Correlation between ADC Histogram-Derived Metrics and the Time to Metastases in Resectable Pancreatic Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
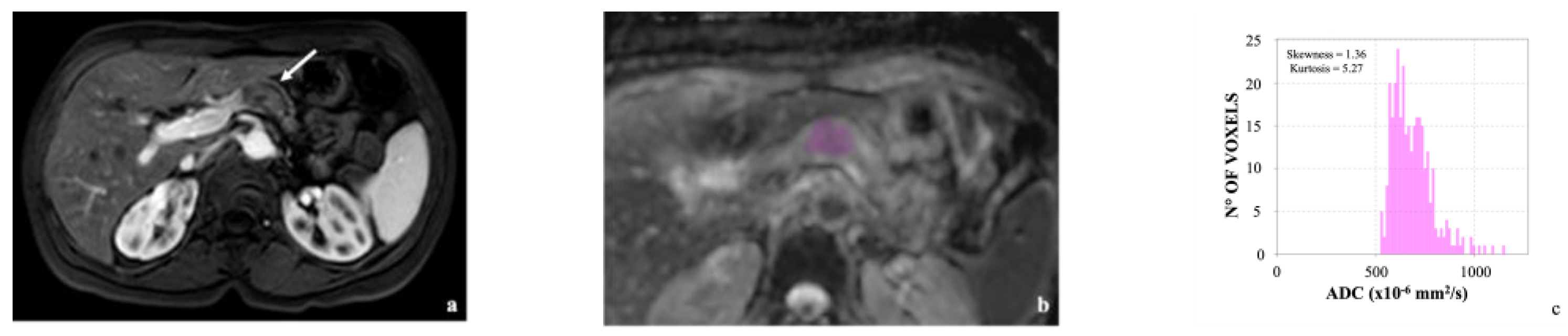
3.2. Image Analysis
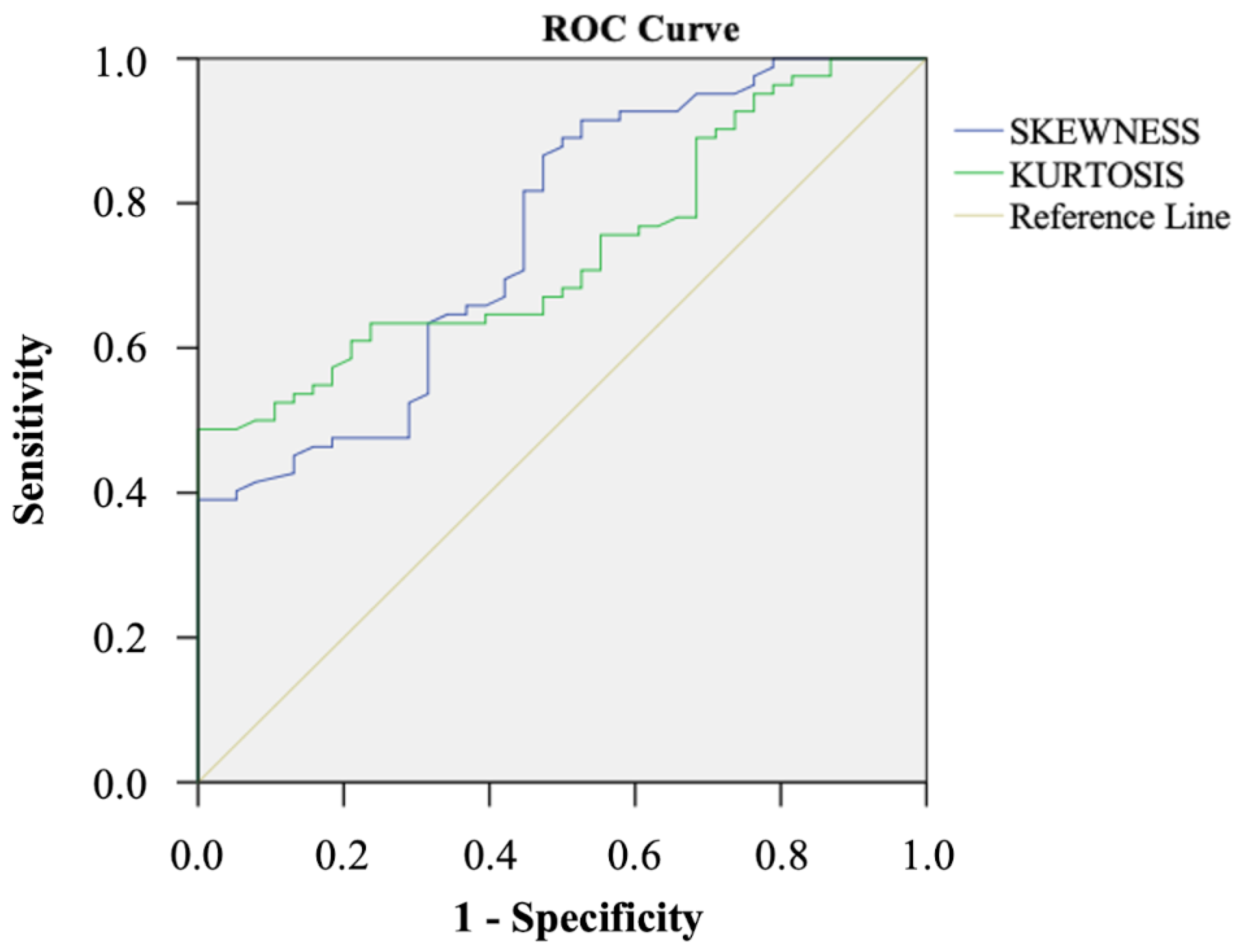
3.3. Correlation with the TTM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Surveillance, Epidemiology, and End Results Program, Cancer Stat Facts: Pancreatic Cancer. NIH. 2022. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 14 October 2022).
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Adbelghani, M.; Wei, A.C.; Raoul, J.L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 20, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; van Laethem, J.L.; Conroy, T.; et al. Cancer of the Pancreas: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2015, 26, v56–v68. [Google Scholar] [CrossRef] [PubMed]
- Nishio, K.; Kimura, K.; Amano, R.; Yamazoe, S.; Ohrira, G.; Nakata, B.; Hirakawa, K.; Ohira, M. Preoperative Predictors for Early Recurrence of Resectable Pancreatic Cancer. World J. Surg. Oncol. 2017, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Fujimoto, M.; Ito, N.; Takahashi, Y.; Kitago, M.; Gotoh, M.; Hiraoka, N.; Yoshida, T.; Kitagawa, Y.; Kanai, Y.; et al. Clinicopathological Impacts of DNA Methylation Alterations on Pancreatic Ductal Adenocarcinoma: Prediction of Early Recurrence Based on Genome-Wide DNA Methylation Profiling. J. Cancer Res. Clin. Oncol. 2021, 147, 1341–1354. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Jacob, R.; Manne, U.; Paluri, R. Advances in Pancreatic Cancer Biomarkers. Oncol. Rev. 2019, 13, 410. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, J.M.; Cho, J.Y.; Lee, K.B.; Kim, J.E.; Moon, S.K.; Kim, S.J.; Baek, J.H.; Kim, S.H.; Kim, S.H.; et al. Small (≤20 Mm) Pancreatic Adenocarcinomas: Analysis of Enhancement Patterns and Secondary Signs with Multiphasic Multidetector CT. Radiology 2011, 259, 442–452. [Google Scholar] [CrossRef]
- Bae, J.S.; Kim, J.H.; Kang, H.-J.; Han, J.K. Prediction of Residual Tumor and Overall Survival after First-Line Surgery in Patients with Pancreatic Ductal Adenocarcinoma Using Preoperative Magnetic Resonance Imaging Findings. Acta Radiol. 2022, 63, 435–446. [Google Scholar] [CrossRef]
- Just, N. Improving Tumour Heterogeneity MRI Assessment with Histograms. Br. J. Cancer 2014, 111, 2205–2213. [Google Scholar] [CrossRef]
- Pereira, J.A.S.; Rosado, E.; Bali, M.; Metens, T.; Chao, S.-L. Pancreatic Neuroendocrine Tumors: Correlation between Histogram Analysis of Apparent Diffusion Coefficient Maps and Tumor Grade. Abdom. Imaging 2015, 40, 3122–3128. [Google Scholar] [CrossRef]
- Hoffman, D.H.; Ream, J.M.; Hajdu, C.H.; Rosenkrantz, A.B. Utility of Whole-Lesion ADC Histogram Metrics for Assessing the Malignant Potential of Pancreatic Intraductal Papillary Mucinous Neoplasms (IPMNs). Abdom. Radiol. 2017, 42, 1222–1228. [Google Scholar] [CrossRef]
- De Robertis, R.; Maris, B.; Cardobi, N.; Tinazzi Martini, P.; Gobbo, S.; Capelli, P.; Ortolani, S.; Cingarlini, S.; Paiella, S.; Landoni, L.; et al. Can Histogram Analysis of MR Images Predict Aggressiveness in Pancreatic Neuroendocrine Tumors? Eur. Radiol. 2018, 28, 2582–2591. [Google Scholar] [CrossRef]
