Early Referral for Breast-Cancer-Related Lymphedema: Do We Follow the Evidence? A Two-Year Prospective Multicenter Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
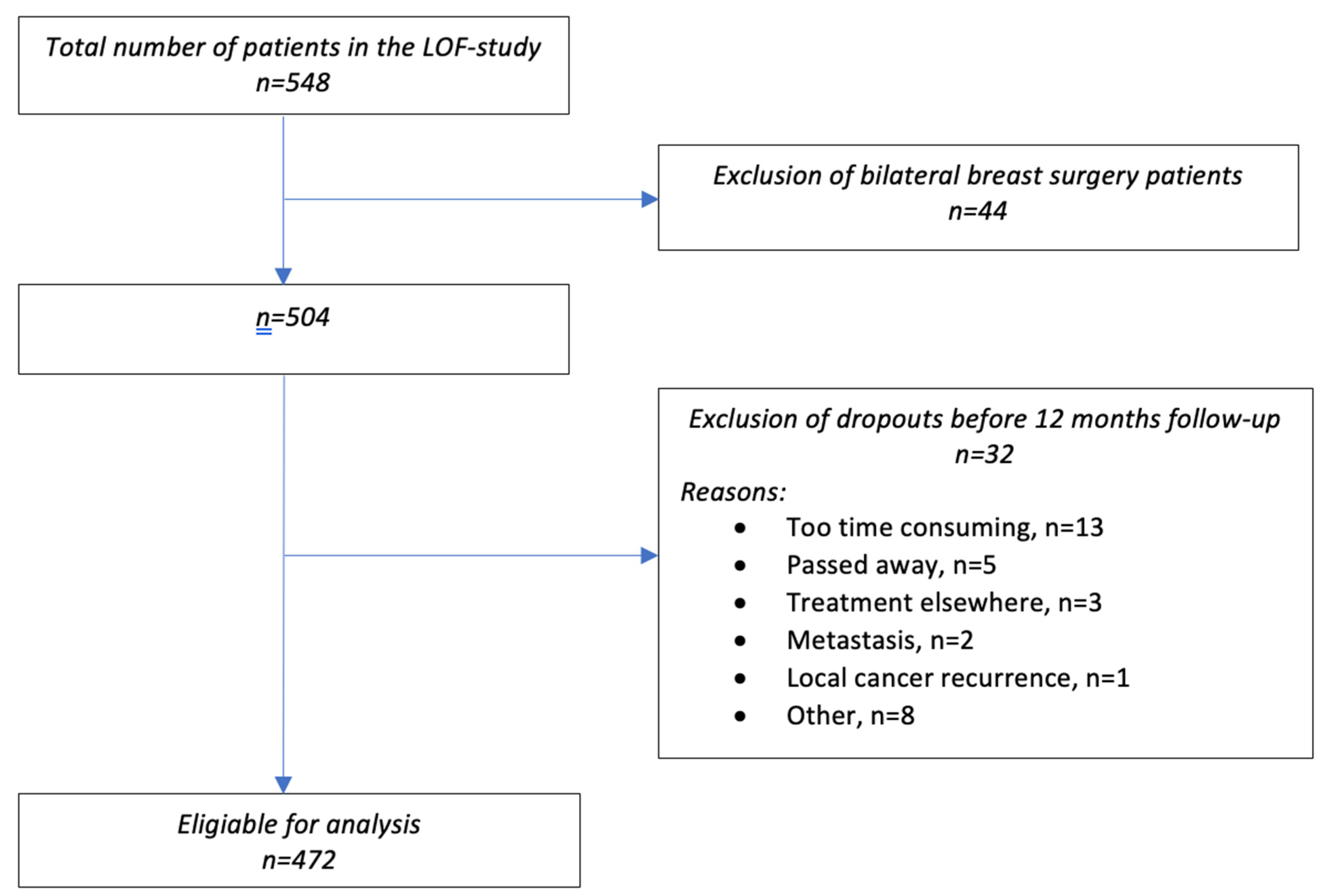
2.2. Participants
2.3. Study Outcomes
2.4. Arm Volume Measurement
2.5. Early Therapy Referral
2.6. Self-Reported Signs and Symptoms
2.7. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Relative Volume Change Data
3.3. Therapy Referral
3.4. Self-Reported Signs and Symptoms
3.5. Assessment of the Mean RVC in Time within the LE Group
4. Discussion
4.1. Key Findings
4.2. Consideration of Possible Mechanisms and Explanations
4.3. Relevant Findings from Other Published Studies
4.4. Potential Implications of Our Results for Daily Practice
4.5. Strength, Limitations, and Recommendations
4.6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Janda, M.; Cornish, B.; Battistutta, D.; Newman, B. Lymphedema After Breast Cancer: Incidence, Risk Factors, and Effect on Upper Body Function. J. Clin. Oncol. 2008, 26, 3536–3542. [Google Scholar] [CrossRef]
- Fu, M.R.; Kang, Y. Psychosocial Impact of Living with Cancer-Related Lymphedema. Semin. Oncol. Nurs. 2013, 29, 50–60. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, T.; Nevelsteen, I.; Thomis, S.; De Groef, A.; Tjalma, W.A.A.; Gebruers, N.; Devooglt, N. What are the economic burden and costs associated with the treatment of breast cancer-related lymphoedema? A systematic review. Support. Care Cancer 2020, 28, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Koelmeyer, L.; Gaitatzis, K.; Ridner, S.H.; Boyages, J.; Nelms, J.; Hughes, T.M.; Elder, E.; French, J.; Ngui, N.; Hsu, J.; et al. Implementing a prospective surveillance and early intervention model of care for breast cancer–related lymphedema into clinical practice: Application of the RE-AIM framework. Support. Care Cancer 2020, 29, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Koelmeyer, L.A.; Borotkanics, R.J.; Alcorso, J.; Prah, P.; Winch, C.J.; Nakhel, K.; Dean, C.M.; Boyages, J. Early surveillance is associated with less incidence and severity of breast cancer–related lymphedema compared with a traditional referral model of care. Cancer 2018, 125, 854–862. [Google Scholar] [CrossRef]
- Gençay Can, A.; Ekşioǧlu, E.; Çakçl, F.A. Early Detection and Treatment of Subclinical Lymphedema in Patients with Breast Cancer. Lymphat. Res. Biol. 2019, 17, 368–373. [Google Scholar] [CrossRef]
