Morphofunctional and Molecular Assessment of Nutritional Status in Head and Neck Cancer Patients Undergoing Systemic Treatment: Role of Inflammasome in Clinical Nutrition
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blood Sampling and Processing to Isolate PBMCs
2.3. RNA Extraction, Quantification and Reverse Transcription
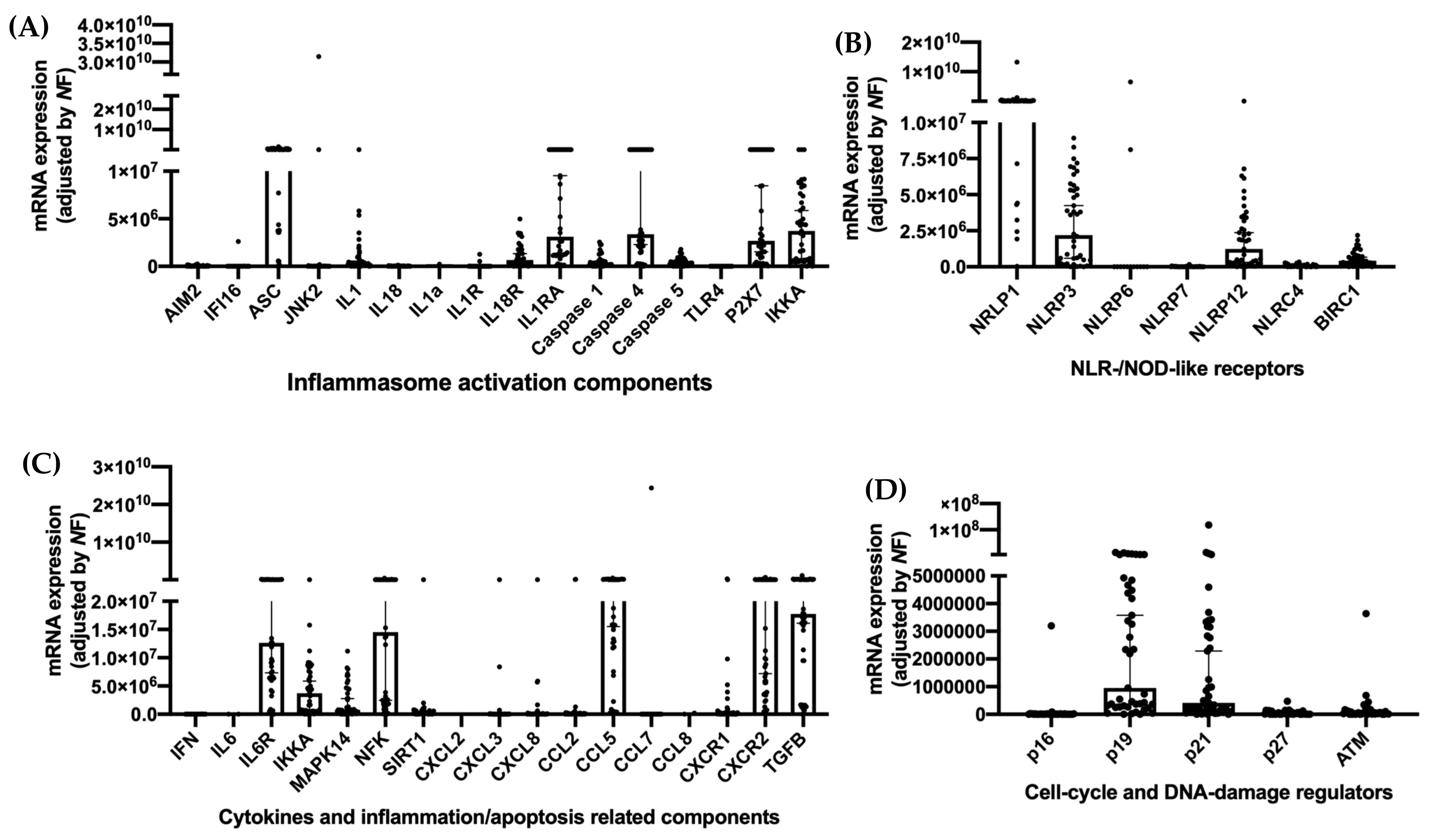
2.4. Analysis of Components of the Inflammasome Machinery by qPCR Dynamic Array Based on Microfluidic Technology
2.5. Statistical Analysis
3. Results
3.1. Patient Population and Clinical Evolution
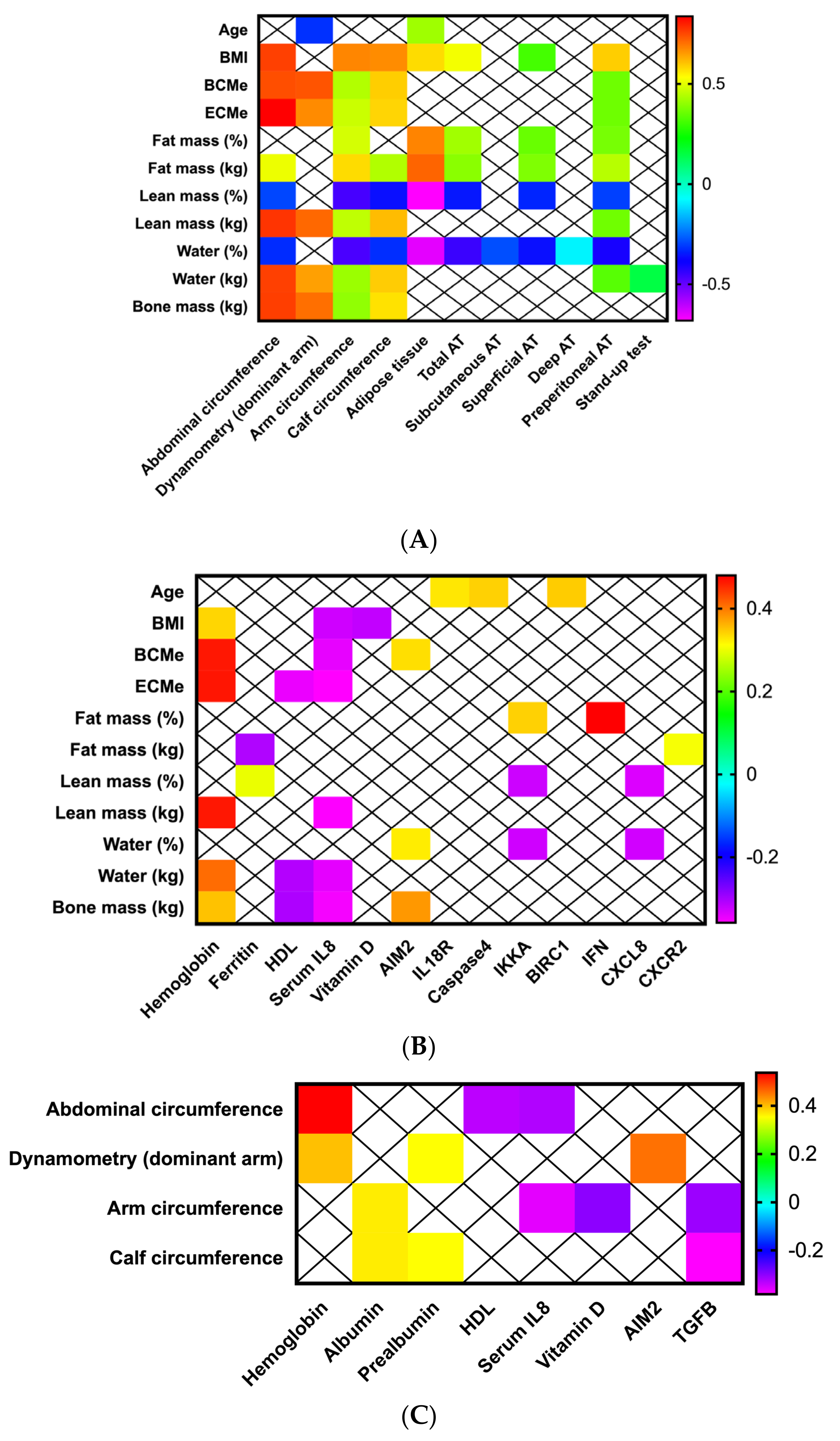
3.2. Anthropometric-Bioimpedance Analysis Is Complemented with the Functional, Ultrasound Evaluation of Adipose-Muscle Tissue, and with the Molecular Expression of Inflammasome Components
3.3. Adipose and Muscle Tissue Evaluation Using Ultrasound Provides Additional Information about Nutritional Status in Patients with Head and Neck Cancers
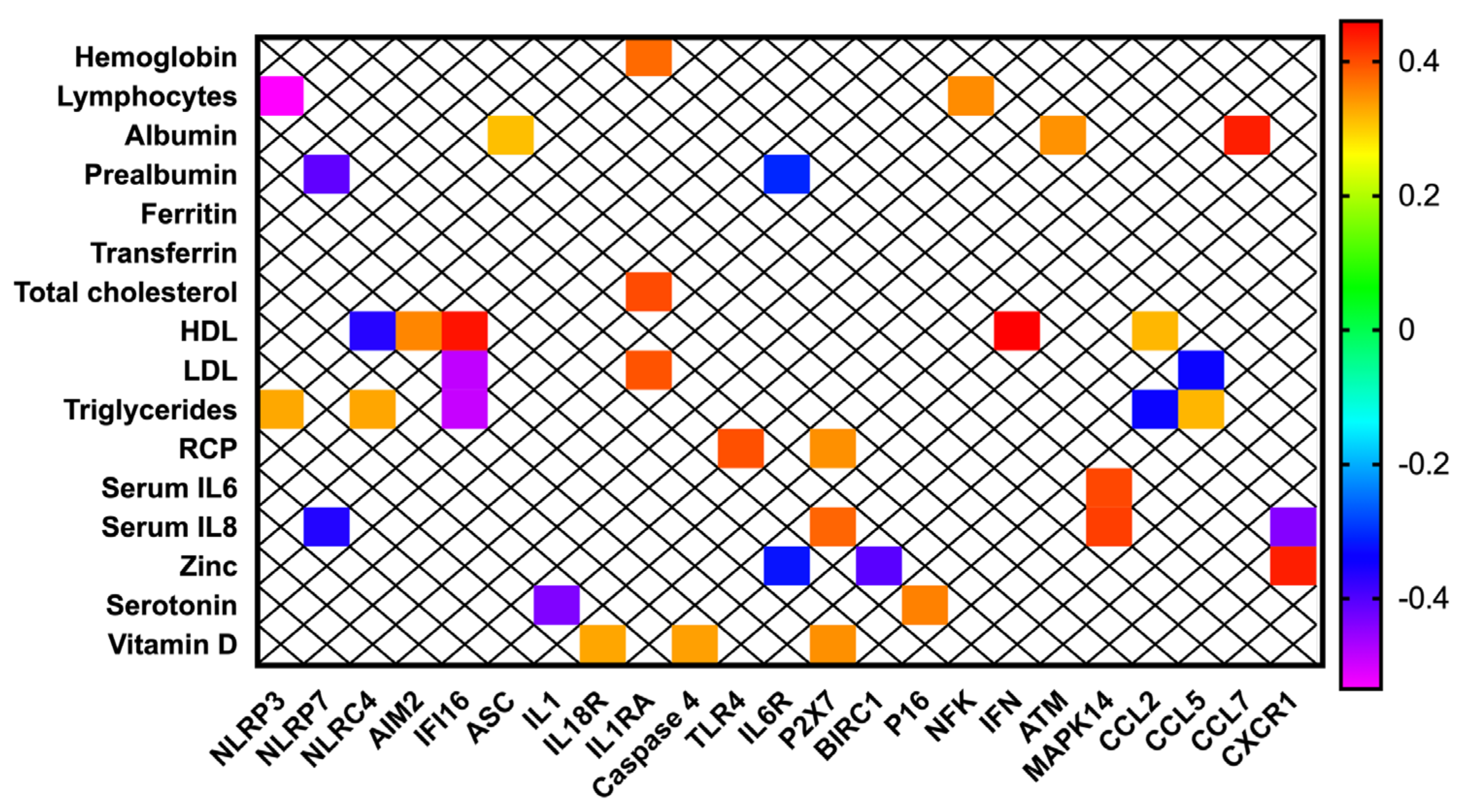
3.4. Inflammasome Components Are Correlated with Biochemical Nutritional Parameters in Patients with Head and Neck Cancer
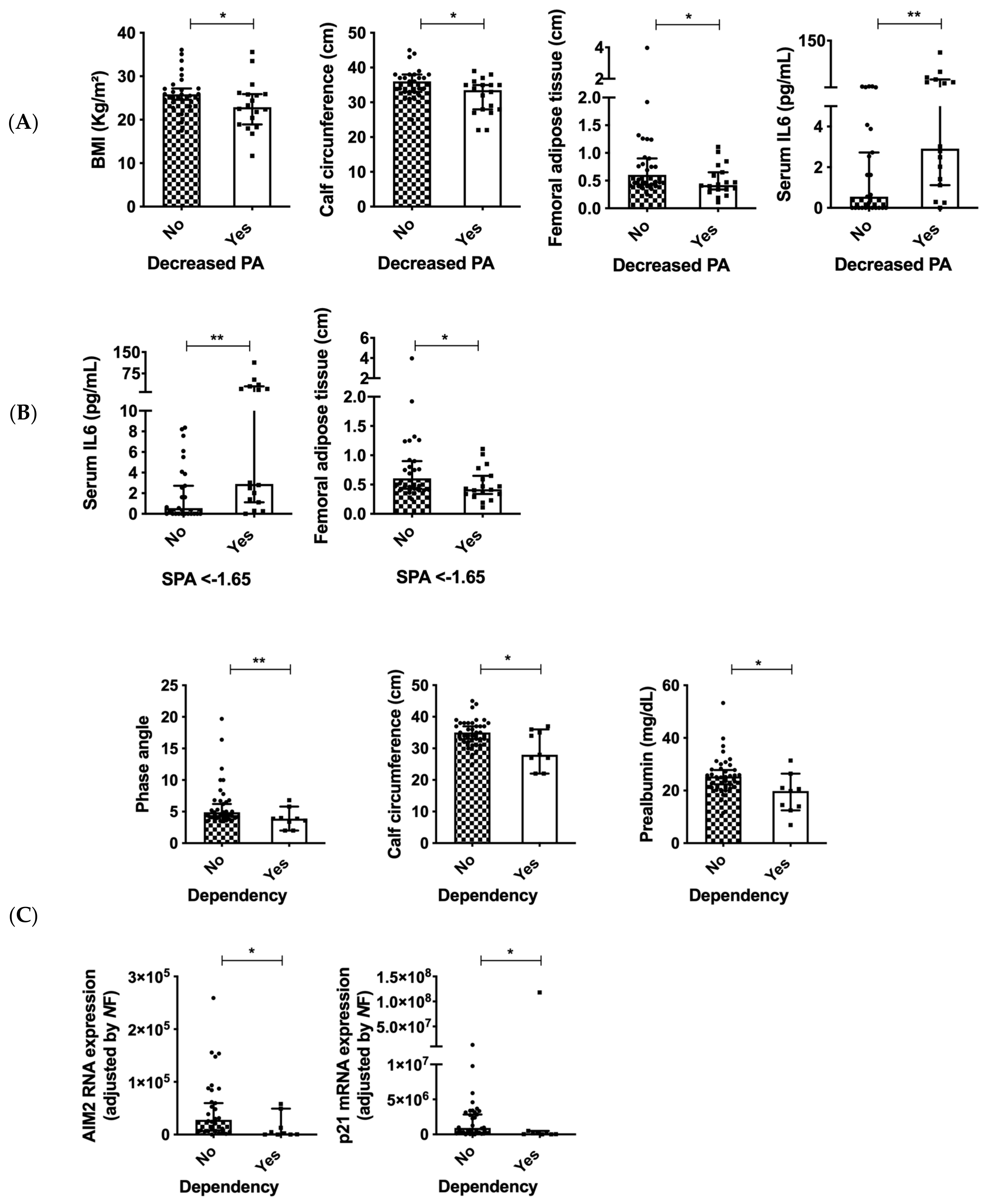
3.5. Decreased BMI and Malnutrition Are Associated with Clinical and Molecular Variables in Patients with Head and Neck Cancers
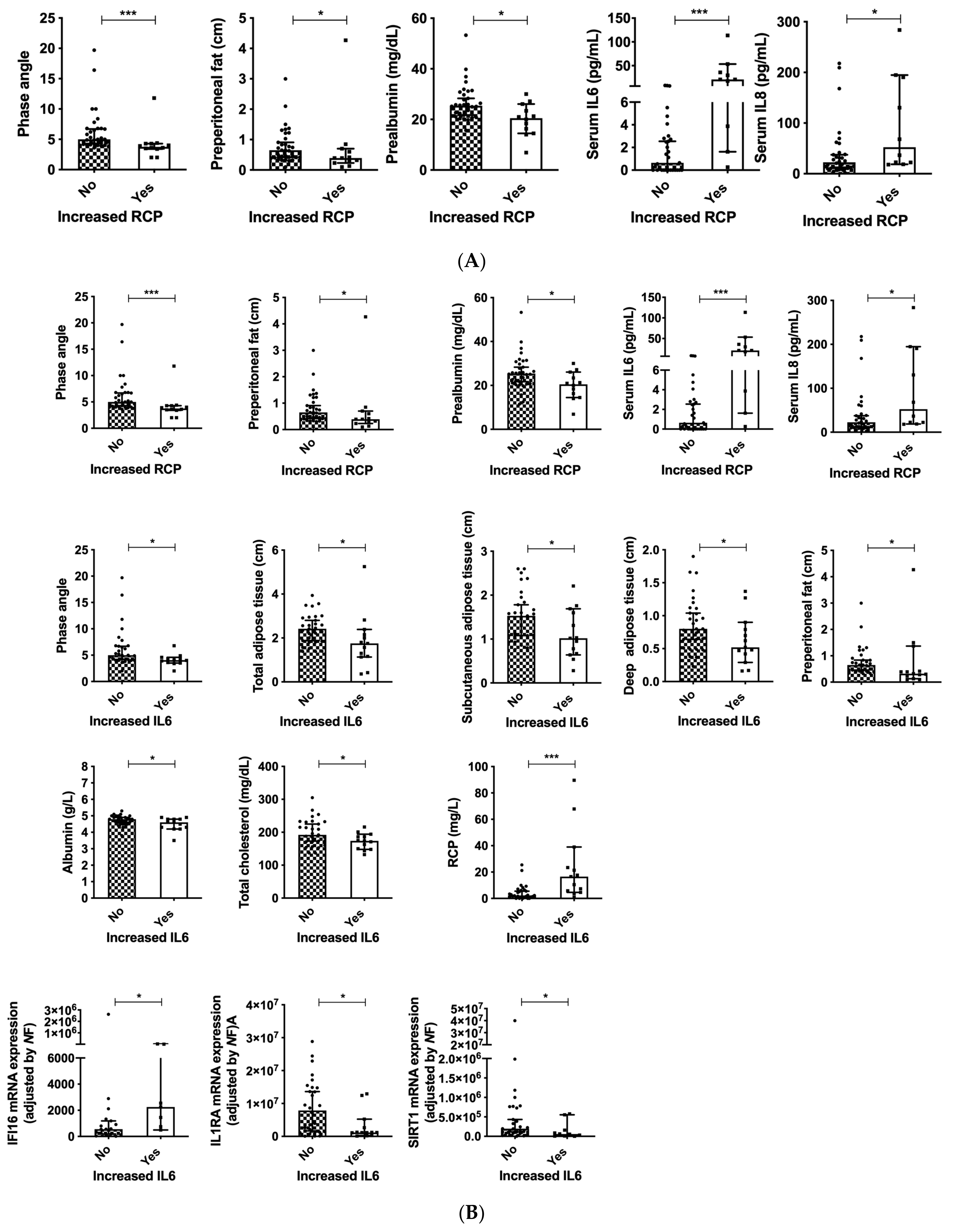
3.6. PA Is Associated with Serum IL6 and Dependency in Patients with Head and Neck Cancers
3.7. Serum Inflammation Markers as Indicators of Nutritional Status in Patients with Head and Neck Cancers
3.8. Inflammasome Components Are Correlated to Standardized PA and Malnutrition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Wayenburg, C.A.M.; Rasmussen-Conrad, E.L.; van den Berg, M.G.A.; Merkx, M.A.W.; van Staveren, W.A.; van Weel, C.; van Binsbergen, J.J. Weight loss in head and neck cancer patients little noticed in general practice. J. Prim. Heal. Care 2010, 2, 16–21. [Google Scholar] [CrossRef]
- Expósito, M.R.A.; Herrera-Martínez, A.D.; García, G.M.; Calvo, M.E.; Serrano, C.M.B.; Moreno, M.Á.G. Early nutrition support therapy in patients with head-neck cancer. Nutr. Hosp. 2018, 35, 505–510. [Google Scholar]
- Bossola, M. Nutritional Interventions in Head and Neck Cancer Patients Undergoing Chemoradiotherapy: A Narrative Review. Nutrients 2015, 7, 265–276. [Google Scholar] [CrossRef] [PubMed]
