Lotus (Nelumbo nucifera Gaertn.) and Its Bioactive Phytocompounds: A Tribute to Cancer Prevention and Intervention
Abstract
:Simple Summary
Abstract
1. Introduction
2. Chemical Constituents of N. nucifera
2.1. Leaves
2.2. Plumules
2.3. Seeds and Rhizomes
2.4. Flowers
| Phytochemicals | Plant Part | References |
|---|---|---|
| Aromatic phenolic compounds | ||
| Arbutin | Stamens | Mukherjee et al., 2009 [31] |
| Gallic acid | Stamens and petals | Noysang and Boonmatit, 2019 [43] |
| (E)-Ferulic acid | Seeds | Rho and Yoon, 2017 [69] |
| (E)-p-Coumaric acid | Seeds | Rho and Yoon, 2017 [69] |
| (E)-Sinapate-4-O-β-d-glucopyranoside | Seeds | Rho and Yoon, 2017 [69] |
| p-Hydroxybenzoic acid | Seeds | Rho and Yoon, 2017 [69] |
| Protocatechuic acid | Seeds | Rho and Yoon, 2017 [69] |
| Tannic acid | Stamens and petals | Noysang and Boonmatit, 2019 [43] |
| Megastigmane/sesquiterpenes compounds | ||
| (−)-Boscialin | Leaves | Ahn et al., 2013 [45] |
| (+)-Dehydrovomifoliol | Leaves | Ahn et al., 2013 [45] |
| (+)-Epiloliolide | Leaves | Ahn et al., 2013 [45] |
| (E)-3-Hydroxymegastigm-7-en-9-one | Leaves | Ahn et al., 2013 [45] |
| 3-oxo-Retro-α-ionol I | Leaves | Ahn et al., 2013 [45] |
| 3S,5R-Dihydroxy-6S,7-megastigmadien-9-one | Leaves | Ahn et al., 2013 [45] |
| 5,6-epoxy-3-Hydroxy-7-megastigmen-9-one | Leaves | Ahn et al., 2013 [45] |
| Annuionone D | Leaves | Ahn et al., 2013 [45] |
| Byzantionoside A | Leaves | Ahn et al., 2013 [45] |
| Grasshopper ketone | Leaves | Ahn et al., 2013 [45] |
| Icariside B2 | Leaves | Ahn et al., 2013 [45] |
| Nelumnucifoside A | Leaves | Ahn et al., 2013 [45] |
| Vomifoliol | Leaves | Ahn et al., 2013 [45] |
| Nelumnucifoside B | Leaves | Ahn et al., 2013 [45] |
| Alkaloids | ||
| (−)-1(R)-N-methylcoclaurine | Leaves | Kashiwada et al., 2005 [40] |
| (−)-Lirinidine (5-demethylnuciferine) | Flower buds, stamen, and leaves | Nakamura et al., 2013 [33]; Paudel & Panth, 2015 [33] |
| (−)-Anonaine | Leaves | Wang et al., 2011 [70] |
| (−)-Asimilobine | Leaves | Wang et al., 2011 [70] |
| (−)-Caaverine | Leaves | Wang et al., 2011 [70] |
| (−)-N-Methylasimilobine | Leaves | Wang et al., 2011 [70] |
| (−)-nor-Nuciferine | Leaves | Wang et al., 2011 [70] |
| (−)-Nuciferine | Leaves | Wang et al., 2011 [70] |
| (−)-Roemerine | Leaves | Wang et al., 2011 [70] |
| (6R,6aR)-Roemerine-Nβ-oxide | Leaves | Ahn et al., 2013 [45] |
| (R)-Roemerine | Leaves | Agnihotri et al., 2008 [71] |
| 2-Hydroxy-1-methoxy-6a,7-dehydroaporphine | Flower buds and leaves | Nakamura et al., 2013 [66] |
| 3-Indoleacetic acid | Seeds | Rho and Yoon, 2017 [69] |
| 4′-Methyl-N-methylcoclaurine | Plumule | Zhou et al., 2013 [57] |
| 7-Hydroxydehydronuciferine | Leaves | Wang et al., 2011 [69] |
| Anisic acid | Seeds | Itoh et al., 2011 [55] |
| Anonaine | Leaves | Agnihotri et al., 2008 [71] |
| Anonaine | Stamen | Paudel & Panth, 2015 [33] |
| Armepavine | Plumule | Zhou et al., 2013 [57] |
| Armepavine | Stamen | Paudel & Panth, 2015 [33] |