- Kilgore, L.J.; Korentager, S.S.; Hangge, A.N.; Amin, A.; Balanoff, C.R.; Larson, K.E.; Mitchell, M.P.; Chen, J.G.; Burgen, E.; Khan, Q.J.; et al. Reducing Breast Cancer-Related Lymphedema (BCRL) Through Prospective Surveillance Monitoring Using Bioimpedance Spectroscopy (BIS) and Patient Directed Self-Interventions. Ann. Surg. Oncol. 2018, 25, 2948–2952. [Google Scholar] [CrossRef]
- McLaughlin, S.A.; Staley, A.C.; Vicini, F.; Thiruchelvam, P.; Hutchison, N.A.; Mendez, J.; MacNeill, F.; Rockson, S.G.; DeSnyder, S.M.; Klimberg, S.; et al. Considerations for Clinicians in the Diagnosis, Prevention, and Treatment of Breast Cancer-Related Lymphedema: Recommendations from a Multidisciplinary Expert ASBrS Panel: Part 1: Definitions, Assessments, Education, and Future Directions. Ann. Surg. Oncol. 2017, 24, 2818–2826. [Google Scholar] [CrossRef]
- Rockson, S.G. Update on the Biology and Treatment of Lymphedema. Curr. Treat. Options Cardiovasc. Med. 2012, 14, 184–192. [Google Scholar] [CrossRef]
- Gillespie, T.C.; Sayegh, H.E.; Brunelle, C.L.; Daniell, K.M.; Taghian, A.G. Breast cancer-related lymphedema: Risk factors, precautionary measures, and treatments. Gland Surg. 2018, 7, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Keeley, V. The Early Detection of Breast Cancer Treatment-Related Lymphedema of the Arm. Lymphat. Res. Biol. 2021, 19, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Bucci, L.K.; Brunelle, C.L.; Bernstein, M.C.; Shui, A.M.; Gillespie, T.C.; Roberts, S.A.; Naoum, G.E.; Taghian, A.G. Subclinical Lymphedema After Treatment for Breast Cancer: Risk of Progression and Considerations for Early Intervention. Ann. Surg. Oncol. 2021, 28, 8624–8633. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Arthur, D.W.; Wazer, D.; Khan, A.; Ridner, S.; Vicini, F. The impact of early detection and intervention of breast cancer-related lymphedema: A systematic review. Cancer Med. 2016, 5, 1154–1162. [Google Scholar] [CrossRef]
- Stout, N.L.; Pfalzer, L.A.; Springer, B.; Levy, E.; McGarvey, C.L.; Danoff, J.V.; Gerber, L.; Soballe, P.W. Breast Cancer–Related Lymphedema: Comparing Direct Costs of a Prospective Surveillance Model and a Traditional Model of Care. Phys. Ther. 2012, 92, 152–163. [Google Scholar] [CrossRef]
- Stout Gergich, N.L.; Pfalzer, L.A.; McGarvey, C.; Springer, B.; Gerber, L.H.; Soballe, P. Preoperative assessment enables the early 426 diagnosis and successful treatment of lymphedema. Cancer 2008, 112, 2809–2819. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.R.; Killelea, B.K.; Sanft, T. Prevention Is Key: Importance of Early Recognition and Referral in Combating Breast Cancer–Related Lymphedema. J. Oncol. Pract. 