- De Luis, D.A.; Izaola, O.; Aller, R. Nutritional status in head and neck cancer patients. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 239–243. [Google Scholar]
- Isenring, E.A.; Capra, S.; Bauer, J. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br. J. Cancer 2004, 91, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Tchekmedyian, N.S.; Zahyna, D.; Halpert, C.; Heber, D. Assessment and maintenance of nutrition in older cancer patients. Oncology 1992, 6, 105–111. [Google Scholar]
- Ackerman, D.; Laszlo, M.; Provisor, A.; Yu, A. Nutrition Management for the Head and Neck Cancer Patient. Cancer Treat. Res. 2018, 174, 187–208. [Google Scholar]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.M.G.; García, C.G.; Aguilar, I.M.V.; Castañeda, V.B.; Guerrero, D.B. Morphofunctional assessment of patient s nutritional status: A global approach. Nutr. Hosp. 2021, 38, 592–600. [Google Scholar]
- Hamagawa, K.; Matsumura, Y.; Kubo, T.; Hayato, K.; Okawa, M.; Tanioka, K.; Yamasaki, N.; Kitaoka, H.; Yabe, T.; Nishinaga, M.; et al. Abdominal Visceral Fat Thickness Measured by Ultrasonography Predicts the Presence and Severity of Coronary Artery Disease. Ultrasound Med. Biol. 2010, 36, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kanneganti, T.-D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef] [Green Version]
- Yi, Y.-S. Role of inflammasomes in inflammatory autoimmune rheumatic diseases. Korean J. Physiol. Pharmacol. 2018, 22, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Gahete, M.D.; del Rio-Moreno, M.; Camargo, A.; Alcala-Diaz, J.F.; Alors-Perez, E.; Delgado-Lista, J.; Reyes, O.; Ventura, S.; Perez-Martínez, P.; Castaño, J.P.; et al. Changes in Splicing Machinery Components Influence, Precede, and Early Predict the Development of Type 2 Diabetes: From the CORDIOPREV Study. EBioMedicine 2018, 37, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Talwar, B.; Donnelly, R.; Skelly, R.; Donaldson, M. Nutritional management in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S32–S40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Paiva, S.I.; Borges, L.R.; Halpern-Silveira, D.; Assunção, M.C.F.; Barros, A.J.D.; Gonzalez, M.C. Standardized phase angle from bioelectrical impedance analysis as prognostic factor for survival in patients with cancer. Support. Care Cancer 2010, 19, 187–192. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.G.; Barros, A.J.D.; Wang, J.; Heymsfield, S.B.; Pierson, R.N., Jr. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am. J. Clin. Nutr. 2005, 82, 49–52. [Google Scholar] [CrossRef]