- Lu, J.Y.; Yu, H.; Zou, X.L.; Li, Z.; Hu, X.M.; Shen, Y.Q.; Hu, D.Y. Apparent Diffusion Coefficient-Based Histogram Analysis Differentiates Histological Subtypes of Periampullary Adenocarcinoma. World J. Gastroenterol. 2019, 25, 6116–6128. [Google Scholar] [CrossRef]
- De Robertis, R.; Beleù, A.; Cardobi, N.; Frigerio, I.; Ortolani, S.; Gobbo, S.; Maris, B.; Melisi, D.; Montemezzi, S.; D’Onofrio, M. Correlation of MR Features and Histogram-Derived Parameters with Aggressiveness and Outcomes after Resection in Pancreatic Ductal Adenocarcinoma. Abdom. Radiol. 2020, 45, 3809–3818. [Google Scholar] [CrossRef]
- Igarashi, T.; Shiraishi, M.; Watanabe, K.; Ohki, K.; Takenaga, S.; Ashida, H.; Ojiri, H. 3D Quantitative Analysis of Diffusion-Weighted Imaging for Predicting the Malignant Potential of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pol. J. Radiol. 2021, 86, 298–308. [Google Scholar] [CrossRef]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef]
- Kimura, K.; Yoshida, S.; Tsuchiya, J.; Yamada, I.; Tanaka, H.; Yokoyama, M.; Matsuoka, Y.; Yoshimura, R.; Tateishi, U.; Fujii, Y. Usefulness of Texture Features of Apparent Diffusion Coefficient Maps in Predicting Chemoradiotherapy Response in Muscle-Invasive Bladder Cancer. Eur. Radiol. 2022, 32, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ibsi.readthedocs.io/en/latest/03_Image_features.html#intensity-histogram-features (accessed on 22 November 2022).