2019, 15, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Gebruers, N.; Verbelen, H.; De Vrieze, T.; Vos, L.; Devoogdt, N.; Fias, L.; Tjalma, W. Current and future perspectives on the evaluation, prevention and conservative management of breast cancer related lymphoedema: A best practice guideline. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 216, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Specht, M.C.; Miller, C.L.; Russell, T.A.; Horick, N.; Skolny, M.N.; O’Toole, J.A.; Jammallo, L.S.; Niemierko, A.; Sadek, B.T.; Shenouda, M.N.; et al. Defining a threshold for intervention in breast cancer-related lymphedema: What level of arm volume increase predicts progression? Breast Cancer Res. Treat. 2013, 140, 485–494. [Google Scholar] [CrossRef]
- Damstra, R.J.; Halk, A.-B.; Halk, B.; Berg, J.V.D.; Born, Y.; Butter, E.; van Dorst, E.; van Everdingen, J.; Feenstra, C.; Gielink, P.; et al. The Dutch lymphedema guidelines based on the International Classification of Functioning, Disability, and Health and the chronic care model. J. Vasc. Surgery: Venous Lymphat. Disord. 2017, 5, 756–765. [Google Scholar] [CrossRef]
- Fu, M.R.; Chen, C.M.; Haber, J.; Guth, A.A.; Axelrod, D. The Effect of Providing Information about Lymphedema on the Cognitive and Symptom Outcomes of Breast Cancer Survivors. Ann. Surg. Oncol. 2010, 17, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Borman, P.; Yaman, A.; Yasrebi, S.; Özdemir, O. The Importance of Awareness and Education in Patients with Breast Cancer-Related Lymphedema. J. Cancer Educ. 2016, 32, 629–633. [Google Scholar] [CrossRef]
- DeSnyder, S.M.; Yi, M.; Boccardo, F.; Feldman, S.; Klimberg, V.S.; Smith, M.; Thiruchelvam, P.T.R.; McLaughlin, S. American Society of Breast Surgeons’ Practice Patterns for Patients at Risk and Affected by Breast Cancer-Related Lymphedema. Ann. Surg. Oncol. 2021, 28, 5742–5751. [Google Scholar] [CrossRef] [PubMed]
- Reichart, K. yLmphedema: Improving Screening and Treatment among At-Risk Breast Cancer Survivors. Clin. J. Oncol. Nurs. 2017, 21, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Tam, E.K.; Shen, L.; Munneke, J.R.; Ackerson, L.M.; Partee, P.N.; Somkin, C.P.; André, M.; Kutner, S.E.; Thiadens, S.R.J.; Kwan, M.L. Clinician awareness and knowledge of breast cancer-related lymphedema in a large, integrated health care delivery setting. Breast Cancer Res. Treat. 2012, 131, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, I.; Wisotzky, E.; Cheville, A.L. Differences in Perceived Risk at Which Clinician and Patient Stakeholders Initiate Activities to Prevent Late Effects Among Breast Cancer Survivors. Arch. Rehabil. Res. Clin. Transl. 2019, 1, 100006. [Google Scholar] [CrossRef] [PubMed]