- Berger, J.; Bunout, D.; Barrera, G.; de la Maza, M.P.; Henriquez, S.; Leiva, L.; Hirsch, S. Rectus femoris (RF) ultrasound for the assessment of muscle mass in older people. Arch. Gerontol. Geriatr. 2015, 61, 33–38. [Google Scholar] [CrossRef]
- del Rio-Moreno, M.; Alors-Pérez, E.; González-Rubio, S.; Ferrín, G.; Reyes, O.; Rodríguez-Perálvarez, M.; Sánchez-Frías, M.E.; Sánchez-Sánchez, R.; Ventura, S.; Lopez-Miranda, J.; et al. Dysregulation of the Splicing Machinery Is Associated to the Development of Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2019, 104, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Del Río-Moreno, M.; Luque, R.M.; Rangel-Zúñiga, O.A.; Alors-Pérez, E.; Alcalá-Diaz, J.F.; Roncero-Ramos, I.; Camargo, A.; Gahete, M.D.; López-Miranda, J.; Castaño, J.P. Dietary Intervention Modulates the Expression of Splicing Machinery in Cardiovascular Patients at High Risk of Type 2 Diabetes Development: From the CORDIOPREV Study. Nutrients 2020, 12, 3528. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Borrego, M.C.; Fuentes-Fayos, A.C.; Herrera-Martínez, A.D.; L-López, F.; Ibáñez-Costa, A.; Moreno-Moreno, P.; Alhambra-Expósito, M.R.; Barrera-Martín, A.; Blanco-Acevedo, C.; Dios, E.; et al. Biguanides exert antitumoral actions in pituitary tumor cells through AMPK-dependent and independent mechanisms. J. Clin. Endocrinol. Metab. 2019, 104, 3501–3513. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, A.D.; Pedraza-Arevalo, S.; L-López, F.; Gahete, M.D.; Gálvez-Moreno, M.A.; Castaño, J.P.; Luque, R.M. Type 2 diabetes in neuroendocrine tumors: Are biguanides and statins part of the solution? J. Clin. Endocrinol. Metab. 2019, 104, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Aguayo, V.; Jiménez-Vacas, J.M.; Sáez-Martínez, P.; Gómez-Gómez, E.; López-Cánovas, J.L.; Garrido-Sánchez, L.; Herrera-Martínez, A.D.; García-Bermejo, L.; Macías-González, M.; López-Miranda, J.; et al. Influence of Obesity in the miRNome: miR-4454, a Key Regulator of Insulin Response Via Splicing Modulation in Prostate. J. Clin. Endocrinol. Metab. 2021, 106, e469–e484. [Google Scholar] [CrossRef] [PubMed]
