |
| Age at diagnosis, years | 8.5 (2.2–17.9) | 7 (3–15) |
| Number of MRS per patient | 4 (1–8) | 2.5 (1–7) |
| Survival from diagnosis, months | 13.5 (6.3–23.6) | 11.2 (3.7–37.6) |
| Sex | ||
| Female | 6 (43) | 19 (59) |
| Male | 8 (57) | 13 (41) |
| Pre-vaccine therapy | ||
| Radiotherapy only | 10 (72) | 6 (19) |
| Radiotherapy + bevacizumab | 2 (14) | 1 (3) |
| Radiotherapy + temozolomide | 2(14) | 5 (16) |
| Radiotherapy + unknown/other * | 20 (62) |
| Within 6 Months of Death | Within 12 Months of Death | |||
|---|---|---|---|---|
| Vaccine Cohort (n = 22 Scans in 10 Patients) | Non-Vaccine Cohort (n = 37 Scans in 18 Patients) | Vaccine Cohort (n = 44 Scans in 13 Patients) | Non-Vaccine Cohort (n = 59 Scans in 23 Patients) | |
| Metabolite ratio | Slope (95% CI) | Slope (95% CI) | Slope (95% CI) | Slope (95% CI) |
| mI/Cho | 0.37 (0.11–0.63) | 0.26 (0.04–0.48) | 0.37 (0.21–0.54) | 0.19 (0.05–0.34) |
| Cr/Cho | 0.12 (0.05–0.19) | 0.06 (-0.02–0.13) | 0.11 (0.06–0.17) | 0.06 (0.02–0.10) |
| NAA/Cho | 0.06 (−0.06–0.18) | 0.02 (−0.06–0.11) | 0.07 (0.02–0.11) | 0.08 (0.04–0.12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panigrahy, A.; Jakacki, R.I.; Pollack, I.F.; Ceschin, R.; Okada, H.; Nelson, M.D.; Kohanbash, G.; Dhall, G.; Bluml, S. Magnetic Resonance Spectroscopy Metabolites as Biomarkers of Disease Status in Pediatric Diffuse Intrinsic Pontine Gliomas (DIPG) Treated with Glioma-Associated Antigen Peptide Vaccines. Cancers 2022, 14, 5995. https://doi.org/10.3390/cancers14235995
Panigrahy A, Jakacki RI, Pollack IF, Ceschin R, Okada H, Nelson MD, Kohanbash G, Dhall G, Bluml S. Magnetic Resonance Spectroscopy Metabolites as Biomarkers of Disease Status in Pediatric Diffuse Intrinsic Pontine Gliomas (DIPG) Treated with Glioma-Associated Antigen Peptide Vaccines. Cancers. 2022; 14(23):5995. https://doi.org/10.3390/cancers14235995
Chicago/Turabian StylePanigrahy, Ashok, Regina I. Jakacki, Ian F. Pollack, Rafael Ceschin, Hideho Okada, Marvin D. Nelson, Gary Kohanbash, Girish Dhall, and Stefan Bluml. 2022. "Magnetic Resonance Spectroscopy Metabolites as Biomarkers of Disease Status in Pediatric Diffuse Intrinsic Pontine Gliomas (DIPG) Treated with Glioma-Associated Antigen Peptide Vaccines" Cancers 14, no. 23: 5995. https://doi.org/10.3390/cancers14235995
APA StylePanigrahy, A., Jakacki, R. I., Pollack, I. F., Ceschin, R., Okada, H., Nelson, M. D., Kohanbash, G., Dhall, G., & Bluml, S. (2022). Magnetic Resonance Spectroscopy Metabolites as Biomarkers of Disease Status in Pediatric Diffuse Intrinsic Pontine Gliomas (DIPG) Treated with Glioma-Associated Antigen Peptide Vaccines. Cancers, 14(23), 5995. https://doi.org/10.3390/cancers14235995







