The Normal, the Radiosensitive, and the Ataxic in the Era of Precision Radiotherapy: A Narrative Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Radiotherapy Toxicities
2.1. Overview
2.2. Mechanisms of RT Toxicities
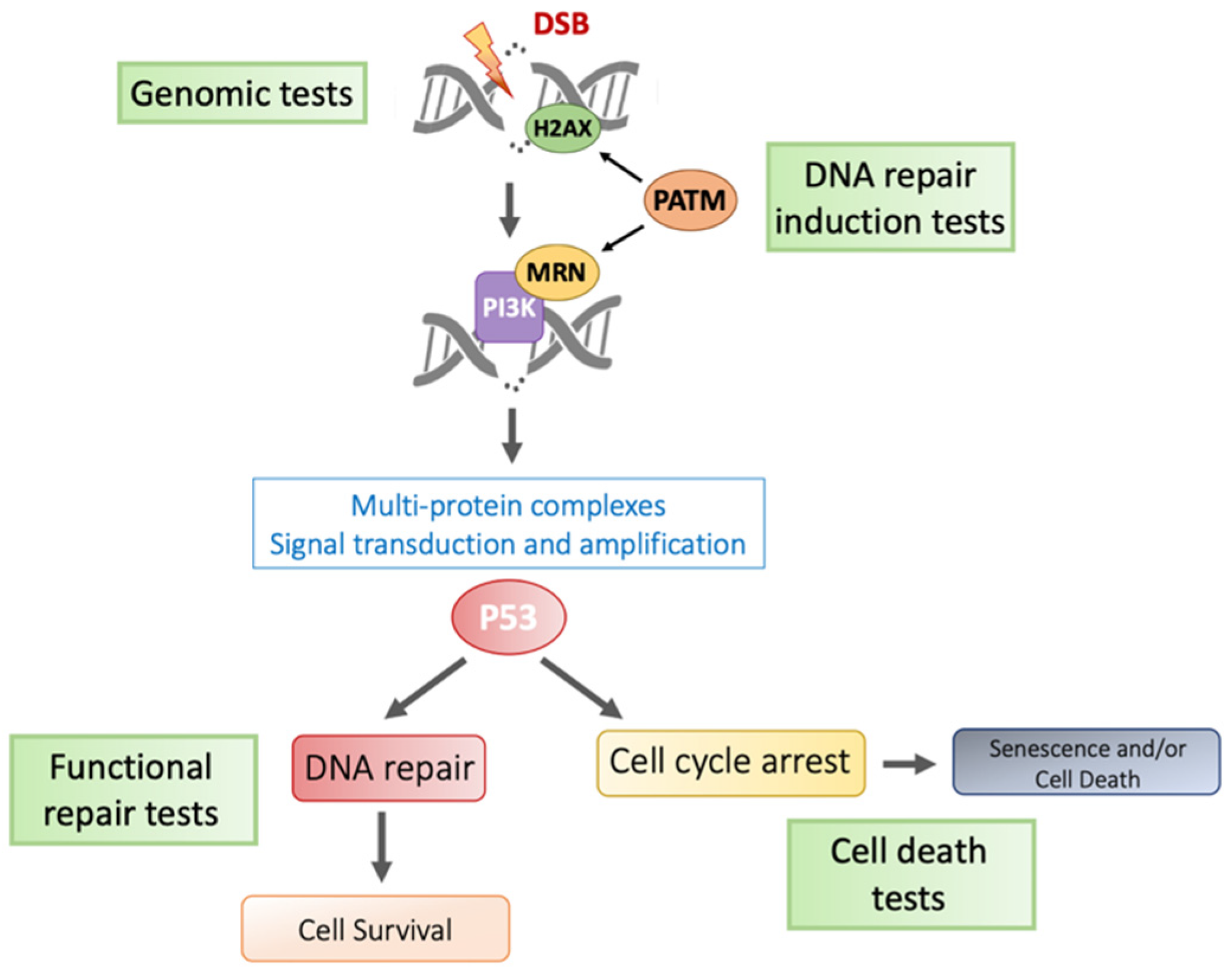
2.2.1. Direct Effects: The DNA Damage Response
2.2.2. Indirect Effects: Oxidative Stress Response Mechanisms
2.2.3. Systemic Mechanisms: Inflammatory and Remodeling Processes
2.3. Risk Factors/Determinants of RT Toxicity
2.3.1. Dosimetric Factors
2.3.2. Other Therapeutic Factors
2.3.3. Non-Genetic Clinical Factors
2.3.4. Radiosensitive Syndromes
2.4. Molecular Events Affecting iRS in Patients with a Normal Phenotype
2.4.1. Polymorphisms and Haplotypes
2.4.2. Gene Expression and Alternative Splicing: An Invisible Burden That Matters
2.4.3. Role of Epigenetic Marks
3. Predictive/Prognostic iRS Biomarker Research
3.1. Systemic and Radiological Biomarkers
3.2. Genetic Assays
3.3. ATM-Based Assays
3.4. Apoptotic Assays
3.5. 8-Oxo-Guanine Assays
3.6. Pros and Cons of Available Methods
4. Practical Consequences of a Priori Knowledge of iRS for Precision Radiotherapy and Patient Selection
4.1. Screening of Individual Radiation Sensitivity
4.2. Possible Therapeutic Adaptations in Patients Screened “iRS Positive”
4.2.1. Tissue Sparing Techniques
- Photon-based techniques
- Tissue volume-sparing with other forms of radiotherapy than IMRT or SBRT
4.2.2. Biological Mitigation
- Hyperfractionation
- New Frontiers: temporal fractionation (such as FLASH) and spatial fractionation
4.2.3. Adapted Toxicity Management
- (1)
- Anti-inflammatory drugs or angiotensin II receptor antagonists [168]; corticosteroid therapy at a minimum dose of 1 mg/kg/day (equivalent prednisolone) is recommended for a minimum of 4 to 6 weeks.
- (2)
- Long-term use of antioxidant drugs such as superoxide dismutase or tocopherol (vitamin E), preferably combined with pentoxifylline, to mitigate fibrosis, as evidenced in randomized trials [169].
- (3)
- (4)
- Endoscopic or minimally invasive procedures, may help to relief stenoses (e.g., esophagus, ureter), adhesions, and strictures (bowel). Argon plasma electrocoagulation of telangiectasia is an effective approach for skin or bleeding mucosae [165]. Several sessions are often necessary, especially for extended lesions. MRI-guided laser thermal ablation of brain radionecrosis was recently investigated.
- (5)
- Hyperbaric oxygen (HBO) is effective in better oxygenating fibrotic/hypoxic tissues, especially lymphedema, jaw osteoradionecrosis, and proctitis [166]. Clinical cases and open studies have reported a positive impact on bleeding after an average of 24 to 67 sessions. Some contraindications (claustrophobia, cardiac conduction disorders, uncontrolled epilepsy, bronchopathy, pneumothorax, etc.) may be observed.
- (6)
- In situ injection of autologous mesenchymal stem cells, recently advocated in the frame of accidental irradiation [167].