| Asimilobine | Flower buds and leaves | Nakamura et al., 2013 [66] |
| Asimilobine | Stamen | Paudel & Panth, 2015 [33] |
| Cepharadione B | Leaves | Wang et al., 2011 [70] |
| cis-N-Coumaroyltyramine | Leaves | Ahn et al., 2013 [45] |
| cis-N-Feruloyltyramine | Leaves | Ahn et al., 2013 [45] |
| Coclaurine | Seeds | Kashiwada et al., 2005 [40] |
| d,l-Armepavine | Flower buds and leaves | Kashiwada et al., 2005 [40]; Nakamura et al., 2013 [65] |
| Dauricine | Seeds | Paudel & Panth, 2015 [33] |
| Dehydroanonaine | Stamen | Paudel & Panth, 2015 [33] |
| Dehydroemerine | Stamen | Paudel & Panth, 2015 [33] |
| Dehydronuciferine | Flower buds and leaves | Nakamura et al., 2013 [65] |
| Dehydronuciferine | Stamen | Paudel & Panth, 2015 [33] |
| Dehydroroemerine | Leaves | Agnihotri et al., 2008 [71] |
| Demethylcoclaurine | Stamen | Paudel & Panth, 2015 [33] |
| Higenamine | Plumule | Zhou et al., 2013 [57] |
| Higenamine 4′-O-β-d-glucoside | Plumule | Kato et al., 2015 [72] |
| Isoliensinine | Seeds, leaves, and stamen | Itoh et al., 2011 [55]; Kashiwada et al., 2005 [40]; Paudel & Panth, 2015 [33] |
| Liensinine | Seeds, leaves, and stamen | Itoh et al., 2011 [55]; Kashiwada et al., 2005 [40]; Paudel & Panth, 2015 [33] |
| Liriodenine | Leaves and stamen | Ahn et al., 2013 [45]; Wang et al., 2011 [70]; Paudel & Panth, 2015 [33] |
| Lotusine | Leaves and seeds | Kashiwada et al., 2005; Paudel & Panth, 2015 [33] |
| Lysicamine | Flower buds, leaves, and leaves | Wang et al., 2011 [70]; Nakamura et al., 2013 [66] |
| Neferine | Seeds and leaves | Kashiwada et al., 2005 [40]; Itoh et al., 2011 [55]; Paudel & Panth, 2015 [33] |
| N-methylasimilobine | Leaves | Agnihotri et al., 2008 [71] |
| N-Methylasimilobine | Flower buds, stamen, and leaves | Nakamura et al., 2013 [66]; Paudel & Panth, 2015 [33] |
| N-Methylcoclaurine | Stamen | Paudel & Panth, 2015 [33] |
| N-Methylisococlaurine | Stamen | Paudel & Panth, 2015 [33] |
| N-Norarmepavine | Stamen | Paudel & Panth, 2015 [33] |
| N-Nornuciferine | Flower buds and leaves | Nakamura et al., 2013 [65] |
| Norjuziphine | Flower | Morikawa et al., 2016 [73] |
| Nornuciferine | Leaves and stamen | Kashiwada et al., 2005 [40]; Paudel & Panth, 2015 [33] |
| Nuciferine | Flower buds, leaves, seeds, and leaves | Kashiwada et al., 2005 [40]; Agnihotri et al., 2008 [71]; Nakamura et al., 2013 [66]; Paudel & Panth, 2015 [33] |
| Nuciferine N-oxide | Flower buds and leaves | Nakamura et al., 2013 [66] |
| Oleracein E | Leaves | Ahn et al., 2013 [45] |
| O-Nornuciferine | Plumule | Zhou et al., 2013 [57] |
| Pronuciferine | Leaves | Ahn et al., 2013 [45]; Nakamura et al., 2013 [66] |
| Reserpine | Stamens and petals | Noysang and Boonmatit, 2019 [43] |
| Roemerin | Stamen, leaves, and plumule | Kashiwada et al., 2005 [40]; Zhou et al., 2013 [57]; Paudel & Panth, 2015 [33] |
| trans-N-Coumaroyltyramine | Leaves | Ahn et al., 2013 [45] |
| trans-N-Feruloyltyramine | Leaves | Ahn et al., 2013 [45] |