- López-Cánovas, J.L.; del Rio-Moreno, M.; García-Fernandez, H.; Jiménez-Vacas, J.M.; Moreno-Montilla, M.; Sánchez-Frias, M.E.; Amado, V.; L-López, F.; Fondevila, M.F.; Ciria, R.; et al. Splicing factor SF3B1 is overexpressed and implicated in the aggressiveness and survival of hepatocellular carcinoma. Cancer Lett. 2021, 496, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Vacas, J.M.; Herrero-Aguayo, V.; Hidalgo, A.J.M.; Gómez-Gómez, E.; Fuentes-Fayos, A.C.; León-González, A.J.; Sáez-Martínez, P.; Alors-Pérez, E.; Pedraza-Arévalo, S.; González-Serrano, T.; et al. Dysregulation of the splicing machinery is directly associated to aggressiveness of prostate cancer. eBioMedicine 2020, 51, 102547. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Aguayo, V.; Sáez-Martínez, P.; López-Cánovas, J.L.; Prados-Carmona, J.J.; Alcántara-Laguna, M.D.; López, F.L.; Molina-Puerta, M.J.; Calañas-Continente, A.; Membrives, A.; Castilla, J.; et al. Dysregulation of Components of the Inflammasome Machinery After Bariatric Surgery: Novel Targets for a Chronic Disease. J. Clin. Endocrinol. Metab. 2021, 106, e4917–e4934. [Google Scholar] [CrossRef]
- Jiménez-Vacas, J.M.; Herrero-Aguayo, V.; Montero-Hidalgo, A.J.; Sáez-Martínez, P.; Gómez-Gómez, E.; León-González, A.J.; Fuentes-Fayos, A.C.; Yubero-Serrano, E.M.; Requena-Tapia, M.J.; López, M.; et al. Clinical, Cellular, and Molecular Evidence of the Additive Antitumor Effects of Biguanides and Statins in Prostate Cancer. J. Clin. Endocrinol. Metab. 2021, 106, e696–e710. [Google Scholar] [CrossRef]
- Fuentes-Fayos, A.C.; Vázquez-Borrego, M.C.; Jiménez-Vacas, J.M.; Bejarano, L.; Pedraza-Arévalo, S.; L. -López, F.; Blanco-Acevedo, C.; Sánchez-Sánchez, R.; Reyes, O.; Ventura, S.; et al. Splicing machinery dysregulation drives glioblastoma development/aggressiveness: Oncogenic role of SRSF3. Brain 2020, 143, 3273–3293. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.1–14.10.91. [Google Scholar] [CrossRef] [PubMed]
- SENPE; SEGG. Valoracion nutricional en el anciano. Recomendaciones prácticas de los expertos en geriatría y nutrición. Consensus Docunment. 2007. Available online: https://www.segg.es/media/descargas/Acreditacion%20de%20Calidad%20SEGG/Residencias/valoracion_nutricional_anciano.pdf (accessed on 10 October 2021).