- Orlhac, F.; Lecler, A.; Savatovski, J.; Goya-Outi, J.; Nioche, C.; Charbonneau, F.; Ayache, N.; Frouin, F.; Duron, L.; Buvat, I. How Can We Combat Multicenter Variability in MR Radiomics? Validation of a Correction Procedure. Eur. Radiol. 2021, 31, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and Its Associated Cutoff Point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Shindo, T.; Fukukura, Y.; Umanodan, T.; Takumi, K.; Hakamada, H.; Nakajo, M.; Umanodan, A.; Ideue, J.; Kamimura, K.; Yoshiura, T. Histogram Analysis of Apparent Diffusion Coefficient in Differentiating Pancreatic Adenocarcinoma and Neuroendocrine Tumor. Medicine 2016, 95, e2574. [Google Scholar] [CrossRef]
- Noda, Y.; Tomita, H.; Ishihara, T.; Tsuboi, Y.; Kawai, N.; Kawaguchi, M.; Kaga, T.; Hyodo, F.; Hara, A.; Kambadakone, A.R.; et al. Prediction of Overall Survival in Patients with Pancreatic Ductal Adenocarcinoma: Histogram Analysis of ADC Value and Correlation with Pathological Intratumoral Necrosis. BMC Med. Imaging 2022, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhong Hou, S.; Wu, Z.; Huang, X.; Wang, Z.; Tian, B. Prognostic nomogram for patients undergoing radical pancreaticoduodenectomy for adenocarcinoma of the pancreatic head. BMC Cancer 2021, 27, 624. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, P.D.; Wadsley, P.J.; et al. Comparison of Adjuvant Gemcitabine and Capecitabine with Gemcitabine Monotherapy in Patients with Resected Pancreatic Cancer (ESPAC-4): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Pappalardo, A.; Giunta, E.F.; Tirino, G.; Pompella, L.; Federico, P.; Daniele, B.; de Vita, F.; Petrillo, A. Adjuvant Treatment in Pancreatic Cancer: Shaping the Future of the Curative Setting. Front. Oncol. 2021, 11, 695627. [Google Scholar] [CrossRef] [PubMed]


| Features | |
|---|---|
| Sex | |
| Male | 72 (60%) |
| Female | 48 (40%) |
| Age a (years) | 65 (42–86) |
| pT1b | 1 (0.8%) |
| pT1c | 40 (33.3%) |
| pT2 | 62 (51.7%) |
| pT3 | 17 (14.2%) |
| pN1 | 5 (4.2%) |
| pN2 | 115 (95.8%) |
| Tumor stage | |
| IIB | 5 (4.2%) |
| III | 115 (95.8%) |
| Follow-up a (months) | 29 (3–54) |
| Metastases | |
| Yes | 82 (68.3%) |
| Liver | 42 (51.2%) |
| Lung | 10 (12.2%) |
| Other sites | 8 (9.8%) |
| Multiple sites | 22 (26.8%) |
| No | 38 (31.7%) |
| Feature | Total | M+ | M− | p |
|---|---|---|---|---|
| Site | 0.308 | |||
| Head | 99 (82.5%) | 70 (85.4%) | 29 (76.3%) | |
| Body | 19 (15.8%) | 11 (13.4%) | 8 (21.1%) | |
| Tail | 2 (1.7%) | 1 (1.2%) | 1 (2.6%) | |
| T1w SI | 1 | |||
| Hypointense | 117 (97.5%) | 80 (97.6%) | 37 (97.4%) | |
| Isointense | 3 (2.5%) | 2 (2.4%) | 1 (2.6%) | |
| T2w SI | 0.399 | |||
| Hypointense | 11 (9.2%) | 7 (8.5%) | 4 (10.5%) | |
| Isointense | 32 (26.6%) | 25 (30.5%) | 7 (18.4%) | |
| Hyperintense | 77 (64.2%) | 50 (61%) | 27 (71.1%) | |