| Tryptophan | Seeds | Rho and Yoon, 2017 [69] |
| Flavonoids | ||
| (−)-Catechin | Leaves | Ahn et al., 2013 [45] |
| 5,7,3′,5′-Tetrahydroxyflavanone | Leaves | Ahn et al., 2013 [45] |
| Chrysoeriol 7-O-β-d-glucopyranoside | Leaves | Wang et al., 2008 [74]; Ahn et al., 2013 [45] |
| Elephantorrhizol | Leaves | Ahn et al., 2013 [45] |
| Epitaxifolin | Leaves | Ahn et al., 2013 [45] |
| Hyperoside | Leaves, and plumule | Wang et al., 2008 [74]; Kashiwada et al., 2005 [40]; Zhou et al., 2013 [57]; Liu et al., 2016 [75] |
| Isoquercitrin (Hirsutrin) | Receptacles, Stamen, plumule, and leaves | Wang et al., 2008 [73]; Kashiwada et al., 2005 [40]; Zhou et al., 2013 [57]; Paudel & Panth, 2015 [33]; Liu et al., 2016 [75] |
| Isorhamnetin | Leaves | Wang et al., 2008 [74] |
| Isorhamnetin 3-O-β-d-glucopyranoside | Receptacles and stamens | Mukherjee et al., 2009 [31]; Liu et al., 2016 [75] |
| Isorhamnetin 3-O-rutinoside | Leaves | Kihyun et al., 2009 [51] |
| Isorhamnetin 3-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside | Stamens | Mukherjee et al., 2009 [31] |
| Isoschaftoside | Seeds | Rho and Yoon, 2017 [69] |
| Kaempferol | Leaves and stamens | Ahn et al., 2013 [45]; Wang et al., 2008 [74]; Mukherjee et al., 2009 [31] |
| Kaempferol 3-O-robinobioside | Receptacles | Liu et al., 2016 [75] |
| Kaempferol 3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside | Stamens | Mukherjee et al., 2009 [31] |
| Kaempferol 3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucuronopyranoside | Stamens | Mukherjee et al., 2009 [31] |
| Kaempferol 3-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside | Stamens | Mukherjee et al., 2009 [31] |
| Kaempferol 3-O-β-d-galactopyranoside | Receptacles and stamens | Mukherjee et al., 2009 [31]; Liu et al., 2016 [75] |
| Kaempferol 3-O-β-d-glucopyranoside/astragalin | Receptacles, leaves, and stamens | Wang et al., 2008 [74]; Mukherjee et al., 2009 [31]; Ahn et al., 2013 [45]; Liu et al., 2016 [75] |
| Kaempferol 3-O-β-d-glucuronopyranoside | Stamens | Mukherjee et al., 2009 [31]; Paudel & Panth, 2015 [33] |
| Kaempferol 3-O-β-d-glucuronopyranosyl methylester | Stamens | Mukherjee et al., 2009 [31] |
| Kaempferol 7-O-β-d-glucopyranoside | Stamens | Mukherjee et al., 2009 [31] |
| Luteolin/luteolin glucoside | Leaves, plumule, and stamen | Ahn et al., 2013 [45]; Zhou et al., 2013 [57]; Paudel & Panth, 2015 [33] |
| Myricetin 3′,5′-dimethylether 3-O-β-d-glucopyranoside | Stamens | Mukherjee et al., 2009 [31] |
| Myricetin 3-O-galactoside | Receptacles | Liu et al., 2016 [75] |
| Myricetin 3-O-glucoside | Receptacles | Liu et al., 2016 [75] |
| Myricetin 3-O-glucuronide | Receptacles | Liu et al., 2016 [75] |
| Nelumboroside A | Stamens | Mukherjee et al., 2009 [31] |
| Nelumboroside B | Stamens | Mukherjee et al., 2009 [31] |
| Quercetin | Leaves, stamens and petals | Wang et al., 2008 [74]; Ahn et al., 2013 [45]; Paudel & Panth, 2015 [33]; Liu et al., 2016 [75]; Noysang and Boonmatit, 2019 [43] |