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef]
- Martín, C.A.G.; Zelaya, R.D.C.U.; Zepeda, E.M.; Méndez, O.A.L. ROUNDS Studies: Relation of OUtcomes with Nutrition Despite Severity—Round One: Ultrasound Muscle Measurements in Critically Ill Adult Patients. J. Nutr. Metab. 2018, 2018, 7142325. [Google Scholar]
- CDC. Body Composition Procedures Manual. Available online: https://www.cdc.gov/nchs/data/nhanes/bc.pdf (accessed on 10 October 2021).
- Araújo, M.L.D.; Cabral, P.C.; de Arruda, I.K.G.; Falcão, A.P.S.T.; Diniz, A.S. Body fat assessment by bioelectrical impedance and its correlation with anthropometric indicators. Nutr. Hosp. 2012, 27, 1999–2005. [Google Scholar]
- Omura-Ohata, Y.; Son, C.; Makino, H.; Koezuka, R.; Tochiya, M.; Tamanaha, T.; Kishimoto, I.; Hosoda, K. Efficacy of visceral fat estimation by dual bioelectrical impedance analysis in detecting cardiovascular risk factors in patients with type 2 diabetes. Cardiovasc. Diabetol. 2019, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wagner, D.R. Ultrasound as a Tool to Assess Body Fat. J. Obes. 2013, 2013, 280713. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Inose, H.; Ushio, S.; Yuasa, M.; Hirai, T.; Yoshii, T.; Okawa, A. Body Mass Index and Modified Glasgow Prognostic Score Are Useful Predictors of Surgical Site Infection After Spinal Instrumentation Surgery: A Consecutive Series. Spine (Phila Pa 1976) 2020, 45, E148–E154. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-R.; Kim, A.-S.; Choi, H.-I.; Jung, J.-H.; Park, J.Y.; Ko, H.-J. Inflammatory markers for predicting overall survival in gastric cancer patients: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0236445. [Google Scholar] [CrossRef]
- Guerra, L.T.; Rosa, A.R.; Romani, R.F.; Gurski, R.R.; Schirmer, C.C.; Kruel, C.D.P. Serum transferrin and serum prealbumin as markers of response to nutritional support in patients with esophageal cancer. Nutr. Hosp. 2009, 24, 241–242. [Google Scholar]
- Gámez, J.L.V.; García-Almeida, J.M.; De Damas, J.M.G.; Vázquez, R.R.; Fernández, D.O.; Rodríguez-García, L.M.; Mata, J.D.R.; García, C.O.; Gutiérrez-Bedmar, M. [INFORNUT process: Validation of the filter phase-FILNUT--and comparison with other methods for the detection of early hospital hyponutrition]. Nutr. Hosp. 2006, 21, 491–504. [Google Scholar]
- Alvarez, J.; Del Río, J.; Planas, M.; Peris, P.G.; De Lorenzo, A.G.; Calvo, V.; Olveira, G.; A Irles, J.; Piñeiro, G.; Senpe, G.D.D.D. SENPE-SEDOM document on coding of hospital hyponutrition. Nutr. Hosp. 2008, 23, 536–540. [Google Scholar]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P.; Jensen, G.L. The ASPEN Malnutrition Committee The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr. Clin. Pract. 2021, 36, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Mahmoud, F.; Barna, B. Assessment of nutritional status and prognosis in advanced cancer: Interleukin-6, C-reactive protein, and the prognostic and inflammatory nutritional index. Support. Care Cancer 2003, 11, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Padhke, R.; Dew, T.; Sidhu, P.S.; et al. Acute Skeletal Muscle Wasting in Critical Illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef] [Green Version]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Redman, L.M.; Rochon, J.; Das, S.K.; Fontana, L.; Kraus, W.E.; Romashkan, S.; Williamson, D.A.; Meydani, S.N.; Villareal, D.T.; et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1097–1104. [Google Scholar] [CrossRef]
- Esser, N.; L’Homme, L.; De Roover, A.; Kohnen, L.; Scheen, A.J.; Moutschen, M.; Piette, J.; Legrand-Poels, S.; Paquot, N. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia 2013, 56, 2487–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Deng, T.; Peterson, L.E.; Yu, R.; Lin, J.; Hamilton, D.J.; Reardon, P.R.; Sherman, V.; Winnier, G.E.; Zhan, M.; et al. Transcriptome analysis of human adipocytes implicates the NOD-like receptor pathway in obesity-induced adipose inflammation. Mol. Cell. Endocrinol. 2014, 394, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Bando, S.; Fukuda, D.; Soeki, T.; Nishimoto, S.; Uematsu, E.; Matsuura, T.; Ise, T.; Tobiume, T.; Yamaguchi, K.; Yagi, S.; et al. Expression of NLRP3 in subcutaneous adipose tissue is associated with coronary atherosclerosis. Atherosclerosis 2015, 242, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kursawe, R.; Dixit, V.D.; Scherer, P.E.; Santoro, N.; Narayan, D.; Gordillo, R.; Giannini, C.; Lopez, X.; Pierpont, B.; Nouws, J.; et al. A Role of the Inflammasome in the Low Storage Capacity of the Abdominal Subcutaneous Adipose Tissue in Obese Adolescents. Diabetes 2016, 65, 610–618. [Google Scholar] [CrossRef] [Green Version]