| Arterial phase SI | 0.678 | |||
| Hypointense | 113 (94.2%) | 78 (95.1%) | 35 (92.1%) | |
| Isointense | 7 (5.8%) | 4 (4.9%) | 3 (7.9%) | |
| Portal phase SI | 0.242 | |||
| Hypointense | 110 (91.7%) | 77 (93.9%) | 33 (86.8%) | |
| Isointense | 9 (7.5%) | 4 (4.9%) | 5 (13.2%) | |
| Hyperintense | 1 (0.8%) | 1 (1.2%) | 0 (0%) | |
| Delayed phase SI | 0.487 | |||
| Hypointense | 104 (86.6%) | 73 (89%) | 31 (81.6%) | |
| Isointense | 11 (9.2%) | 6 (7.3%) | 4 (10.5%) | |
| Hyperintense | 5 (4.2%) | 3 (3.7%) | 3 (7.9%) |
| Parameter | M+ | M− | p |
|---|---|---|---|
| Age | 65 (42–86) | 66 (46–83) | 0.66 |
| Size | 27.5 (10–58) | 28.4 (7–60) | 0.81 |
| ADCmin | 677.3 (1–1541) | 666.2 (16–1206) | 0.95 |
| ADCmax | 2363 (1049–3607) | 2164 (249–3541) | 0.10 |
| ADCmean | 1361.6 (658–1881) | 1341.9 (175–1875) | 0.99 |
| SD | 295.1 (35–848) | 280.4 (24–707) | 0.52 |
| ADCmedian | 1329.4 (652–1871) | 1320.6 (177–1831) | 0.80 |
| ADC25 | 1157.4 (18;1793) | 1157.7 (165–1587) | 0.74 |
| ADC75 | 1529.9 (725;2124) | 1509.6 (190–2202) | 0.82 |
| Skewness | 0.6 (−0.6;3.3) | 0.2 (−1.2;1.8) | 0.005 |
| Kurtosis | 4.3 (1.7; 17.3) | 3.8 (2.1; 11.1) | 0.032 |
| Entropy | 6.5 (1.3–9.3) | 6.4 (1.2–9.4) | 0.31 |
| Uniformity | 0.1 (0–0.1) | 0.1 (0–0.4) | 0.36 |
| ADC Skewness | ADC Kurtosis | |
|---|---|---|
| Optimal Cut-off | 0.23 | 3.90 |
| Sensitivity | 98.6 (92.5–100) | 47.6 (36.4–58.9) |
| Specificity | 41.7 (27.6–56.8) | 100 (91–100) |
| PPV | 71.7 (66.6–76.3) | 100 (-) |
| NPV | 95.2 (73.5–99.3) | 46.9 (41.8–52.1) |
| Accuracy | 75.8 (67.2–83.2) | 64.2 (54.9–72.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Robertis, R.; Tomaiuolo, L.; Pasquazzo, F.; Geraci, L.; Malleo, G.; Salvia, R.; D’Onofrio, M. Correlation between ADC Histogram-Derived Metrics and the Time to Metastases in Resectable Pancreatic Adenocarcinoma. Cancers 2022, 14, 6050. https://doi.org/10.3390/cancers14246050
De Robertis R, Tomaiuolo L, Pasquazzo F, Geraci L, Malleo G, Salvia R, D’Onofrio M. Correlation between ADC Histogram-Derived Metrics and the Time to Metastases in Resectable Pancreatic Adenocarcinoma. Cancers. 2022; 14(24):6050. https://doi.org/10.3390/cancers14246050
Chicago/Turabian StyleDe Robertis, Riccardo, Luisa Tomaiuolo, Francesca Pasquazzo, Luca Geraci, Giuseppe Malleo, Roberto Salvia, and Mirko D’Onofrio. 2022. "Correlation between ADC Histogram-Derived Metrics and the Time to Metastases in Resectable Pancreatic Adenocarcinoma" Cancers 14, no. 24: 6050. https://doi.org/10.3390/cancers14246050
APA StyleDe Robertis, R., Tomaiuolo, L., Pasquazzo, F., Geraci, L., Malleo, G., Salvia, R., & D’Onofrio, M. (2022). Correlation between ADC Histogram-Derived Metrics and the Time to Metastases in Resectable Pancreatic Adenocarcinoma. Cancers, 14(24), 6050. https://doi.org/10.3390/cancers14246050