| Quercetin 3-O-glucuronide/Quercetin 3-O-β-d-glucuronide | Receptacles and leaves | Kashiwada et al., 2005 [40]; Kihyun et al., 2009 [51]; Liu et al., 2016 [75] |
| Quercetin 3-O-β-d-glucopyranoside | Leaves and stamens | Agnihotri et al., 2008 [71]; Mukherjee et al., 2009 [31]; Kihyun et al., 2009 [51]; Ahn et al., 2013 [45] |
| Quercetin 3-O-β-d-xylopyranosyl-(1→2)-β-d-galactopyranoside | Leaves | Kashiwada et al., 2005 [40] |
| quercetin-3-O-β-d-xylopyranosyl-(1→2)-β-d-glucopyranosyl glycoside | Leaves | Wang et al., 2008 [74] |
| Rutin | Leaves, stamens and petals | Kashiwada et al., 2005 [40]; Noysang and Boonmatit, 2019 [43] |
| Syringetin 3-O-glucoside | Receptacles | Liu et al., 2016 [75] |
| Taxifolin | Leaves | Ahn et al., 2013 [45] |
| Sterols and triterpenoids | ||
| 24(R)-Ethylcholest-6-ene-5α-ol-3-O-β-d-glucopyranoside | Leaves | Agnihotri et al., 2008 [71] |
| Stigmasta-4,22-dien-3-one | Leaves | Wang et al., 2011 [70] |
| Β-Sitostenone | Leaves | Wang et al., 2011 [70] |
| β-Sitosterol | Rhizome | Chaudhuri & Singh, 2009 [61] |
| β-Sitosterol-3-O-glucoside/β-Sitosterol-3-O-β-d-glucopyranoside | Rhizome, leaves, and stamens | Agnihotri et al., 2008 [71]; Chaudhuri & Singh, 2009 [62]; Mukherjee et al., 2009 [31] |
| Betulinic acid | Rhizome | Chaudhuri & Singh, 2009 [61] |
| α-Amyrin | Rhizome | Chaudhuri & Singh, 2009 [61] |
| Aliphatic open chain compounds | ||
| 10-Eicosanol | Leaves | Agnihotri et al., 2008 [71] |
| 3,7,11,15-Tetramethyl-1-hexadecen- 3-ol (isophytol) | Leaves | Agnihotri et al., 2008 [71] |
| 3,7,11,15- Tetramethyl-2-hexadecen-1-ol (trans-phytol) | Leaves | Agnihotri et al., 2008 [71] |
| 7,11,15-Trimethyl-2-hexadecanone | Leaves | Agnihotri et al., 2008 [71] |
| Nonacosan-10-ol | Leaves | Mukherjee et al., 2009 [31] |
| Triacontan-7-ol | Leaves | Mukherjee et al., 2009 [31] |
| Nonacosane-4,10-diol | Leaves | Mukherjee et al., 2009 [31] |
| Nonacosane-5,10-diol | Leaves | Mukherjee et al., 2009 [31] |
| Nonacosane-10,13-diol | Leaves | Mukherjee et al., 2009 [31] |
| Hentriacontane-12,15-diol | Leaves | Mukherjee et al., 2009 [31] |
| Tritriacontane-9,10-diol | Leaves | Mukherjee et al., 2009 [31] |
| Octadecanoic acid | Leaves | Mukherjee et al., 2009 [31] |
| Palmitic acid | Rhizome | Chaudhuri & Singh, 2009 [62] |
| Linoleic acid | Rhizome | Chaudhuri & Singh, 2009 [62] |
| 9E,12E,15E-Octadecatrienoic acid | Rhizome | Chaudhuri & Singh, 2009 [62] |
| Linalool | Stamen | Paudel & Panth, 2015 [33] |
| Tartaric acid | Leaves | Paudel & Panth, 2015 [33] |
| Gluconic acid | Leaves | Paudel & Panth, 2015 [33] |
| Acetic acid | Leaves | Paudel & Panth, 2015 [33] |
| Malic acid | Leaves | Paudel & Panth, 2015 [33] |
| Ginnol | Leaves | Paudel & Panth, 2015 [33] |
| Nonadecane | Leaves | Paudel & Panth, 2015 [33] |
| Succinic acid | Leaves | Paudel & Panth, 2015 [33] |
| Miscellaneous compounds | ||
| Dihydrophaseic acid | Seeds | Rho and Yoon, 2017 [69] |