- Damstra, R.J.; Glazenburg, E.J.; Hop, W.C.J. Validation of the inverse water volumetry method: A new gold standard for arm volume measurements. Breast Cancer Res. Treat. 2006, 99, 267–273. [Google Scholar] [CrossRef]
- Hidding, J.T.; Viehoff, P.B.; Beurskens, C.H.G.; van Laarhoven, H.W.M.; Nijhuis-van der Sanden, M.W.G.; van der Wees, P.J. Measurement Properties of Instruments for Measuring of Lymphedema: Systematic Review. Phys. Ther. 2016, 96, 1965–1981. [Google Scholar] [CrossRef] [PubMed]
- Ancukiewicz, M.; Russell, T.A.; Otoole, J.; Specht, M.; Singer, M.; Kelada, A.; Murphy, C.D.; Pogachar, J.; Gioioso, V.; Patel, M.; et al. Standardized Method for Quantification of Developing Lymphedema in Patients Treated for Breast Cancer. Int. J. Radiat. Oncol. 2011, 79, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Arts, D.L.; Voncken, A.G.; Medlock, S.; Abu-Hanna, A.; van Weert, H.C. Reasons for intentional guideline non-adherence: A systematic review. Int. J. Med. Inform. 2016, 89, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Branje, E. Arm lymphoedema in a cohort of breast cancer survivors 10 years after diagnosis. Acta Oncol. 2010, 49, 166–173. [Google Scholar] [CrossRef]
- Michelotti, A.; Invernizzi, M.; Lopez, G.; Lorenzini, D.; Nesa, F.; de Sire, A.; Fusco, N. Tackling the diversity of breast cancer related lymphedema: Perspectives on diagnosis, risk assessment, and clinical management. Breast 2019, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kilbreath, S.L.; Lee, M.-J.; Refshauge, K.M.; Beith, J.M.; Ward, L.C.; Simpson, J.M.; Black, D. Transient swelling versus lymphoedema in the first year following surgery for breast cancer. Support. Care Cancer 2013, 21, 2207–2215. [Google Scholar] [CrossRef]
- Norman, S.A.; Localio, A.R.; Potashnik, S.L.; Torpey, H.A.S.; Kallan, M.J.; Weber, A.L.; Miller, L.T.; DeMichele, A.; Solin, L.J. Lymphedema in Breast Cancer Survivors: Incidence, Degree, Time Course, Treatment, and Symptoms. J. Clin. Oncol. 2009, 27, 390–397. [Google Scholar] [CrossRef]
- Zou, L.; Liu, F.; Shen, P.; Hu, Y.; Liu, X.; Xu, Y.; Pen, Q.; Wang, B.; Zhu, Y.; Tian, Y. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: A 2-year follow-up prospective cohort study. Breast Cancer 2018, 25, 309–314. [Google Scholar] [CrossRef]