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries; Global Cancer Observatory, IARC: Lyon, France, 2020. [Google Scholar]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Mohanti, B.K.; Bansal, M. Late sequelae of radiotherapy in adults. Support. Care Cancer 2005, 13, 775–780. [Google Scholar] [CrossRef]
- Sperk, E.; Welzel, G.; Keller, A.; Kraus-Tiefenbacher, U.; Gerhardt, A.; Sütterlin, M.; Wenz, F. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: Results from the randomized phase III trial TARGIT A. Breast Cancer Res. Treat. 2012, 135, 253–260. [Google Scholar] [CrossRef]
- Foray, N.; Bourguignon, M.; Hamada, N. Individual response to ionizing radiation. Mutat. Res. Mol. Mech. Mutagen. 2016, 770, 369–386. [Google Scholar] [CrossRef]
- Burnet, N.G.; Johansen, J.; Turesson, I.; Nyman, J.; Peacock, J.H. Describing patients’ normal tissue reactions: Concerning the possibility of individualising radiotherapy dose prescriptions based on potential predictive assays of normal tissue radiosensitivity. Steering Committee of the BioMed2 European Union Concerted Action Programme on the Development of Predictive Tests of Normal Tissue Response to Radiation Therapy. Int. J. Cancer 1998, 79, 606–613. [Google Scholar]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Prim. 2019, 5, 13, Nat. Rev. Dis. Prim.2019, 5, 15. [Google Scholar] [CrossRef]
- Borrelli, M.R.; Shen, A.H.; Lee, G.K.; Momeni, A.; Longaker, M.T.; Wan, D.C. Radiation-Induced Skin Fibrosis: Pathogenesis, Current Treatment Options, and Emerging Therapeutics. Ann. Plast. Surg. 2019, 83 (Suppl. 1), S59–S64. [Google Scholar] [CrossRef]
- Vogin, G. Description and Management of Radiotherapy-Induced Long-Term Effects. In Survivorship Care for Cancer Patients; Rauh, S., Ed.; Springer: Cham, Switzerland, 2021; pp. 257–285. [Google Scholar] [CrossRef]
- Vogin, G. Radiosensibilité, radiocurabilité et réparation [Radiosensitivity, radiocurability and DNA repair]. Cancer Radiother. 2011, 15, 294–306. [Google Scholar] [CrossRef]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R., 3rd; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- San Filippo, J.; Sung, P.; Klein, H. Mechanism of Eukaryotic Homologous Recombination. Annu. Rev. Biochem. 2008, 77, 229–257. [Google Scholar] [CrossRef]
- Lieber, M.R. The Mechanism of Human Nonhomologous DNA End Joining. J. Biol. Chem. 2008, 283, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Mirzayans, R.; Andrais, B.; Scott, A.; Wang, Y.W.; Murray, D. Ionizing Radiation-Induced Responses in Human Cells with Differing TP53 Status. Int. J. Mol. Sci. 2013, 14, 22409–22435. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-S.; Wang, H.-J.; Qian, H.-L. Biological effects of radiation on cancer cells. Mil. Med. Res. 2018, 5, 20. [Google Scholar] [CrossRef]
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. 2013, 25, 578–585. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of Reactive Oxygen Species: An Emerging Approach for Cancer Therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.P.; Amstad, P.; He, P.; Robles, A.; Lupold, S.; Kaneko, I.; Ichimiya, M.; Sengupta, S.; Mechanic, L.; Okamura, S.; et al. p53-Induced Up-Regulation of MnSOD and GPx but not Catalase Increases Oxidative Stress and Apoptosis. Cancer Res. 2004, 64, 2350–2356. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, C.; Wu, R.; Sun, Y.; Levine, A.; Feng, Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc. Natl. Acad. Sci. USA 2010, 107, 7455–7460. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Bhatt, D.; Oltvai, Z.N.; Greenberger, J.S.; Bahar, I. Significance of p53 dynamics in regulating apoptosis in response to ionizing radiation and polypharmacological strategies. Sci. Rep. 2014, 4, srep06245. [Google Scholar] [CrossRef] [Green Version]
- Rey, S.; Schito, L.; Koritzinsky, M.; Wouters, B.G. Molecular targeting of hypoxia in radiotherapy. Adv. Drug Deliv. Rev. 2017, 109, 45–62. [Google Scholar] [CrossRef]
- Movafagh, S.; Crook, S.; Vo, K. Regulation of Hypoxia-Inducible Factor-1a by Reactive Oxygen Species: New Developments in an Old Debate. J. Cell. Biochem. 2015, 116, 696–703. [Google Scholar] [CrossRef]
- Yarnold, J.; Brotons, M.-C.V. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 2010, 97, 149–161. [Google Scholar] [CrossRef]
- Wynn, T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.; Lefaix, J.; Delanian, S. TGF-beta1 and radiation fibrosis: A master switch and a specific therapeutic target? Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 277–290. [Google Scholar] [CrossRef]
- Wang, M.; Saha, J.; Hada, M.; Anderson, J.A.; Pluth, J.M.; O’Neill, P.; Cucinotta, F.A. Novel Smad proteins localize to IR-induced double-strand breaks: Interplay between TGFbeta and ATM pathways. Nucleic Acids Res. 2013, 41, 933–942. [Google Scholar] [CrossRef] [Green Version]
- Bentzen, S.M. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef]
- Rubin, P.; Johnston, C.J.; Williams, J.P.; McDonald, S.; Finkelstein, J.N. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int. J. Radiat. Oncol. 1995, 33, 99–109. [Google Scholar] [CrossRef]
- Mikkelsen, R.B.; Wardman, P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene 2003, 22, 5734–5754. [Google Scholar] [CrossRef] [Green Version]
- Nahum, A.E. The radiobiology of hypofractionation. Clin. Oncol. (R Coll. Radiol.) 2015, 27, 260–269. [Google Scholar] [CrossRef]
- Hall, E.J. Radiobiology for the Radiologist, 8th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2018. [Google Scholar]
- Emami, B.; Lyman, J.; Brown, A.; Cola, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Ten Haken, R.K.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. Use of Normal Tissue Complication Probability Models in the Clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S10–S19. [Google Scholar] [CrossRef] [Green Version]
- Rancati, T.; Fiorino, C. Modelling Radiotherapy Side Effects Practical Applications for Planning Optimisation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Stewart, F.A.; Akleyev, A.; Hauerjensen, M.; Hendry, J.; Kleiman, N.; MacVittie, T.; Aleman, B.; Edgar, A.; Mabuchi, K.; Muirhead, C.; et al. ICRP PUBLICATION 118: ICRP Statement on Tissue Reactions and Early and Late Effects of Radiation in Normal Tissues and Organs—Threshold Doses for Tissue Reactions in a Radiation Protection Context. Ann. ICRP 2012, 41, 1–322. [Google Scholar] [CrossRef]