| Dihydrophaseic acid 3′-O-β-d-glucopyranoside | Seeds | Rho and Yoon, 2017 [69] |
| Pheophytin-a (chlorophyll derivative) | Leaves | Wang et al., 2011 [70] |
| Aristophyll-C (chlorophyll derivative) | Leaves | Wang et al., 2011 [70] |
3. N. nucifera Extracts, Fractions and Pure Compounds in Cancer Research
3.1. Literature Search Methodology
3.2. Preclinical Studies (In Vitro and In Vivo)
3.2.1. Breast Cancer
3.2.2. Cervical Cancer
3.2.3. Colon Cancer
3.2.4. Esophageal Cancer
3.2.5. Eye Cancer
3.2.6. Gallbladder Cancer
3.2.7. Gastric Cancer
3.2.8. Head and Neck Cancers
3.2.9. Hematological Cancers
3.2.10. Laryngeal Cancer
3.2.11. Liver Cancer
3.2.12. Lung Cancer
| Materials Tested | Cell Lines Used | Conc. (Duratdion) | Anticancer Effects | Mechanisms | References |
|---|---|---|---|---|---|
| Breast cancer | |||||
| Aqueous rhizome extract | MDA-MB-231 (human breast cancer) | 1–1000 µg/mL (24 h) | Inhibited proliferation and migration | ↓MMP-2; ↓MMP-9 | Karki et al., 2008 [78] |
| Flavonoid-rich leaf extract | MCF-7 (human breast cancer) | 0.5–3 mg/mL (24 h) | Inhibited proliferation | ↑p16; ↑p21; ↑p27; ↓cyclin E; ↓cyclin D1; ↓CDK2; ↓CDK4; cell cycle arrest in G0/G1 phase; ↓cyclin D1/CDK4 complex; ↓cyclin E/CDK2 complex; ↑Rb-E2Fcomplex; ↓Fas | Yang et al., 2011 [79] |
| Aqueous leaf extract | MDA-MB-231 (human breast cancer) | 0.5–5 mg/mL (24 h) | Inhibited angiogenesis; inhibited proliferation; decreased migration rate; reduced cell invasion properties | ↓MMP2; ↑TIMP; ↓VEGF, ↓CTGF; ↓VEGFR2; ↓NF-κB p65; ↓PI3K-Akt-ERK; ↓RAS; ↓MEK; ↓ERK; ↓Akt | Chang et al., 2016 [80] |
| Aqueous and methanol leaf extracts | 4T-1 (mouse mammary carcinoma); MDA-MB-231 (human breast cancer) | 2–4 mg/mL (24 h) | Reduced cell viability and attenuated migration | ↑Apoptosis; ↓RhoA; ↓Rac1; ↓Cdc42; ↓ERK; ↓p38; ↓MAPK | Wu et al., 2017 [81] |
| Methanol and acetone leaf and flower extracts | MCF-7 (human breast cancer) | 6.25–100 µg/mL (24 h) | Inhibited proliferation and reduced viability | Not reported | Arjun et al., 2012 [82] |
| Methanol floral receptacle extract | MCF-7 (human breast cancer) | 200–600 µg/mL (24 h) | Induced cytotoxicity | Antioxidant activity | Krubha & Vasan, 2016 [83] |
| Isoliensinine, liensinine & neferine | MCF-10A (human breast cancer) | 5–20 μM (48 h) | Induced cell death | ↑Apoptosis; ↑oxidative stress; ↑p38; ↑MAPK; ↑JNK | Zhang et al., 2015 [84] |
| Neferine | MCF-7 (human breast cancer) | 2–20 mg/mL (48 h) | Inhibited proliferation; reduced cell viability | ↑Apoptosis; ↑caspase-3; ↑caspase-8; ↑caspase-9; ↑Bax; ↑p53; ↑p21; ↑E2F1; ↑Fas; ↑FasL; ↓Bcl-2; ↓Bcl-xL; ↓HIAP-1; ↓HIAP-2 | Yang et al., 2016 [85] |
| Neferine | MCF-7 (human breast cancer) | 0.039–100 μM (72 h) | Induced cytotoxicity | ↑R123 uptake; ↓P-gp | Kadioglu et al., 2017 [86] |
| Neferine | MCF-7 (human breast cancer) | 1–5 μM IC50 = 41.1 μM | Induced autophagy | ↑Ryr; ↑cytosolic Ca2+; ↑AMPk-mTOR; ↑GFP-LC3 puncta; ↑CXCR-4; ↑p-PERK; ↑PERK; ↑SQSTM; ↑Ulk-1 | Law et al., 2019 [87] |