- Serena, C.; Keiran, N.; Ceperuelo-Mallafre, V.; Ejarque, M.; Fradera, R.; Roche, K.; Nuñez-Roa, C.; Vendrell, J.; Fernández-Veledo, S. Obesity and Type 2 Diabetes Alters the Immune Properties of Human Adipose Derived Stem Cells. Stem Cells 2016, 34, 2559–2573. [Google Scholar] [CrossRef]
- Camell, C.; Goldberg, E.; Dixit, V.D. Regulation of Nlrp3 inflammasome by dietary metabolites. Semin. Immunol. 2015, 27, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Dalvi, P.S.; Yang, S.; Swain, N.; Kim, J.; Saha, S.; Bourdon, C.; Zhang, L.; Chami, R.; Bandsma, R.H.J. Long-term metabolic effects of malnutrition: Liver steatosis and insulin resistance following early-life protein restriction. PLoS ONE 2018, 13, e0199916. [Google Scholar] [CrossRef]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachex-Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Blanc, R.S.; Kallenbach, J.G.; Bachman, J.F.; Mitchell, A.; Paris, N.D.; Chakkalakal, J.V. Inhibition of inflammatory CCR2 signaling promotes aged muscle regeneration and strength recovery after injury. Nat. Commun. 2020, 11, 4167. [Google Scholar] [CrossRef]
- Baracos, V.E. Regulation of skeletal-muscle–protein turnover in cancer-associated cachexia. Nutrition 2000, 16, 1015–1018. [Google Scholar] [CrossRef]
- Rawat, R.; Cohen, T.V.; Ampong, B.; Francia, D.; Henriques-Pons, A.; Hoffman, E.P.; Nagaraju, K. Inflammasome Up-Regulation and Activation in Dysferlin-Deficient Skeletal Muscle. Am. J. Pathol. 2010, 176, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Truax, A.D.; Chen, L.; Tam, J.W.; Cheng, N.; Guo, H.; Koblansky, A.A.; Chou, W.-C.; Wilson, J.E.; Brickey, W.J.; Petrucelli, A.; et al. The Inhibitory Innate Immune Sensor NLRP12 Maintains a Threshold against Obesity by Regulating Gut Microbiota Homeostasis. Cell Host Microbe 2018, 24, 364–378.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Palombaro, M.; Pulcini, G.; Gasbarrini, A.; Mele, M. Nutritional Interventions Targeting Gut Microbiota during Cancer Therapies. Microorganisms 2021, 9, 1469. [Google Scholar] [CrossRef]
- Daniel, C.R.; McQuade, J.L. Nutrition and Cancer in the Microbiome Era. Trends Cancer 2019, 5, 521–524. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | Total (n = 45) | No Malnutrition (GLIM Criteria) (n = 27) | Malnutrition (GLIM Criteria) (n = 18) | p1 | SPA > −1.65 (n = 26) | SPA < −1.65 (n = 18) | p2 |
|---|---|---|---|---|---|---|---|
| Sex (♂/♀) | 62.2/37.8% (28/17) | 51.9/48.1% (14/13) | 77.8/22.2% (14/4) | 0.07 | 50/50% (13/13) | 77.8/22.2% (14/18) | 0.06 |
| Age at diagnosis (years) | 64.5 (61.4–73) | 64 (58.7–73.8) | 65 (54.8–82.4) | 0.7 | 65 (57–76) | 64 (56–78) | 0.3 |
| Tobacco exposure | 0.9 | 0.5 | |||||
| No | 21.1% (8/38) | 22.7% (5/22) | 18.8% (3/16) | 28.6% (6/21) | 12.5% (2/16) | ||
| Active | 31.1.% (4/38) | 31.8% (7/22) | 43.8% (7/16) | 28.6% (6/21) | 43.8% (7/16) | ||
| Previous exposure | 35.6% (16/38) | 45.5% (10/22) | 37.5% (6/16) | 42.9% (9/21) | 43.8% (7/16) | ||
| Comorbidities | |||||||
| Hypertension | 37.8 (17/45) | 37 (10/27) | 38.9 (7/18) | 0.6 | 38.5 (10/26) | 33.3 (6/18) | 0.8 |
| Diabetes | 20 (9/45) | 18.5 (5/27) | 22.2 (4/14) | 0.5 | 15.4 (4/26) | 22.2 (4/18) | 0.7 |
| Dyslipidemia | 31.1 (14/45) | 29.8 (8/27) | 33.3 (6/18) | 0.5 | 30.8 (8/26) | 27.8 (5/18) | 0.6 |
| Heart disease | 6.7 (3/45) | 3.7 (1/27) | 11.1 (2/18) | 0.4 | 3.8 (1/26) | 11.1 (2/18) | 0.4 |
| Lung disease | 8.9 (4/45) | 7.4 (2/27) | 11.1 (2/18) | 0.5 | 3.8 (1/26 | 16.7 (3/18) | 0.3 |
| Other neoplasms | 11.1% (5/40) | 14.8% (4/23) | 5.6% (1/17) | 15.4% (4/26) | 5.6% (1/16) | ||
| Tumor localization | 0.9 | 0.9 | |||||
| Oral cavity | 60% (27/45) | 59.1 (16/27) | 61.1% (11/18) | 61.5% (16/26) | 61.1% (11/18) | ||
| Supraglottic larynx | 13.3% (6/45) | 11.1 (3/27) | 16.7% (3/18) | 7.7% (2/26) | 16.7% (3/18) | ||
| Glottic larynx | 11.1% (5/45) | 18.5 (5/27) | 0 | 19.2% (5/26) | 0 | ||
| Subglottic larynx | 8.9% (4/45) | 3.7 (1/27) | 16.7% (3/18) | 3.8% (1/26) | 16.7% (3/18) | ||
| Neck metastasis from unknown primary | 6.6% (3/45) | 7.4 (2/27) | 5.6% (1/18) | 7.7% (2/26) | 5.6% (1/18) | ||
| Treatment | |||||||
| Surgery | 53.3% (24/45) | 55.6% (15/27) | 50% (9/18) | 0.5 | 53.8% (14/26) | 50% (9/18) | 0.5 |
| Chemotherapy | 55.6% (25/45) | 48.1% (13/27) | 66.7 (12/18) | 0.2 | 46.2% (12/26) | 66.7% (12/18) | 0.2 |
| Radiotherapy | 91.1% (42/45) | 88.9% (24/17) | 94.4 (17/18) | 0.5 | 88.5% (23/26) | 94.4% (17/18) | 0.4 |