- Seibold, P.; Webb, A.; Aguado-Barrera, M.E.; Azria, D.; Bourgier, C.; Brengues, M.; Briers, E.; Bultijnck, R.; Calvo-Crespo, P.; Carballo, A.; et al. REQUITE: A prospective multicentre cohort study of patients undergoing radiotherapy for breast, lung or prostate cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 138, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Kierkels, R.G.; Wopken, K.; Visser, R.; Korevaar, E.W.; van der Schaaf, A.; Bijl, H.P.; Langendijk, J.A. Multivariable normal tissue complication probability model-based treatment plan optimization for grade 2–4 dysphagia and tube feeding dependence in head and neck radiotherapy. Radiother. Oncol. 2016, 121, 374–380. [Google Scholar] [CrossRef]
- Vai, A.; Molinelli, S.; Rossi, E.; Iacovelli, N.A.; Magro, G.; Cavallo, A.; Pignoli, E.; Rancati, T.; Mirandola, A.; Russo, S.; et al. Proton Radiation Therapy for Nasopharyngeal Cancer Patients: Dosimetric and NTCP Evaluation Supporting Clinical Decision. Cancers 2022, 14, 1109. [Google Scholar] [CrossRef]
- Massi, M.C.; Gasperoni, F.; Ieva, F.; Paganoni, A.M.; Zunino, P.; Manzoni, A.; Franco, N.R.; Veldeman, L.; Ost, P.; Fonteyne, V.; et al. A Deep Learning Approach Validates Genetic Risk Factors for Late Toxicity After Prostate Cancer Radiotherapy in a REQUITE Multi-National Cohort. Front. Oncol. 2020, 10, 541281. [Google Scholar] [CrossRef]
- Franco, N.R.; Massi, M.C.; Ieva, F.; Manzoni, A.; Paganoni, A.M.; Zunino, P.; Veldeman, L.; Ost, P.; Fonteyne, V.; Talbot, C.J.; et al. Development of a method for generating SNP interaction-aware polygenic risk scores for radiotherapy toxicity. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 159, 241–248. [Google Scholar] [CrossRef]
- Deichaite, I.; Hopper, A.; Krockenberger, L.; Sears, T.J.; Sutton, L.; Ray, X.; Sharabi, A.; Navon, A.; Sanghvi, P.; Carter, H.; et al. Germline genetic biomarkers to stratify patients for personalized radiation treatment. J. Transl. Med. 2022, 20, 360. [Google Scholar] [CrossRef]
- Deneuve, S.; Bastogne, T.; Duclos, M.; Mirjolet, C.; Bois, P.; Bachmann, P.; Nokovitch, L.; Roux, P.-E.; Girodet, D.; Poupart, M.; et al. Predicting acute severe toxicity for head and neck squamous cell carcinomas by combining dosimetry with a radiosensitivity biomarker: A pilot study. Tumori J. 2022. [Google Scholar] [CrossRef]
- Rancati, T.; Massi, M.C.; Franco, N.R.; Avuzzi, B.; Azria, D.; Choudhury, A.; Cicchetti, A.; de Ruysscher, D.; Dunning, A.M.; Elliott, R.; et al. West Prediction of toxicity after prostate cancer RT: The value of a SNP-interaction polygenic risk score. Radiother. Oncol. 2021, 161, S526–S528. [Google Scholar] [CrossRef]
- Tucker, S.L.; Li, M.; Xu, T.; Gomez, D.; Yuan, X.; Yu, J.; Liu, Z.; Yin, M.; Guan, X.; Wang, L.-E.; et al. Incorporating Single-nucleotide Polymorphisms into the Lyman Model to Improve Prediction of Radiation Pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Girinsky, T.; Cosset, J.M. Pulmonary and cardiac late effects of ionizing radiations alone or combined with chemotherapy. Cancer Radiother. 1997, 1, 735–743. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Skoczylas, J.Z.; Overgaard, M.; Overgaard, J. Radiotherapy-Related Lung Fibrosis Enhanced by Tamoxifen. Gynecol. Oncol. 1996, 88, 918–922. [Google Scholar] [CrossRef]
- Barcellini, A.; Loap, P.; Murata, K.; Villa, R.; Kirova, Y.; Okonogi, N.; Orlandi, E. PARP Inhibitors in Combination with Radiotherapy: To Do or Not to Do? Cancers 2021, 13, 5380. [Google Scholar] [CrossRef]
- Niyazi, M.; Maihoefer, C.; Krause, M.; Rodel, C.; Budach, W.; Belka, C. Radiotherapy and “new” drugs-new side effects? Radiat. Oncol. 2011, 6, 177. [Google Scholar] [CrossRef] [Green Version]
- Voong, K.R.; Hazell, S.Z.; Fu, W.; Hu, C.; Lin, C.T.; Ding, K.; Suresh, K.; Hayman, J.; Hales, R.K.; Alfaifi, S.; et al. Relationship Between Prior Radiotherapy and Checkpoint-Inhibitor Pneumonitis in Patients with Advanced Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2019, 20, e470–e479. [Google Scholar] [CrossRef]
- Paulino, A.C.; Constine, L.S.; Rubin, P.; Williams, J.P. Normal Tissue Development, Homeostasis, Senescence, and the Sensitivity to Radiation Injury Across the Age Spectrum. Semin. Radiat. Oncol. 2010, 20, 12–20. [Google Scholar] [CrossRef]
- Rübe, C.E.; Fricke, A.; Schneider, R.; Simon, K.; Kühne, M.; Fleckenstein, J.; Gräber, S.; Graf, N.; Rübe, C. DNA Repair Alterations in Children with Pediatric Malignancies: Novel Opportunities to Identify Patients at Risk for High-Grade Toxicities. Int. J. Radiat. Oncol. 2010, 78, 359–369. [Google Scholar] [CrossRef]
- Bernier-Chastagner, V.; Hettal, L.; Gillon, V.; Fernandes, L.; Huin-Schohn, C.; Vazel, M.; Tosti, P.; Salleron, J.; François, A.; Cérimèle, E.; et al. Validation of a high performance functional assay for individual radiosensitivity in pediatric oncology: A prospective cohort study (ARPEGE). BMC Cancer 2018, 18, 719. [Google Scholar] [CrossRef] [Green Version]
- Hernández, L.; Terradas, M.; Camps, J.; Martín, M.; Tusell, L.; Genescà, A. Aging and radiation: Bad companions. Aging Cell 2015, 14, 153–161. [Google Scholar] [CrossRef]
- Amoaku, W.M.K.; Archer, D.B. Cephalic radiation and retinal vasculopathy. Eye 1990, 4, 195–203. [Google Scholar] [CrossRef]
- Sun, X.-S.; Liang, Y.-J.; Jia, G.-D.; Liu, S.-L.; Liu, L.-T.; Guo, S.-S.; Sun, R.; Luo, D.-H.; Chen, Q.-Y.; Tang, L.-Q.; et al. Establishment of a prognostic nomogram to identify optimal candidates for local treatment among patients with local recurrent nasopharyngeal carcinoma. Oral Oncol. 2020, 106, 104711. [Google Scholar] [CrossRef]
- Li, Y.Q.; Tian, Y.M.; Tan, S.H.; Liu, M.Z.; Kusumawidjaja, G.; Ong, E.H.; Zhao, C.; Tan, T.W.; Fong, K.W.; Sommat, K.; et al. Prognostic Model for Stratification of Radioresistant Nasopharynx Carcinoma to Curative Salvage Radiotherapy. J. Clin. Oncol. 2018, 36, 891–899. [Google Scholar] [CrossRef]
- Maruyama, Y.; van Nagell, J.R.; Utley, J.; Vider, M.L.; Parker, J.C. Radiation and small bowel complications in cervical carcinoma therapy. Radiology 1974, 112, 699–703. [Google Scholar] [CrossRef]
- Mukesh, M.; Harris, E.; Jena, R.; Evans, P.; Coles, C. Relationship between irradiated breast volume and late normal tissue complications: A systematic review. Radiother. Oncol. 2012, 104, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chak, L.Y.; Gill, P.S.; Levine, A.M.; Meyer, P.R.; Anselmo, J.A.; Petrovich, Z. Radiation therapy for acquired immunodeficiency syndrome-related Kaposi’s sarcoma. J. Clin. Oncol. 1988, 6, 863–867. [Google Scholar] [CrossRef]
- Gold, D.G.; Miller, R.C.; Pinn, M.E.; Osborn, T.G.; Petersen, I.A.; Brown, P.D. Chronic toxicity risk after radiotherapy for patients with systemic sclerosis (systemic scleroderma) or systemic lupus erythematosus: Association with connective tissue disorder severity. Radiother. Oncol. 2008, 87, 127–131. [Google Scholar] [CrossRef]