| Neferine | MDA-MB-231 (human breast cancer) | 2–10 μM (24 h) | Reduced proliferation | ↑Apoptosis; ↓miR-374a; ↓PI3K; ↓Akt; ↓MEK; ↓ERK | Liu et al., 2019 [88] |
| Liensinine and nuciferine | MCF-7; MDA-MB-231 (human breast cancer) | 10–60 µM (24 h) | Inhibited proliferation | ↑Apoptosis; ↑Bax/Bcl-2; ↑caspase-3 | Kang et al., 2017 [89] |
| Liensinine | MCF-7; MDA-MB-231 (human breast cancer) | 20 µM (24 h) | Reduced viability | ↓Autophagy; ↓autophagosome-lysosome fusion; ↓recruitment of RAB7A to lysosomes; ↑mitochondrial fission; ↑DNM1L dephosphorylation; ↑DNM1L translocation | Zhou et al., 2015 [90] |
| Seco-neferine F | MDA-MB-231 (human breast cancer) | IC50: 39 µM (48 h) | Induced cytotoxicity | Not reported | Huang et al., 2021 [91] |
| Cervical cancer | |||||
| Ethanolic petal extract; (−)-lirinidine | HeLa (human cervical cancer) | PC50: 2–11 μM (24 h) | Displayed antiausterity activities | ↑Apoptosis; ↑caspase-3; ↓Bcl-2; ↓p-Akt; ↓p-mTOR | Maneenet et al., 2021 [67] |
| Isoliensinine | Caski, C33A, HeLa, SiHa (human cervical cancer) | 5–25 μM (24 h and 48 h) | Inhibited cell proliferation and colony formation | ↑Apoptosis; ↑G0/G1 phase arrest; ↑p21; ↑caspase-9; ↓Mcl-1; ↓CDK2; ↓cyclin E; ↓p-Akt; ↓GSK3α | Li et al., 2022 [92] |
| Neferine | HeLa, SiHa (human cervical cancer) | 5–50 μM (48 h) | Suppressed viability; induced cytotoxicity; reduced migration | ↑Apoptosis; ↑Bax; ↑cyt c; ↑cleaved-caspase-3; ↑cleaved-caspase-9; ↑PARP-cleavage; ↓Bcl-2; ↓procaspase-3; ↓procaspase-9; ↓TCTP; ↑beclin-1, ↑atg-4, ↑atg-5; ↑atg-12; ↑LC-3 activation; ↑P62/SQSTM1; ↑G1-G0 phase arrest; ↑ROS | Dasari et al., 2019 [93] |
| Colon cancer | |||||
| Ethanolic stamen extract | HCT-116 (human colon cancer) | 100–400 µg/mL (24 h) | Showed cytotoxic activity | ↑Apoptosis; ↑Fas; ↑FasL; ↑TRAIL; ↑DR4; ↑DR5; ↑caspase-3, ↑caspase-8; ↑caspase-9; ↑Bax; ↓Bcl-2; ↓Bcl-xL; ↓MMP-2; ↓MMP-9; ↑TIMP-1; ↑TIMP-2; ↓iNOS; ↓COX-2; ↓NF-κB; ↑IκBα | Zhao et al., 2017 [94] |
| Neferine | HCT-8 (human colon cancer) | 0.039–100 μM (72 h) | Reduced cell viability | ↑R123 uptake; ↓P-gp | Kadioglu et al., 2017 [86] |
| Neferine; isoliensinine | HCT-15 (human colon cancer) | 2–12 µM (24 h) | Showed cytotoxicity | ↑Apoptosis; ↑ROS; ↑p38; ↑MAPK; ↑Bax; ↑caspase-9; ↑caspase-3; ↑cleaved PARP; ↑membrane permeability; ↓Bcl2; ↑[Ca2+]; ↓∆ΨM | Manogaran et al., 2019 [95] |
| Nuciferine | CT26 (murine colon carcinoma); HT29 and HCT116 (human colon cancer) | 0.05–1.0 mg/mL (24 h) | Reduced cell viability and inhibited cell invasion | ↓PI3K; ↓IL1B; ↓p-Akt (CT26 cells only) | Qi et al., 2016 [96] |
| Liensinine | HT29, DLD-1 (human colorectal cancer) | 5–20 µM (24–48 h) | Suppressed cell proliferation | ↑Apoptosis; ↑cleaved caspase-3; ↑cleaved PARP; ↑G2/M cell arrest; ↑p-CDK1; ↑cyclin A2; ↑p-JNK; ↑Bax; ↓Bcl-2; ↓Bcl-xL | Wang et al., 2018 [97] |
| Hyperoxide; rutin | HT29 (human colorectal cancer) | 100–200 µM (24 h) | Exhibited cytotoxicity, reduced cell viability and inhibited cell proliferation | ↑Apoptosis; ↑Bax; ↓Bcl-2; ↑Bax/Bcl-2 ratio; ↑caspase-3; ↑caspase-8; ↑caspase-9 | Guon and Chung, 2016 [98] |