- de Sire, A.; Losco, L.; Lippi, L.; Spadoni, D.; Kaciulyte, J.; Sert, G.; Ciamarra, P.; Marcasciano, M.; Cuomo, R.; Bolletta, A.; et al. Surgical Treatment and Rehabilitation Strategies for Upper and Lower Extremity Lymphedema: A Comprehensive Review. Medicina 2022, 58, 954. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.L.; Binkley, J.M.; Schmitz, K.; Andrews, K.; Hayes, S.; Campbell, K.L.; Pt, M.L.M.; Soballe, P.W.; Berger, A.M.; Cheville, A.L.; et al. A prospective surveillance model for rehabilitation for women with breast cancer. Cancer 2012, 118, 2191–2200. [Google Scholar] [CrossRef]
- Whitworth, P.W.; Cooper, A. Reducing chronic breast cancer-related lymphedema utilizing a program of prospective surveillance with bioimpedance spectroscopy. Breast J. 2017, 24, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, P.W.; Shah, C.; Vicini, F.; Cooper, A. Preventing Breast Cancer-Related Lymphedema in High-Risk Patients: The Impact of a Structured Surveillance Protocol Using Bioimpedance Spectroscopy. Front. Oncol. 2018, 8, 197. [Google Scholar] [CrossRef]
- Jeffs, E.; Ream, E.; Taylor, C.; Bick, D. Clinical effectiveness of decongestive treatments on excess arm volume and patient-centered outcomes in women with early breast cancer-related arm lymphedema: A systematic review. JBI Database Syst. Rev. Implement Rep. 2018, 16, 453–506. [Google Scholar] [CrossRef]
| Characteristics | Mean ± SD | Number of Patients | Frequency % |
|---|---|---|---|
| Demographic & general health related | |||
| Age (years) | 59.2 ± 10.4 | ||
| Smoking | |||
| 60 | 12.7 | |
| 208 | 44.2 | |
| 203 | 43.1 | |
| ASA classification | |||
| 85 | 18.1 | |
| 294 | 62.6 | |
| 88 | 18.7 | |
| 3 | 0.6 | |
| BMI preoperative | 27.3 ± 5.1 | ||
| 162 | 34.5 | |
| 198 | 42.1 | |
| 110 | 23.4 | |
| Dominant side surgery | 226 | 49.9 | |
| Tumor related | |||
| Tumor stage | |||
| 3 | 0.9 | |
| 72 | 21.5 | |
| 167 | 49.9 | |
| 93 | 27.8 | |
| Number of removed lymphnodes | |||
| 428 | 90.7 | |
| 44 | 9.3 | |
| Total number of positive nodes ≥1 | 141 | 29.9 | |
| Treatment related | |||
| Breast conserving therapy (BCT) | 361 | 76.5 | |
| Mastectomy | 120 | 25.4 | |
| Sentinel node procedure | 450 | 95.3 | |
| Axillar lymph node dissection | 34 | 7.2 | |
| Neo-adjuvant chemotherapy | 111 | 23.5 | |
| Adjuvant chemotherapy | 107 | 22.7 | |
| Hormonal therapy | 287 | 60.8 | |
| Radiation | 400 | 84.7 | |
| 132 | 33.0 |
| Follow-Up | RVC < 5% | RVC ≥ 5% | ||
|---|---|---|---|---|
| Proportion | 95% CI | Proportion | 95% CI | |
| 3 months (n = 455) | 90.5 | 87.4, 93.0 | 9.5 | 6.9, 12.6 |
| 6 months (n = 449) | 91.3 | 88.2, 93.7 | 8.7 | 6.3, 11.8 |
| 12 months (n = 448) | 91.3 | 88.2, 93.7 | 8.7 | 6.3, 11.8 |
| 24 months (n = 437) | 89.5 | 86.1, 92.1 | 10.5 | 7.9, 13.9 |
| Therapy Referral | RVC < 5% | RVC ≥ 5% |
|---|---|---|
| n (%) | n (%) | |
| 3 months | ||