- Giaj-Levra, N.; Sciascia, S.; Fiorentino, A.; Fersino, S.; Mazzola, R.; Ricchetti, F.; Roccatello, D.; Alongi, F. Radiotherapy in patients with connective tissue diseases. Lancet Oncol. 2016, 17, e109–e117. [Google Scholar] [CrossRef]
- Riva, G.; Vischioni, B.; Gandini, S.; Cavalieri, S.; Ronchi, S.; Barcellini, A.; Bonora, M.; Chalaszczyk, A.; Ingargiola, R.; Vitolo, V.; et al. Particle Beam Therapy Tolerance and Outcome on Patients with Autoimmune Diseases: A Single Institution Matched Case–Control Study. Cancers 2021, 13, 5183. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Piccirillo, J.F.; Tierney, R.M.; Costas, I.; Grove, L.; Spitznagel, E.L., Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. J. Am. Med. Assoc. 2004, 291, 2441–2447. [Google Scholar] [CrossRef] [Green Version]
- Takeda, Y.; Dynan, W.S. Autoantibodies against DNA double-strand break repair proteins. Front. Biosci. 2001, 6, D1412–D1422. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Lehrer, E.J.; Rosenberg, J.; Trifiletti, D.M.; Zaorsky, N.G. Toxicity after radiotherapy in patients with historically accepted contraindications to treatment (CONTRAD): An international systematic review and meta-analysis. Radiother. Oncol. 2019, 135, 147–152. [Google Scholar] [CrossRef]
- Peppone, L.J.; Mustian, K.M.; Morrow, G.R.; Dozier, A.M.; Ossip, D.J.; Janelsins, M.C.; Sprod, L.K.; McIntosh, S. The Effect of Cigarette Smoking on Cancer Treatment–Related Side Effects. Oncologist 2011, 16, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Andreassen, C.N.; Alsner, J. Genetic variants and normal tissue toxicity after radiotherapy: A systematic review. Radiother. Oncol. 2009, 92, 299–309. [Google Scholar] [CrossRef]
- Taylor, A.M.R.; Harnden, D.G.; Arlett, C.F.; Harcourt, S.A.; Lehmann, A.R.; Stevens, S.; Bridges, B.A. Ataxia telangiectasia: A human mutation with abnormal radiation sensitivity. Nature 1975, 258, 427–429. [Google Scholar] [CrossRef]
- Joubert, A.; Zimmerman, K.M.; Bencokova, Z.; Gastaldo, J.; Chavaudra, N.; Favaudon, V.; Arlett, C.F.; Foray, N. DNA double-strand break repair defects in syndromes associated with acute radiation response: At least two different assays to predict intrinsic radiosensitivity? Int. J. Radiat. Biol. 2008, 84, 107–125. [Google Scholar] [CrossRef]
- Varela, I.; Pereira, S.; Ugalde, A.P.; Navarro, C.L.; Suárez, M.F.; Cau, P.; Cadiñanos, J.; Osorio, F.G.; Foray, N.; Cobo, J.; et al. Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat. Med. 2008, 14, 767–772. [Google Scholar] [CrossRef]
- Ferlazzo, M.L.; Sonzogni, L.; Granzotto, A.; Bodgi, L.; Lartin, O.; Devic, C.; Vogin, G.; Pereira, S.; Foray, N. Mutations of the Huntington’s Disease Protein Impact on the ATM-Dependent Signaling and Repair Pathways of the Radiation-Induced DNA Double-Strand Breaks: Corrective Effect of Statins and Bisphosphonates. Mol. Neurobiol. 2014, 49, 1200–1211. [Google Scholar] [CrossRef]
- Ferlazzo, M.L.; Bach-Tobdji, M.K.E.; Djerad, A.; Sonzogni, L.; Devic, C.; Granzotto, A.; Bodgi, L.; Bachelet, J.-T.; Djefal-Kerrar, A.; Hennequin, C.; et al. Radiobiological Characterization of Tuberous Sclerosis: A Delay in the Nucleo-Shuttling of ATM May Be Responsible for Radiosensitivity. Mol. Neurobiol. 2018, 55, 4973–4983. [Google Scholar] [CrossRef]
- Devic, C.; Ferlazzo, M.L.; Berthel, E.; Foray, N. Influence of Individual Radiosensitivity on the Hormesis Phenomenon: Toward a Mechanistic Explanation Based on the Nucleoshuttling of ATM Protein. Dose-Response 2020, 18, 155932582091378. [Google Scholar] [CrossRef]
- Berthel, E.; Ferlazzo, M.L.; Devic, C.; Bourguignon, M.; Foray, N. What Does the History of Research on the Repair of DNA Double-Strand Breaks Tell Us?—A Comprehensive Review of Human Radiosensitivity. Int. J. Mol. Sci. 2019, 20, 5339. [Google Scholar] [CrossRef] [Green Version]
- Averbeck, D.; Candéias, S.; Chandna, S.; Foray, N.; Friedl, A.A.; Haghdoost, S.; Jeggo, P.A.; Lumniczky, K.; Paris, F.; Quintens, R.; et al. Establishing mechanisms affecting the individual response to ionizing radiation. Int. J. Radiat. Biol. 2020, 96, 297–323. [Google Scholar] [CrossRef] [Green Version]
- Khanna, K.K. Cancer Risk and the ATM Gene: A Continuing Debate. JNCI J. Natl. Cancer Inst. 2000, 92, 795–802. [Google Scholar] [CrossRef]
- Maréchal, A.; Zou, L. DNA Damage Sensing by the ATM and ATR Kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Ho, A.Y.; Fan, G.; Atencio, D.P.; Green, S.; Formenti, S.C.; Haffty, B.G.; Iyengar, P.; Bernstein, J.L.; Stock, R.G.; Cesaretti, J.A.; et al. Possession of ATM Sequence Variants as Predictor for Late Normal Tissue Responses in Breast Cancer Patients Treated with Radiotherapy. Int. J. Radiat. Oncol. 2007, 69, 677–684. [Google Scholar] [CrossRef]
- Andreassen, C.N.; Rosenstein, B.S.; Kerns, S.L.; Ostrer, H.; De Ruysscher, D.; Cesaretti, J.A.; Barnett, G.C.; Dunning, A.M.; Dorling, L.; West, C.M.L.; et al. Individual patient data meta-analysis shows a significant association between the ATM rs1801516 SNP and toxicity after radiotherapy in 5456 breast and prostate cancer patients. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2016, 121, 431–439. [Google Scholar] [CrossRef]
- Kerns, S.L.; Ostrer, H.; Stock, R.; Li, W.; Moore, J.; Pearlman, A.; Campbell, C.; Shao, Y.; Stone, N.; Kusnetz, L.; et al. Genome-Wide Association Study to Identify Single Nucleotide Polymorphisms (SNPs) Associated with the Development of Erectile Dysfunction in African-American Men After Radiotherapy for Prostate Cancer. Int. J. Radiat. Oncol. 2010, 78, 1292–1300. [Google Scholar] [CrossRef]
- Angèle, S.; Romestaing, P.; Moullan, N.; Vuillaume, M.; Chapot, B.; Friesen, M.; Jongmans, W.; Cox, D.G.; Pisani, P.; Gérard, J.-P.; et al. ATM haplotypes and cellular response to DNA damage: Association with breast cancer risk and clinical radiosensitivity. Cancer Res. 2003, 63, 8717–8725. [Google Scholar]
- Andreassen, C.N.; Alsner, J.; Overgaard, M.; Overgaard, J. Prediction of normal tissue radiosensitivity from polymorphisms in candidate genes. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2003, 69, 127–135. [Google Scholar] [CrossRef]
- Zschenker, O.; Raabe, A.; Boeckelmann, I.K.; Borstelmann, S.; Szymczak, S.; Wellek, S.; Rades, D.; Hoeller, U.; Ziegler, A.; Dikomey, E.; et al. Association of single nucleotide polymorphisms in ATM, GSTP1, SOD2, TGFB1, XPD and XRCC1 with clinical and cellular radiosensitivity. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2010, 97, 26–32. [Google Scholar] [CrossRef]