| Esophageal cancer | |||||
| Neferine | KYSE30, KYSE150 and KYSE510 (human esophageal squamous cell carcinoma) | 5–30 µM (24–48 h) | Suppressed cell proliferation and colony formation | ↑Apoptosis; ↑G2/M arrest; ↑cleaved PARP; ↑cleaved caspase-3; ↑cleaved caspase-9; ↑p21; ↓cyclin B1; ↓Bcl-2; ↑ROS; ↑p-JNK; ↓Nrf2 | An et al., 2020 [99] |
| Eye cancer | |||||
| Neferine | WERI-Rb-1 (human retinoblastoma) | 0.1 to 200 μM (24 h) | Inhibited cell proliferation, migration, and viability | ↓Ki-67; ↓Survivin; ↓microtubule-like structures; ↓nodes/HPF; ↓VEGF; ↓SOD; ↓GSH; ↑MDA; ↓Bcl-2; ↓c-myc; ↑Bax; ↑cleaved caspase-3; ↑cleaved caspase-9 | Wang et al. 2020 [100] |
| Gallbladder cancer | |||||
| Liensinine | GBC-SD and NOZ (human gallbladder carcinoma) | 40–120 μM (24 and 48 h) | Inhibited proliferation and suppressed colony formation | ↑Apoptosis; ↑G2/M phase arrest; ↓cyclin B1; ↓CDK1; ↓CDC25C; ↑cleaved-caspase 3; ↑cleaved-caspase 9; ↑cleaved-PARP; ↑Bax; ↓Bcl-2; ↓PI3K; ↓ZFX; ↓p-Akt | Shen et al., 2019 [101] |
| Gastric cancer | |||||
| Water-soluble polysaccharides from seeds | MFC (human gastric cancer) | 50–200 μg/mL (48 h) | Showed growth inhibition | Not reported | Zheng et al., 2016 [102] |
| Neferine | Adriamycin resistant SGC7901/ADM (human gastric cancer) | 2.5–40 μg/mL (24 h) | Exerted cytotoxicity and reversed drug resistance | ↓P-gp expression; ↓MDR-1 mRNA | Huang et al., 2011 [103] |
| Neferine | GIST-1 (human gastrointestinal stromal tumor) | 1–10 µM (24 h) | Inhibited cell viability, proliferation and migration | ↑Apoptosis; ↑p15; ↑p16; ↑p21; ↓cyclinD1; ↑Bax; ↓Bcl-2; ↑cleaved caspase-3; ↑cleaved caspase-9; ↓MMP-2; ↓MMP-9;↑miR-449a; ↓p-PI3K; ↓p-Akt; ↓Notch1; ↓Notch2; ↓Notch3 | Xue et al., 2019 [104] |
| 7-Hydroxydehydronuciferine | AGS (human gastric cancer) | IC50: 62.9 μM (duration not specified) | Inhibited cell proliferation | Antioxidant activity | Liu et al., 2014 [105] |
| Liensinine | BGC823 and SGC7901(human gastric cancer) | 40–80 µM (48 h) | Inhibited cell proliferation | ↑Apoptosis; ↑cleaved caspase-3; ↑cleaved caspase-9; ↑cleaved PARP; ↓p-Akt; ↓Bcl-2; ↑Bax; ↑ROS; ↑G0/G1 cell arrest; ↓cyclin D1; ↓CDK4 | Yang et al. 2019 [106] |
| Head and neck cancers | |||||
| Neferine | HN6, CAL27 (tongue squamous cell carcinoma), HN30 (pharyngeal squamous cell carcinoma) | 7.5–30 µM (24 h) | Reduced cell viability and inhibited colony formation | ↑Apoptosis; ↑G1 arrest; ↓Bcl-1; ↑Bax; ↑ROS; ↑autophagosome formation; ↑LC3; ↑p62; ↑p-JNK; ↑p-ASK1 | Zhu et al., 2021 [107] |
| Hematological cancers | |||||
| Kaempferol (from methanolic stamen extract) | KU81F (chronic myelocytic leukemia) | 3.5–35 µM (24 h) | Did not display cytotoxic effect | ↓FcεR1 expression; ↓FcεR1 α- and γ-chains; ↓intracellular Ca2+; ↓histamine release | Shim et al., 2009 [108] |