| No | 411 (99.8) | 35 (81.4) |
| Yes | 1 (0.2) | 8 (18.6) |
| 6 months | ||
| No | 371 (97.9) | 17 (89.5) |
| Yes | 8 (2.1) | 2 (10.5) |
| 12 months | ||
| No | 340 (97.1) | 11 (78.6) |
| Yes | 10 (2.9) | 3 (21.4) |
| 24 months | ||
| No | 314 (98.4) | 10 (83.3) |
| Yes | 5 (1.6) | 2 (16.7) |
| Follow-Up | % (95% CI) |
|---|---|
| 3 months (n = 43) | 25.6 (14.0, 41.5) |
| Not referred | 45.5 (18.1, 75.4) |
| Referred | 54.5 (24.6, 81.9) |
| 6 months (n = 19) | 26.3 (10.1, 51.4) |
| Not referred | 60.0 (17.0, 92.7) |
| Referred | 40.0 (7.3, 83.0) |
| 12 months (n = 14) | 28.6 (9.6, 58.0) |
| Not referred | 25.0 (1.3, 78.1) |
| Referred | 75.0 (21.9, 98.7) |
| 24 months (n = 12) | 58.3 (28.6, 83.5) |
| Not referred | 71.4 (30.3, 94.9) |
| Referred | 28.6 (5.1, 69.7) |
| Follow Up. | Mean (SD) RVC % | T24, Mean (SD) RVC % | Mean Difference (%, SD) | p Value |
|---|---|---|---|---|
| No treatment referral | ||||
| T3 (n = 33) | 8.7 (4.6) | 5.3 4.0) | −3.4 (5.8) | 0.002 * |
| T6 (n = 16) | 9.9 (11.6) | 3.6 (3.2) | −6.3 (11.6) | 0.045 * |
| T12 (n = 11) | 7.8 (2.6) | 2.4 (4.3) | −5.4 (5.7) | 0.011 * |
| Treatment referral | ||||
| T3 (n = 7) | 10.8 (5.9) | 15.9 (16.5) | 5.1 (17.2) | 0.467 |
| T6 (n = 2) | 8.2 (1.2) | 9.2 (10.3) | 1.0 (9.1) | 0.899 |
| T12 (n = 3) | 9.4 (2.2) | 6.6 (3.4) | −2.8 (2.2) | 0.163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendrickx, A.A.; Küthe, S.W.; van der Schans, C.P.; Krijnen, W.P.; Mouës-Vink, C.M.; Damstra, R.J. Early Referral for Breast-Cancer-Related Lymphedema: Do We Follow the Evidence? A Two-Year Prospective Multicenter Cohort Study. Cancers 2022, 14, 6016. https://doi.org/10.3390/cancers14236016
Hendrickx AA, Küthe SW, van der Schans CP, Krijnen WP, Mouës-Vink CM, Damstra RJ. Early Referral for Breast-Cancer-Related Lymphedema: Do We Follow the Evidence? A Two-Year Prospective Multicenter Cohort Study. Cancers. 2022; 14(23):6016. https://doi.org/10.3390/cancers14236016
Chicago/Turabian StyleHendrickx, Ad A., Saskia W. Küthe, Cees P. van der Schans, Wim P. Krijnen, Chantal M. Mouës-Vink, and Robert J. Damstra. 2022. "Early Referral for Breast-Cancer-Related Lymphedema: Do We Follow the Evidence? A Two-Year Prospective Multicenter Cohort Study" Cancers 14, no. 23: 6016. https://doi.org/10.3390/cancers14236016
APA StyleHendrickx, A. A., Küthe, S. W., van der Schans, C. P., Krijnen, W. P., Mouës-Vink, C. M., & Damstra, R. J. (2022). Early Referral for Breast-Cancer-Related Lymphedema: Do We Follow the Evidence? A Two-Year Prospective Multicenter Cohort Study. Cancers, 14(23), 6016. https://doi.org/10.3390/cancers14236016