- Terrazzino, S.; Cargnin, S.; DeAntonio, L.; Pisani, C.; Masini, L.; Canonico, P.L.; Genazzani, A.A.; Krengli, M. Impact of ATM rs1801516 on late skin reactions of radiotherapy for breast cancer: Evidences from a cohort study and a trial sequential meta-analysis. PLoS ONE 2019, 14, e0225685. [Google Scholar] [CrossRef]
- Quarmby, S.; West, C.; Magee, B.; Stewart, A.; Hunter, R.; Kumar, S. Differential Expression of Cytokine Genes in Fibroblasts Derived from Skin Biopsies of Patients who Developed Minimal or Severe Normal Tissue Damage after Radiotherapy. Radiat. Res. 2002, 157, 243–248. [Google Scholar] [CrossRef]
- Moullan, N.; Cox, D.G.; Angèle, S.; Romestaing, P.; Gérard, J.-P.; Hall, J. Polymorphisms in the DNA repair gene XRCC1, breast cancer risk, and response to radiotherapy. Cancer Epidemiol. Biomark. Prev. 2003, 12, 1168–1174. [Google Scholar]
- Andreassen, C.N. Can risk of radiotherapy-induced normal tissue complications be predicted from genetic profiles? Acta Oncol. 2005, 44, 801–815. [Google Scholar] [CrossRef]
- Alsner, J.; Rødningen, O.K.; Overgaard, J. Differential gene expression before and after ionizing radiation of subcutaneous fibroblasts identifies breast cancer patients resistant to radiation-induced fibrosis. Radiother. Oncol. 2007, 83, 261–266. [Google Scholar] [CrossRef]
- Rødningen, O.K.; Børresen-Dale, A.-L.; Alsner, J.; Hastie, T.; Overgaard, J. Radiation-induced gene expression in human subcutaneous fibroblasts is predictive of radiation-induced fibrosis. Radiother. Oncol. 2008, 86, 314–320. [Google Scholar] [CrossRef]
- Forrester, H.B.; Li, J.; Leong, T.; McKay, M.J.; Sprung, C.N. Identification of a radiation sensitivity gene expression profile in primary fibroblasts derived from patients who developed radiotherapy-induced fibrosis. Radiother. Oncol. 2014, 111, 186–193. [Google Scholar] [CrossRef]
- Sprung, C.; McKAY, M. Method of Detecting Radiation Exposure and Adverse Toxicity Thereto. WO2011006214A1, 20 January 2011. [Google Scholar]
- Henderson, N.C.; Sheppard, D. Integrin-mediated regulation of TGFβ in fibrosis. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis 2013, 1832, 891–896. [Google Scholar] [CrossRef]
- Portela, A.; Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef]
- Babalola, O.; Mamalis, A.; Lev-Tov, H.; Jagdeo, J. The role of microRNAs in skin fibrosis. Arch. Dermatol. Res. 2013, 305, 763–776. [Google Scholar] [CrossRef]
- Chung, Y.L.; Wang, A.-J.; Yao, L.-F. Antitumor histone deacetylase inhibitors suppress cutaneous radiation syndrome: Implications for increasing therapeutic gain in cancer radiotherapy. Mol. Cancer Ther. 2004, 3, 317–325. [Google Scholar] [CrossRef]
- Weigel, C.; Veldwijk, M.R.; Oakes, C.C.; Seibold, P.; Slynko, A.; Liesenfeld, D.B.; Rabionet, M.; Hanke, S.A.; Wenz, F.; Sperk, E.; et al. Epigenetic regulation of diacylglycerol kinase alpha promotes radiation-induced fibrosis. Nat. Commun. 2016, 7, 10893. [Google Scholar] [CrossRef] [Green Version]
- Chua, M.L.; Rothkamm, K. Biomarkers of radiation exposure: Can they predict normal tissue radiosensitivity? Clin. Oncol. (R Coll. Radiol.) 2013, 25, 610–616. [Google Scholar] [CrossRef]
- Ferlazzo, M.L.; Bourguignon, M.; Foray, N. Functional Assays for Individual Radiosensitivity: A Critical Review. Semin. Radiat. Oncol. 2017, 27, 310–315. [Google Scholar] [CrossRef]
- Ozsahin, M.; Crompton, N.E.; Gourgou, S.; Kramar, A.; Li, L.; Shi, Y.; Sozzi, W.J.; Zouhair, A.; Mirimanoff, R.O.; Azria, D. CD4 and CD8 T-Lymphocyte Apoptosis Can Predict Radiation-Induced Late Toxicity: A Prospective Study in 399 Patients. Clin. Cancer Res. 2005, 11, 7426–7433. [Google Scholar] [CrossRef] [Green Version]
- Granzotto, A.; Benadjaoud, M.A.; Vogin, G.; Devic, C.; Ferlazzo, M.L.; Bodgi, L.; Pereira, S.; Sonzogni, L.; Forcheron, F.; Viau, M.; et al. Influence of Nucleoshuttling of the ATM Protein in the Healthy Tissues Response to Radiation Therapy: Toward a Molecular Classification of Human Radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 450–460. [Google Scholar] [CrossRef]
- Grossberg, A.J.; Lei, X.; Xu, T.; Shaitelman, S.F.; Hoffman, K.E.; Bloom, E.S.; Stauder, M.C.; Tereffe, W.; Schlembach, P.J.; Woodward, W.A.; et al. Association of Transforming Growth Factor beta Polymorphism C-509T With Radiation-Induced Fibrosis Among Patients With Early-Stage Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1751–1757. [Google Scholar] [CrossRef] [Green Version]
- Bentzen, S.M.; Parliament, M.; Deasy, J.O.; Dicker, A.; Curran, W.J.; Williams, J.P.; Rosenstein, B.S. Biomarkers and Surrogate Endpoints for Normal-Tissue Effects of Radiation Therapy: The Importance of Dose–Volume Effects. Int. J. Radiat. Oncol. 2010, 76, S145–S150. [Google Scholar] [CrossRef]
- Desideri, I.; Loi, M.; Francolini, G.; Becherini, C.; Livi, L.; Bonomo, P. Application of Radiomics for the Prediction of Radiation-Induced Toxicity in the IMRT Era: Current State-of-the-Art. Front. Oncol. 2020, 10, 1708. [Google Scholar] [CrossRef]
- Scalco, E.; Fiorino, C.; Cattaneo, G.M.; Sanguineti, G.; Rizzo, G. Texture analysis for the assessment of structural changes in parotid glands induced by radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2013, 109, 384–387. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Sharifi, H.; Defraene, G.; Kerns, S.L.; Christiaens, M.; De Ruyck, K.; Peeters, S.; Vansteenkiste, J.; Jeraj, R.; Heuvel, F.V.D.; et al. Quantification of radiation-induced lung damage with CT scans: The possible benefit for radiogenomics. Acta Oncol. 2013, 52, 1405–1410. [Google Scholar] [CrossRef] [Green Version]
- Geady, C.; Keller, H.; Siddiqui, I.; Bilkey, J.; Dhani, N.; Jaffray, D. Bridging the gap between micro- and macro-scales in medical imaging with textural analysis—A biological basis for CT radiomics classifiers? Phys. Med. 2020, 72, 142–151. [Google Scholar] [CrossRef]
- Seibold, P.; Hall, P.; Schoof, N.; Nevanlinna, H.; Heikkinen, T.; Benner, A.; Liu, J.; Schmezer, P.; Popanda, O.; Flesch-Janys, D.; et al. Polymorphisms in oxidative stress-related genes and mortality in breast cancer patients—Potential differential effects by radiotherapy? Breast 2013, 22, 817–823. [Google Scholar] [CrossRef]
- Rattay, T.; Seibold, P.; Aguado-Barrera, M.E.; Altabas, M.; Azria, D.; Barnett, G.C.; Bultijnck, R.; Chang-Claude, J.; Choudhury, A.; Coles, C.E.; et al. External Validation of a Predictive Model for Acute Skin Radiation Toxicity in the REQUITE Breast Cohort. Front. Oncol. 2020, 10, 575909. [Google Scholar] [CrossRef]