| Combination therapies | |||||||
| Surgery and Radiotherapy | 28.9% (13/45) | 29.6% (8/27) | 44.4% (8/18) | 0.5 | 46.2% (12/26) | 44.4% (8/18) | 0.5 |
| Surgery and Chemotherapy | 20% (9/45) | 22.2% (6/27) | 16.7% (3/18) | 0.5 | 19.2% (5/26) | 16.7% (3/18) | 0.6 |
| Chemoradiotherapy | 31.1% (14/45) | 25.9% (7/27) | 50% (9/18) | 0.4 | 42.3% (11/26) | 50% (9/18) | 0.2 |
| Surgery and Chemoradiotherapy | 20% (9/45) | 22.2% (6/27) | 16.7% (3/18) | 0.7 | 19.2 (5/26) | 16.7 (3/18) | 0.5 |
| Histology | 0.5 | 0.3 | |||||
| Epidermoid carcinoma | 86.7 (39/45) | 85.3 (23/27) | 88.9 (16/18) | 80.8 (21/26) | 94.4 (17/18) | ||
| Cystic adenoma | 2.2 (1/45) | 3.1 (1/27) | 0 | 3.8 (1/26) | 0 | ||
| Lymphoepithelioma | 2.2 (1/45) | 0 | 5.6 (1/18) | 0 | 5.6 (1/18) | ||
| Polymorphic adenocarcinoma | 4.4 (2/45) | 3.1 (1/27) | 5.6 (1/18) | 7.7 (2/26) | 0 | ||
| Others | 4.4 (2/45) | 7.4 (2/27) | 0 | 7.7 (2/26) | 0 | ||
| Cancer stage | 0.2 | 0.05 | |||||
| I | 13.3 (6/45) | 23.1 (6/26) | 0 | 24 (6/26) | 0 | ||
| II | 6.7 (3/45) | 7.7 (2/26) | 5.9 (1/17) | 12 (3/26) | 0 | ||
| III | 15.6 (7/45) | 15.4 (4/26) | 17.6 (3/17) | 12 (3/26) | 23.5 (4/17) | ||
| IV | 60 (27/45) | 53.8 (14/26) | 76.5 (13/17) | 52 (13/26) | 76.5 (13/17) | ||
| Symptoms | |||||||
| Weight loss (3 months) | 44.4% (20/45) | 37% (10/27) | 55.6% (10/18) | 0.2 | 34.9% (9/26) | 55.6% (10/18) | 0.1 |
| Weight loss kg (3 months) | 4 (2.6–5.6) | 5 (2.3–7.4) | 3 (1.6–4.8) | 0.5 | 5 (1.8–8.1) | 3.5 (2–4.5) | 0.9 |
| Weight loss (6 months) | 46.7% (21/45) | 44.4% (12/27) | 50% (9/18) | 0.5 | 42.3% (11/26) | 55.6% (10/18) | 0.3 |
| Weight loss kg (6 months) | 3.5 (2.5–7.2) | 3 (1–6.7) | 4 (0.6–11.2) | 0.8 | 3.5 (0.8–7.5) | 3.5 (0.9–10) | 0.5 |
| Abdominal pain | 4.4% (2/45) | 7.4% (2/27) | 0 | 0.2 | 3.8% (1/26) | 5.6% (1/18) | 0.7 |
| Nauseas/vomits | 11.1% (5/45) | 11.1% (3/27) | 11.1% (2/18) | 0.7 | 15.4% (4/26) | 5.6% (1/18) | 0.3 |
| Diarrhea | 4.4% (2/45) | 7.4% (2/27) | 0 | 0.4 | 7.7% (2/26) | 0 | 0.3 |
| Dyspnea | 13.3% (6/45) | 14.8% (4/27) | 11.1% (2/18) | 0.6 | 11.5% (3/26) | 16.7% (3/18) | 0.3 |
| Dermatitis | 28.9% (13/45) | 29.6% (8/27) | 27.8% (5/18) | 0.5 | 26.9% (7/26) | 27.8% (5/18) | 0.5 |
| Dysphagia | 66.7% (30/45) | 55.6% (15/27) | 83.3% (15/18) | 0.05 | 57.7%(15/26) | 77.8% (14/18) | 0.2 |
| Mucositis | 40% (18/45) | 40.7% (11/27) | 38.9% (7/18) | 0.6 | 42.3% (11/26) | 33.3% (6/18) | 0.4 |
| Asthenia | 73.3% (33/45) | 77.8% (21/27) | 66.7% (12/18) | 0.3 | 76.9% (20/26) | 66.7% (12/18) | 0.3 |
| Quality of life | |||||||
| KI | 0 (0.05–2) | 0 (−0.2–0.5) | 2 (0.1–4.7) | 0.06 | 0 (0–0) | 2 (0.3–4) | 0.02 |
| Self-rated health score | 70 (54–78) | 70 (58–78) | 50 (27–98) | 0.3 | 70 (56–80) | 60 (37–91) | 0.4 |
Characteristics | Total (n = 45) | No Malnutrition (GLIM Criteria) (n = 27) | Malnutrition (GLIM Criteria) (n = 18) | p1 | SPA > −1.65 (n = 26) | SPA < −1.65 (n = 18) | p2 |
|---|---|---|---|---|---|---|---|
| Bioimpedance analysis | |||||||
| BMI (kg/m2) | 24 (21.8–25.8) | 23.1 (20.6–26.8) | 25.6 (20.2–27.7) | 0.3 | 23.9 (21.8–27.3) | 24.5 (19.3–26.7) | 0.04 |
| BCMe | 26.4 (23–34.2) | 26.2 (21.2–30) | 33.1 (18–47.7) | 0.7 | 26.4 (22.5–30.9) | 28.5 (17.8–43.2) | 0.4 |
| ECMe | 16.3 (14.4–19.2) | 15.5 (12.9–17.9) | 18.9 (13.3–24.4) | 0.7 | 16.3 (13.9–18.3) | 17.3 (12.3–22.9) | 0.9 |
| Fat mass (%) | 24.6 (21.2–32.2) | 26.9 (21.5–35) | 22.6 (11.4–37.5) | 0.7 | 28.9 (21–37.2) | 22.9 (14.3–34.1) | 0.2 |
| Fat mass (kg) | 14.6 (12.6–20.3) | 14.7 (10.7–23.1) | 13.1 (8.6–23.2) | 0.9 | 16.1 (11.6–24.8) | 12.3 (8.5–21) | 0.4 |
| Lean mass (%) | 71.6 (64.6–74.6) | 66.9 (61.2–74.1) | 73.3 (61.3–83.3) | 0.4 | 66.3 (59.2–74.4) | 73 (64.1–80.7) | 0.2 |
| Lean mass (kg) | 40.1 (35.8–50.9) | 39.6 (32.4–45.4) | 49.4 (30.5–68.6) | 0.8 | 40.1 (34.6–46.7) | 43.4 (29–63) | 0.6 |
| Water (%) | 51.4 (47.5–54.9) | 49.7 (45.9–54.5) | 53.4 (43.3–62) | 0.7 | 49.7 (44.5–54.7) | 53.6 (45.8–59.9) | 0.3 |
| Water (kg) | 33.8 (27.6–39.2) | 30 (24–36.1) | 36.9 (24.7–51.5) | 0.7 | 31.1 (25.6–37.5) | 36.6 (22.8–47.7) | 0.6 |
| Bone mass (kg) | 2.2 (1.9–2.7) | 2.1 (1.8–2.4) | 2.6 (1.7–3.5) | 0.9 | 2.2 (1.9–2.5) | 2.4 (1.6–3.3) | 0.8 |
| Anthropometric evaluation | |||||||
| Abdominal circumference | 90.5 (84.5–97.3) | 88 (78.9–92.8) | 95 (86–109) | 0.9 | 89 (81.6–94) | 93 (80.5–107.5) | 0.6 |
| Arm circumference | 26 (24.9–28.2) | 26 (24.1–29) | 26 (23–30) | 0.3 | 26.5 (24.3–29.7) | 25.5 (23.2–29.1) | 0.05 |
| Calf circumference | 33.5 (28.4–34.5) | 33 (27.7–36) | 34 (24–37) | 0.2 | 33.5 (28–37) | 31 (24.6–35.8) | 0.04 |