- Lopez Guerra, J.L.; Gomez, D.; Zhuang, Y.; Hong, D.S.; Heymach, J.V.; Swisher, S.G.; Lin, S.H.; Komaki, R.; Cox, J.D.; Liao, Z. Prognostic impact of radiation therapy to the primary tumor in patients with non-small cell lung cancer and oligometastasis at diagnosis. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e61–e67. [Google Scholar] [CrossRef] [Green Version]
- Pang, Q.; Wei, Q.; Xu, T.; Yuan, X.; Lopez Guerra, J.L.; Levy, L.B.; Liu, Z.; Gomez, D.R.; Zhuang, Y.; Wang, L.-E.; et al. Functional Promoter Variant rs2868371 of HSPB1 Is Associated with Risk of Radiation Pneumonitis After Chemoradiation for Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1332–1339. [Google Scholar] [CrossRef]
- Kerns, S.L.; de Ruysscher, D.; Andreassen, C.N.; Azria, D.; Barnett, G.C.; Chang-Claude, J.; Davidson, S.; Deasy, J.O.; Dunning, A.M.; Ostrer, H.; et al. STROGAR—STrengthening the Reporting of Genetic Association studies in Radiogenomics. Radiother. Oncol. 2014, 110, 182–188. [Google Scholar] [CrossRef]
- Barnett, G.C.; Thompson, D.; Fachal, L.; Kerns, S.; Talbot, C.; Elliott, R.M.; Dorling, L.; Coles, C.E.; Dearnaley, D.P.; Rosenstein, B.S.; et al. A genome wide association study (GWAS) providing evidence of an association between common genetic variants and late radiotherapy toxicity. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 111, 178–185. [Google Scholar] [CrossRef]
- Bourgier, C.; Fenoglietto, P.; Lemanski, C.; Ducteil, A.; Charissoux, M.; Draghici, R.; Azria, D. Techniques d’irradiation du cancer du sein en 2016: Intérêt et indications de la radiothérapie conformationnelle avec modulation d’intensité [Modalities of breast cancer irradiation in 2016: Aims and indications of intensity modulated radiation therapy]. Cancer Radiother. 2016, 20, 572–575. [Google Scholar] [CrossRef]
- Vogin, G.; Bastogne, T.; Bodgi, L.; Gillet-Daubin, J.; Canet, A.; Pereira, S.; Foray, N. The Phosphorylated ATM Immunofluorescence Assay: A High-performance Radiosensitivity Assay to Predict Postradiation Therapy Overreactions. Int. J. Radiat. Oncol. 2018, 101, 690–693. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.; Bodgi, L.; Duclos, M.; Canet, A.; Ferlazzo, M.L.; Devic, C.; Granzotto, A.; Deneuve, S.; Vogin, G.; Foray, N. Fast and Binary Assay for Predicting Radiosensitivity Based on the Theory of ATM Nucleo-Shuttling: Development, Validation, and Performance. Int. J. Radiat. Oncol. 2018, 100, 353–360. [Google Scholar] [CrossRef]
- Deneuve, S.; Mirjolet, C.; Bastogne, T.; Duclos, M.; Retif, P.; Zrounba, P.; Roux, P.-E.; Poupart, M.; Vogin, G.; Foray, N.; et al. Proof of Concept of a Binary Blood Assay for Predicting Radiosensitivity. Cancers 2021, 13, 2477. [Google Scholar] [CrossRef]
- Cain, K.; Brown, D.G.; Langlais, C.; Cohen, G.M. Caspase activation involves the formation of the aposome, a large (approximately 700 kDa) caspase-activating complex. J. Biol. Chem. 1999, 274, 22686–22692. [Google Scholar] [CrossRef] [Green Version]
- Dogu, Y.; Díaz, J. Mathematical model of a network of interaction between p53 and Bcl-2 during genotoxic-induced apoptosis. Biophys. Chem. 2009, 143, 44–54. [Google Scholar] [CrossRef]
- Kuribayashi, K.; Finnberg, N.K.; Jeffers, J.R.; Zambetti, G.P.; El-Deiry, W.S. The relative contribution of pro-apoptotic p53-target genes in the triggering of apoptosis following DNA damage in vitro and in vivo. Cell Cycle 2011, 10, 2380–2389. [Google Scholar] [CrossRef] [Green Version]
- Scaffidi, C.; Fulda, S.; Srinivasan, A.; Friesen, C.; Li, F.; Tomaselli, K.J.; Debatin, K.M.; Krammer, P.H.; Peter, M.E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998, 17, 1675–1687. [Google Scholar] [CrossRef] [Green Version]
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016, 17, 102. [Google Scholar] [CrossRef] [Green Version]
- Biechonski, S.; Olender, L.; Zipin-Roitman, A.; Yassin, M.; Aqaqe, N.; Marcu-Malina, V.; Rall-Scharpf, M.; Trottier, M.; Meyn, M.S.; Wiesmüller, L.; et al. Attenuated DNA damage responses and increased apoptosis characterize human hematopoietic stem cells exposed to irradiation. Sci. Rep. 2018, 8, 6071. [Google Scholar] [CrossRef]
- Azria, D.; Belkacemi, Y.; Lagrange, J.-L.; Chapet, O.; Mornex, F.; Maingon, P.; Hennequin, C.; Rosenstein, B.; Ozsahin, M. Séquelles radio-induites et tests prédictifs. Cancer/Radiothérapie 2008, 12, 619–624. [Google Scholar] [CrossRef]
- Mirjolet, C.; Merlin, J.; Dalban, C.; Maingon, P.; Azria, D. Correlation between radio-induced lymphocyte apoptosis measurements obtained from two French centres. Cancer Radiother. 2016, 20, 391–394. [Google Scholar] [CrossRef]
- Azria, D.; Riou, O.; Castan, F.; Nguyen, T.D.; Peignaux, K.; Lemanski, C.; Lagrange, J.-L.; Kirova, Y.; Lartigau, E.; Belkacemi, Y.; et al. Radiation-induced CD8 T-lymphocyte Apoptosis as a Predictor of Breast Fibrosis After Radiotherapy: Results of the Prospective Multicenter French Trial. eBioMedicine 2015, 2, 1965–1973. [Google Scholar] [CrossRef] [Green Version]
- Fhoghlú, M.N.; Barrett, S. A Review of Radiation-Induced Lymphocyte Apoptosis as a Predictor of Late Toxicity after Breast Radiotherapy. J. Med. Imaging Radiat. Sci. 2019, 50, 337–344. [Google Scholar] [CrossRef]
- Veldwijk, M.R.; Seibold, P.; Botma, A.; Helmbold, I.; Sperk, E.; Giordano, F.A.; Gürth, N.; Kirchner, A.-K.; Behrens, S.; Wenz, F.; et al. Association of CD4+ Radiation-Induced Lymphocyte Apoptosis with Fibrosis and Telangiectasia after Radiotherapy in 272 Breast Cancer Patients with >10-Year Follow-up. Clin. Cancer Res. 2019, 25, 562–572. [Google Scholar] [CrossRef] [Green Version]
- Mirjolet, C.; Merlin, J.; Truc, G.; Noël, G.; Thariat, J.; Domont, J.; Sargos, P.; Renard-Oldrini, S.; Ray-Coquard, I.; Liem, X.; et al. RILA blood biomarker as a predictor of radiation-induced sarcoma in a matched cohort study. eBioMedicine 2019, 41, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Talbot, C.J.; Veldwijk, M.R.; Azria, D.; Batini, C.; Bierbaum, M.; Brengues, M.; Chang-Claude, J.; Johnson, K.; Keller, A.; Smith, S.; et al. Multi-centre technical evaluation of the radiation-induced lymphocyte apoptosis assay as a predictive test for radiotherapy toxicity. Clin. Transl. Radiat. Oncol. 2019, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Muggiolu, G.; Libert, S.; Treillard, B.; Alfonse, G.; Philouze, P.; Rodriguez-Lafrasse, C.; Lauret, A.; Ceruse, P.; Righini, C.A.; Sauvaigo, S. PO-1929, ESTRO2021. Available online: https://www.estro.org/ESTRO/media/ESTRO/Congresses/ESTRO-2021/Scientific-Programme-ESTRO-2021_verion-1.pdf (accessed on 16 November 2022).
- Barnett, G.C.; Kerns, S.L.; Dorling, L.; Fachal, L.; Aguado-Barrera, M.E.; Martínez-Calvo, L.; Jandu, H.K.; Welsh, C.; Tyrer, J.; Coles, C.E.; et al. No association between polygenic risk scores for cancer and development of radiotherapy toxicity. Int. J. Radiat. Oncol. 2022, 114, 494–501. [Google Scholar] [CrossRef]
- Bergom, C.; West, C.M.; Higginson, D.S.; Abazeed, M.E.; Arun, B.; Bentzen, S.M.; Bernstein, J.L.; Evans, J.D.; Gerber, N.K.; Kerns, S.L.; et al. The Implications of Genetic Testing on Radiation Therapy Decisions: A Guide for Radiation Oncologists. Int. J. Radiat. Oncol. 2019, 105, 698–712. [Google Scholar] [CrossRef] [Green Version]
- Evans, E.S.; Hahn, C.A.; Kocak, Z.; Zhou, S.-M.; Marks, L.B. The Role of Functional Imaging in the Diagnosis and Management of Late Normal Tissue Injury. Semin. Radiat. Oncol. 2007, 17, 72–80. [Google Scholar] [CrossRef]
- Fried, D.V.; Das, S.K.; Marks, L.B. Imaging Radiation-Induced Normal Tissue Injury to Quantify Regional Dose Response. Semin. Radiat. Oncol. 2017, 27, 325–331. [Google Scholar] [CrossRef]
- Anscher, M.S.; Kong, F.M.; Andrews, K.; Clough, R.; Marks, L.B.; Bentel, G.; Jirtle, R.L. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 1029–1035. [Google Scholar] [CrossRef]
- Rades, D.; Narvaez, C.A.; Doemer, C.; Janssen, S.; Olbrich, D.; Tvilsted, S.; Conde-Moreno, A.J.; Cacicedo, J. Radiotherapy-related skin toxicity (RAREST-02): A randomized trial testing the effect of a mobile application reminding head-and-neck cancer patients to perform skin care (reminder app) on radiation dermatitis. Trials 2020, 21, 424. [Google Scholar] [CrossRef]
- Zini, E.M.; Lanzola, G.; Quaglini, S.; Bossi, P.; Licitra, L.; Resteghini, C. A pilot study of a smartphone-based monitoring intervention on head and neck cancer patients undergoing concurrent chemo-radiotherapy. Int. J. Med. Inform. 2019, 129, 404–412. [Google Scholar] [CrossRef]
- Evans, D.G.; Woodward, E.R.; Bajalica-Lagercrantz, S.; Oliveira, C.; Frebourg, T. Germline TP53 Testing in Breast Cancers: Why, When and How? Cancers 2020, 12, 3762. [Google Scholar] [CrossRef]
- Schon, K.; Tischkowitz, M. Clinical implications of germline mutations in breast cancer: TP53. Breast Cancer Res. Treat. 2018, 167, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Kry, S.F.; Bednarz, B.; Howell, R.M.; Dauer, L.; Followill, D.; Klein, E.; Paganetti, H.; Wang, B.; Wuu, C.-S.; Xu, X.G. AAPM TG 158: Measurement and calculation of doses outside the treated volume from external-beam radiation therapy. Med. Phys. 2017, 44, e391–e429. [Google Scholar] [CrossRef]
- Cowan, R.A.; McBain, C.A.; Ryder, W.J.; Wylie, J.P.; Logue, J.P.; Turner, S.L.; Van Der Voet, J.; Collins, C.D.; Khoo, V.S.; Read, G.R. Radiotherapy for muscle-invasive carcinoma of the bladder: Results of a randomized trial comparing conventional whole bladder with dose-escalated partial bladder radiotherapy. Int. J. Radiat. Oncol. 2004, 59, 197–207. [Google Scholar] [CrossRef]
- Thariat, J.; Hannoun-Levi, J.-M.; Myint, A.S.; Vuong, T.; Gérard, J.-P. Past, present, and future of radiotherapy for the benefit of patients. Nat. Rev. Clin. Oncol. 2013, 10, 52–60. [Google Scholar] [CrossRef]
- Tinganelli, W.; Durante, M. Carbon Ion Radiobiology. Cancers 2020, 12, 3022. [Google Scholar] [CrossRef]
- Durante, M.; Formenti, S. Harnessing radiation to improve immunotherapy: Better with particles? Br. J. Radiol. 2020, 93, 20190224. [Google Scholar] [CrossRef]
- Durante, M.; Formenti, S.C. Radiation-Induced Chromosomal Aberrations and Immunotherapy: Micronuclei, Cytosolic DNA, and Interferon-Production Pathway. Front. Oncol. 2018, 8, 192. [Google Scholar] [CrossRef] [Green Version]
- Paganetti, H.; Moteabbed, M.; Athar, B.; Adams, J.; Schneider, U.; Yock, T. SU-E-T-258: Assessment of Radiation Induced Second Cancer Risks in Proton Therapy and IMRT for Organs inside the Main Radiation Field. Med. Phys. 2012, 39, 3762–3763. [Google Scholar] [CrossRef] [Green Version]
- Durante, M.; Loeffler, J.S. Charged particles in radiation oncology. Nat. Rev. Clin. Oncol. 2010, 7, 37–43. [Google Scholar] [CrossRef]
- Knopf, A.C.; Czerska, K.; Fracchiolla, F.; Graeff, C.; Molinelli, S.; Rinaldi, I.; Rucincki, A.; Sterpin, E.; Stützer, K.; Trnkova, P.; et al. Clinical necessity of multi-image based (4DMIB) optimization for targets affected by respiratory motion and treated with scanned particle therapy—A comprehensive review. Radiother. Oncol. 2022, 169, 77–85. [Google Scholar] [CrossRef]
- Rousselle, A.; Amelot, A.; Thariat, J.; Jacob, J.; Mercy, G.; de Marzi, L.; Feuvret, L. Metallic implants and CT artefacts in the CTV area: Where are we in 2020? Cancer Radiother. 2020, 24, 658–666. [Google Scholar] [CrossRef]
- Fu, K.K.; Pajak, T.F.; Trotti, A.; Jones, C.U.; Spencer, S.A.; Phillips, T.L.; Garden, A.S.; Ridge, J.A.; Cooper, J.S.; Ang, K. A radiation therapy oncology group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int. J. Radiat. Oncol. 2000, 48, 7–16. [Google Scholar] [CrossRef]
- Moore, J.W.; Woolley, T.E.; Hopewell, J.W.; Jones, B. Further development of spinal cord retreatment dose estimation: Including radiotherapy with protons and light ions. Int. J. Radiat. Biol. 2021, 97, 1657–1666. [Google Scholar] [CrossRef]
- Barnett, G.C.; West, C.; Dunning, A.M.; Elliott, R.M.; Coles, C.E.; Pharoah, P.D.P.; Burnet, N.G. Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat. Rev. Cancer 2009, 9, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Trigilio, A.; Franciosini, G.; Moeckli, R.; Zhang, R.; Böhlen, T.T. FLASH radiotherapy treatment planning and models for electron beams. Radiother. Oncol. 2022, 175, 210–221. [Google Scholar] [CrossRef]
- Kirkby, K.J.; Kirkby, N.F.; Burnet, N.G.; Owen, H.; Mackay, R.I.; Crellin, A.; Green, S. Heavy charged particle beam therapy and related new radiotherapy technologies: The clinical potential, physics and technical developments required to deliver benefit for patients with cancer. Br. J. Radiol. 2020, 93, 20200247. [Google Scholar] [CrossRef]
- Taylor, P.A.; Moran, J.M.; Jaffray, D.A.; Buchsbaum, J.C. A roadmap to clinical trials for FLASH. Med. Phys. 2022, 49, 4099–4108. [Google Scholar] [CrossRef]
- Mazal, A.; Prezado, Y.; Ares, C.; de Marzi, L.; Patriarca, A.; Miralbell, R.; Favaudon, V. FLASH and minibeams in radiation therapy: The effect of microstructures on time and space and their potential application to protontherapy. Br. J. Radiol. 2020, 93, 20190807. [Google Scholar] [CrossRef]
- Moulder, J.; Fish, B.; Cohen, E. ACE Inhibitors and AII Receptor Antagonists in the Treatment and Prevention of Bone Marrow Transplant Nephropathy. Curr. Pharm. Des. 2003, 9, 737–749. [Google Scholar] [CrossRef]
- Billena, C.; Khan, A.J. A Current Review of Spatial Fractionation: Back to the Future? Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 177–187. [Google Scholar] [CrossRef]
- Brady, R.; McSharry, L.; Lawson, S.; Regan, J. The impact of dysphagia prehabilitation on swallowing outcomes post-chemoradiation therapy in head and neck cancer: A systematic review. Eur. J. Cancer Care 2022, 31, e13549. [Google Scholar] [CrossRef]