| Muscle echography | |||||||
| Adipose tissue | 0.53 (0.20–1.5) | 0.69 (0.1–2.3) | 0.47 (0.3–0.6) | 0.3 | 0.7 (−0.2–2.7) | 0.4 (0.3–0.6) | 0.03 |
| Area | 1.8 (1.5–3.2) | 2.15 (1.4–3.7) | 1.7 (0.3–3.9) | 0.3 | 3 (1.4–4) | 1.5 (0.6–3.4) | 0.2 |
| Circunference | 8.6 (6.9–9) | 8.6 (6.4–9.6) | 8.6 (5.7–10.2) | 0.2 | 8.7 (7.5–9.5) | 8 (5.2–9.6) | 0.1 |
| Abdominal echography | |||||||
| Total adipose tissue | 2.23 (1.8–2.7) | 2 (1.7–3) | 2.5 (1.12–3.11) | 0.7 | 2.3 (1.8–3.2) | 2 (1.2–2.8) | 0.2 |
| Subcutaneous adipose tissue | 1.5 (1.1–1.9) | 1.5 (1–2) | 1.6 (0.6–2.6) | 0.6 | 1.6 (0.9–2.1) | 1.3(0.7–2.3) | 0.2 |
| Superficial subcutaneous adipose tissue | 0.49 (0.4–0.7) | 0.5 (0.3–0.7) | 0.5 (0.1–1.1) | 0.9 | 0.5 (0.3–0.7) | 0.5 (0.2–0.9) | 0.6 |
| Deep subcutaneous adipose tissue | 1 (0.8–1.3) | 1.1 (0.8–1.4) | 0.9 (0.6–1−3) | 0.5 | 1.2 (0.8–1.5) | 0.9 (0.5–1.2) | 0.2 |
| Preperitoneal adipose tissue | 0.5 (0.3–1.2) | 0.4 (0.3–0.8) | 0.7 (−0.5–2.4) | 0.4 | 0.5 (0.3–0.9) | 0.6 (−0.2–2) | 0.2 |
| Functional evaluation | |||||||
| Dynamometry (dominant arm) | 18 (15–26) | 19 (14–29) | 17 (8–31) | 0.9 | 22 (14–31) | 17 (10–28) | 0.9 |
| Stand up test | 9 (6–11) | 10 (8–11) | 7 (−0.08–12) | 0.3 | 9.5 (8–10) | 8 (1–12) | 0.6 |
Characteristics | Total (n = 45) | No Malnutrition (GLIM Criteria) (n = 27) | Malnutrition (GLIM Criteria) (n = 18) | p1 | SPA > −1.65 (n =26) | SPA < −1.65 (n =18) | p2 |
|---|---|---|---|---|---|---|---|
| Biochemical parameters | |||||||
| Haemoglobin | 13.1 (11.7–15.3) | 13(11.2–13.7) | 13.4 (10.3–19) | 0.6 | 12.7(10–13) | 13.4 (11.1–18.4) | 0.6 |
| Lymphocytes | 910 (624–1228) | 680 (296–1227) | 1120 (717–1598) | 0.4 | 810(456–1320) | 1000 (965–1562) | 0.9 |
| Albumin (g/dL) | 4.6 (4.3–4.8) | 4.7 (4.5–4.9) | 4.2 (3.9–4.7) | 0.05 | 4.7 (4.4–4.9) | 4.4 (4–4.8) | 0.05 |
| Prealbumin (mg/dL) | 10.7 (17.7–26.7) | 22.4 (19.3–30.6) | 14.5 (9.5–27.2) | 0.2 | 23.8(19–32) | 17.2 (11.9–25) | 0.3 |
| Ferritin (mg/dL) | 70.2 (36.4–121.2) | 76.8 (11–160) | 63.6 (10–128) | 0.7 | 82 (6–183) | 56.6 (15–110) | 0.6 |
| Transferrin (mg/dL) | 260.5 (231–301) | 287 (230–310) | 255 (170–352) | 0.03 | 269 (219–305) | 260 (198–344) | 0.06 |
| Total cholesterol (mg/dL) | 191 (175–231) | 215 (175–268) | 174 (162–193) | 0.02 | 213 (160–267) | 181 (152–232) | 0.1 |
| HDL cholesterol | 59 (51–74) | 55 (45–78) | 63 (33–91) | 0.7 | 54 (45–74) | 63 (41–90) | 0.9 |
| LDL cholesterol | 116 (93–139) | 135 (97–163) | 109 (61–129) | 0.1 | 128 (88–167) | 112(70–138) | 0.2 |
| Triglycerides | 100 (85–160) | 143 (71–206) | 93 (67–135) | 0.3 | 107 (50–213) | 100 (72–155) | 0.9 |
| RCP | 3 (1.5–10.4) | 2.5 (0.8–5.2) | 9.2 (−1.8–21.7) | 0.03 | 2.4 (0.4–4.6) | 7.9 (0.3–18.4) | 0.04 |
| IL-6 | 1.4 (0.4–11.9) | 0 (−0.7–5) | 2.8 (−6–3−28) | 0.01 | 0 (−1.5–5.6) | 2.6(−4–22.8) | 0.007 |
| Zinc (mg/dL) | 70.6 (66.7–90.3) | 70 (58.6–85.8) | 91.7 (60–113) | 0.2 | 67 (59–81) | 93 (68–108) | 0.08 |
| Serotonin | 127.5 (92–209) | 197 (78–262) | 117 (20–226) | 0.9 | 159 (60–229) | 126 (41–272) | 0.5 |
| Vitamin D | 17 (13–28) | 17 (8–36) | 17 (11–27) | 0.3 | 22 (9–40) | 16 (9–24) | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Idougourram, S.; Pérez-Gómez, J.M.; Muñoz Jiménez, C.; L-López, F.; Manzano García, G.; Molina Puertas, M.J.; Herman-Sánchez, N.; Alonso-Echague, R.; Calañas Continente, A.; Gálvez Moreno, M.Á.; et al. Morphofunctional and Molecular Assessment of Nutritional Status in Head and Neck Cancer Patients Undergoing Systemic Treatment: Role of Inflammasome in Clinical Nutrition. Cancers 2022, 14, 494. https://doi.org/10.3390/cancers14030494
León-Idougourram S, Pérez-Gómez JM, Muñoz Jiménez C, L-López F, Manzano García G, Molina Puertas MJ, Herman-Sánchez N, Alonso-Echague R, Calañas Continente A, Gálvez Moreno MÁ, et al. Morphofunctional and Molecular Assessment of Nutritional Status in Head and Neck Cancer Patients Undergoing Systemic Treatment: Role of Inflammasome in Clinical Nutrition. Cancers. 2022; 14(3):494. https://doi.org/10.3390/cancers14030494
Chicago/Turabian StyleLeón-Idougourram, Soraya, Jesús M. Pérez-Gómez, Concepción Muñoz Jiménez, Fernando L-López, Gregorio Manzano García, María José Molina Puertas, Natalia Herman-Sánchez, Rosario Alonso-Echague, Alfonso Calañas Continente, María Ángeles Gálvez Moreno, and et al. 2022. "Morphofunctional and Molecular Assessment of Nutritional Status in Head and Neck Cancer Patients Undergoing Systemic Treatment: Role of Inflammasome in Clinical Nutrition" Cancers 14, no. 3: 494. https://doi.org/10.3390/cancers14030494