- Barcellini, A.; Dominoni, M.; Mas, F.D.; Biancuzzi, H.; Venturini, S.C.; Gardella, B.; Orlandi, E.; Bø, K. Sexual Health Dysfunction After Radiotherapy for Gynecological Cancer: Role of Physical Rehabilitation Including Pelvic Floor Muscle Training. Front. Med. 2022, 8, 813352. [Google Scholar] [CrossRef]
- Sacomori, C.; Araya-Castro, P.; Diaz-Guerrero, P.; Ferrada, I.A.; Martínez-Varas, A.C.; Zomkowski, K. Pre-rehabilitation of the pelvic floor before radiation therapy for cervical cancer: A pilot study. Int. Urogynecol. J. 2020, 31, 2411–2418. [Google Scholar] [CrossRef]
- Loewen, I.; Jeffery, C.C.; Rieger, J.; Constantinescu, G. Prehabilitation in head and neck cancer patients: A literature review. J. Otolaryngol.—Head Neck Surg. 2021, 50, 2. [Google Scholar] [CrossRef]
- Delanian, S.; Porcher, R.; Balla-Mekias, S.; Lefaix, J.-L. Randomized, Placebo-Controlled Trial of Combined Pentoxifylline and Tocopherol for Regression of Superficial Radiation-Induced Fibrosis. J. Clin. Oncol. 2003, 21, 2545–2550. [Google Scholar] [CrossRef]
- Le Rhun, E.; Dhermain, F.; Vogin, G.; Reyns, N.; Metellus, P. Radionecrosis after stereotactic radiotherapy for brain metastases. Expert Rev. Neurother. 2016, 16, 903–914. [Google Scholar] [CrossRef]
- Levin, V.A.; Bidaut, L.; Hou, P.; Kumar, A.J.; Wefel, J.S.; Bekele, B.N.; Prabhu, S.; Loghin, M.; Gilbert, M.R.; Jackson, E.F. Randomized Double-Blind Placebo-Controlled Trial of Bevacizumab Therapy for Radiation Necrosis of the Central Nervous System. Int. J. Radiat. Oncol. 2011, 79, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, S.; O’Connor, H.; O’Morain, C.; Buckley, M. Argon plasma coagulation as first-line treatment for chronic radiation proctopathy. J. Gastroenterol. Hepatol. 2004, 19, 1169–1173. [Google Scholar] [CrossRef]

| OAR | Main Late Toxicities |
|---|---|
| Radiosensitive OAR (Endpoint Occurring with 5-Year Occurrence Probability > 5% for Dose Usually < 20 Gy) | |
| Ovary | Infertility, premature ovarian failure, temporary or permanent castration |
| Testis | Temporary or permanent infertility |
| Lens | Cataract |
| Breast | Breast atrophy |
| Growth plates | Growth retardation or arrest |
| Kidney | Nephritis |
| Liver | Hepatitis, Radiation-Induced liver disease |
| Salivary glands | Temporary or permanent xerostomia |
| Bone marrow | ±Deep/prolonged aplasia or selective blood cell loss (lymphocytes) |
| Mildly sensitive OAR (endpoint occurring with 5-year occurrence probability >5% for dose usually 20 Gy-60 Gy) | |
| Lung | Lung fibrosis—respiratory failure |
| Larynx | Dysphonia |
| Heart | Constrictive pericarditis, coronary artery stenosis, myocardial fibrosis, valvular damage |
| Small bowel | Enteritis, occlusive syndrome, perforation, fistula, malabsorption |
| Stomach | Late gastritis, ulceration, antral stenosis |
| Spinal cord | Late radiation myelitis |
| Hair | Depilation |
| Rectum | Late proctitis, ulceration, perforation, fistula |
| Bladder | Cystitis, micro bladder, ulceration, perforation, fistula |
| Brain—nerves | Necrosis, leukoencephalopathy, dementia, neurocognitive disorders, plexitis, neuropathy |
| Retina | Retinopathy, maculopathy |
| Thyroid | Hypothyroidism |
| Inner ear | Sensorineural deafness |
| Middle ear | Conductive deafness, chronic otitis media, eustachian tube pathology |
| Esophagus | Late esophagitis, ulcerations, fistulas |
| Mucosae | Mucositis, ulcerations, perforation, necrosis |
| Skin | Dystrophy, sclero-atrophic dermatitis, ulcerations |
| Radioresistant OAR (endpoint occurring with 5-year occurrence probability > 5% for dose usually > 60 Gy) | |
| Uterus—vagina | Endo-cervical-vaginal canal stenosis, uterine corpus fibrosis—infertility, vaginal synechiae, ulcerations, dryness, atrophy, vulvodynia |
| Bone | Osteoporosis, fracture, osteonecrosis |
| Muscles | Fibrosis |
| Joints | Ankylosis |
| Main arteries | Arterial disease, moya-moya vasculopathy |
| Connective tissues | Fibrosis |
| Gene | Reference SNP | OR [CI95%] | Localization | Type of Mutation | References |
|---|---|---|---|---|---|
| ATM | rs1801516 | 1.23 [1, 1.51] 1.27 [1.02, 1.58] | Exonic | Missense (D > N) | [82,89] |
| ATM | IVS22–77 T>C | 0.45 [0.24, 0.85] | Intronic | - | [86] |
| ATM | IVS48 + 238 C>G | 0.50 [0.27, 0.94] | Intronic | - | [86] |
| DNMT1 | rs2228611 | 0.26 [0.10, 0.71] | Exonic | Synonymous | [89] |
| TGFB1 | rs1800469 | 3.40 [1.38, 8.40] | Upstream | - | [87,90] |
| TGFB1 | rs1800470 | 2.37 [0.99, 5.60] | Exonic | Missense (P > L) | [87,90] |
| XRCC1 | rs1799782 rs25487 | 4.33 [1.24, 15.12] | Exonic | Missense (R > W + Q > R) | [91] |
| XRCC3 | rs861539 | 1.17 [1.09, 1.26] | Exonic | Missense (T > M) | [87] |
| Selected Differentially Expressed Genes | Assay Used for Gene Selection | References |
|---|---|---|
| FMLP-R-I, TNFα, NGFR, EPHB2, PDGFB, NTRK1, LFNG, DDR1; IFNGR1 | Cytokine array | [90] |
| CDC6, CDON, CXCL12, FAP, FBLN2, LMNB2, LUM, MT1X, MXRA5, SLC1A3, SOD2, SOD3, WISP2 | 15KcDNA microarray | [93] |
| PLAGL1, CCND2, CDC6, DEGS1, CDON, CXCL12, MXRA5, LUM, MT1X, MT1F, MT1H, C1S, NF1, ARID5B, SCL1A3, TM4SF10, MGC33894, ZDHHC5/MFGE8 | 15K cDNA microarray | [94] |
| FBN2, FST, GPRC5B, NOTCH3, PLCB1, DPT, DDIT4L, SGCG | GeneChip Human Exon 1.0 ST Array | [95] |
| Type of Study | Tissue | External Validation (Yes/No) | Predictive Model (Yes/No) | Performance | References |
|---|---|---|---|---|---|
| SNP | Breast | Yes | Yes | OR = 4.47 | [113] |
| multivariate odds ratio | |||||
| yes | No | [OR] = 0.77, p = 0.02 | [82] | ||
| No | Yes | OR = 1.78 | [106] | ||
| Yes | Yes | AUC = 0.65 | [114] | ||
| Lung | Yes | No | p = 0.031 | [115] | |
| Yes | No | p = 0.02 to 0.023 | [82,85] | ||
| Prostate | No | No | OR = 1.3 | [43,44,114] | |
| No | Yes | AUC from 0.76 to 0.8 | |||
| Yes | Yes | AUC from 0.63 to 0.78 | |||
| GWAS | Prostate | No | Yes | OR from 6.42 to 33.95 | [116] |
| Yes | Yes | OR from 2.71 to 3.12 | [117] | ||
| Breast | Yes | No | RR from 1.56 to 3.28 | [118] | |
| No | No | OR from 4.19 to 7.52 | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.; Orlandi, E.; Deneuve, S.; Barcellini, A.; Chalaszczyk, A.; Behm-Ansmant, I.; Hettal, L.; Rancati, T.; Vogin, G.; Thariat, J. The Normal, the Radiosensitive, and the Ataxic in the Era of Precision Radiotherapy: A Narrative Review. Cancers 2022, 14, 6252. https://doi.org/10.3390/cancers14246252
Pereira S, Orlandi E, Deneuve S, Barcellini A, Chalaszczyk A, Behm-Ansmant I, Hettal L, Rancati T, Vogin G, Thariat J. The Normal, the Radiosensitive, and the Ataxic in the Era of Precision Radiotherapy: A Narrative Review. Cancers. 2022; 14(24):6252. https://doi.org/10.3390/cancers14246252
Chicago/Turabian StylePereira, Sandrine, Ester Orlandi, Sophie Deneuve, Amelia Barcellini, Agnieszka Chalaszczyk, Isabelle Behm-Ansmant, Liza Hettal, Tiziana Rancati, Guillaume Vogin, and Juliette Thariat. 2022. "The Normal, the Radiosensitive, and the Ataxic in the Era of Precision Radiotherapy: A Narrative Review" Cancers 14, no. 24: 6252. https://doi.org/10.3390/cancers14246252
APA StylePereira, S., Orlandi, E., Deneuve, S., Barcellini, A., Chalaszczyk, A., Behm-Ansmant, I., Hettal, L., Rancati, T., Vogin, G., & Thariat, J. (2022). The Normal, the Radiosensitive, and the Ataxic in the Era of Precision Radiotherapy: A Narrative Review. Cancers, 14(24), 6252. https://doi.org/10.3390/cancers14246252