| Neferine | Imatinib-resistant K562/G01 cells (human myelogenous leukemia) | 4–64 µM (48 h) | Reduced cell survival rate and reversed drug resistance | ↓P-gp expression; ↓MDR-1 mRNA | Qin et al., 2011 [109] |
| Laryngeal cancer | |||||
| Nuciferine | AMC-HN-8, TU-212 (Laryngeal squamous cell carcinoma) | 25–100 µM (24 h) | Inhibited cell survival | ↓TRIM44; ↓TLR4; ↓Akt signaling | Li et al., 2021 [110] |
| Liver cancer | |||||
| Water-soluble polysaccharides from seeds | HuH-7 (human liver cancer); H22 (mouse hepatocarcinoma) | 50–200 μg/mL (48 h) | Inhibited cell proliferation | Not reported | Zheng et al., 2016 [102] |
| Polyphenolic seed extract | HepG2 (human liver cancer) | 6.25–50 µg/mL (24–48 h) | Showed cytotoxicity | Antioxidant activity | Shen et al., 2019 [111] |
| Procyanidins from seedpod extract | HepG2 (human liver cancer) | 12.5–400 μg/mL (6–96 h) | Decreased viability and inhibited cell proliferation | ↑Autophagy; S phase arrest; ↑LC3; ↑GFP-LC3; ↑ROS | Duan et al., 2016 [112] |
| Neferine | HepG2 (human hepatocarcinoma) | 10–40 µM (24 h) | Reversed thermotolerance of tumor cells | ↑Apoptosis; ↓Bcl-2 | Xiao-Hong et al., 2007 [113] |
| Neferine | Hep3B (human liver cancer); Sk-hep-1 (human hepatic adenocarcinoma) | 5–30 µM (24 h) | Induced growth inhibition and decreased cell viability | ↑Apoptosis; ↓c-Myc; ↓cyclin D1; ↓cyclin D3; ↓CDK4; ↓E2F-1; ↑Bim; ↑Bid; ↑Bax; ↑Bak; ↑Puma; ↑caspase-3; ↑caspase-6; ↑caspase-7; caspase-8; ↑PARP | Yoon et al., 2013 [114] |
| Neferine | HepG2 (human liver cancer) | 2–25 µM (48 h) | Reduced cell viability | ↑Apoptosis; ↑Bax; ↑Bad; ↑cleaved caspase-3; ↑caspase-9; ↑PARP; ↓Bcl2; ↑p53, ↑PTEN; ↓p-Akt; ↑TNF-α; ↑p38; ↑ERK1/2 MAP kinases; ↑ROS | Poornima et al., 2013 [115] |
| Neferine | HepG2 (human liver cancer) | 2.5–100 µM (24–48 h) | Exhibited cytotoxicity and suppressed migration and invasion | ↑Apoptosis; ↑E-cadherin; ↓Vimentin; ↓Snail; ↓N-cadherin | Deng et al., 2017 [116] |
| Neferine | HepG2, Hep3B (human liver cancer) | 1–5 µM (2 weeks) | Induced cytotoxicity | ↑Autophagy; ↑Ryr; ↑cytosolic [Ca2+]; ↑Ulk-1-PERK; ↑AMPK-mTOR | Law et al., 2019 [87] |
| Isoliensinine | HepG2, Huh-7 and H22 (human liver cancer) | 3–10 µg/mL (24–48 h) | Inhibited cell proliferation | ↑Apoptosis; ↑sub-G1 DNA; ↑caspase-3; ↓Bcl-2; ↓Bcl-xL; ↓MMP-9; ↓NF-κB activity; ↓p65 phosphorylation; ↑p65/PP2A binding | Shu et al., 2015 [117]; Shu et al., 2016 [118] |
| Lung cancer | |||||
| Ethanolic seed pod extract | A549, H460 (human non-small cell lung cancer) | 10–80 µM (24, 48 h) | Inhibited cell proliferation and colony formation | ↑Apoptosis; ↑cleaved PARP; ↑γ-H2AX; ↓Axl | Kim et al., 2021 [121] |
| Neferine | A-549 (human lung carcinoma) | 1–30 µM (12–72 h) | Inhibited cell proliferation | ↑Autophagy; ↓PI3K; ↓Akt; ↓mTOR; ↑ROS; ↓GSH | Poornima et al., 2013 [122] |
| Neferine | A-549, H520, H661, H44 (human lung carcinoma) | 1–30 µM (48 h) | Reduced cell viability | ↑Apoptosis; G1 cell cycle arrest; ↓Bcl-2; ↑Bax; ↑Bad; ↑cyt c; ↑cleaved caspase-3; ↑cleaved caspase-9; ↓ΔΨM; ↑p53; ↑p27; ↓cyclin D1; ↓NF-κB; ↑PTEN; ↑p-JNK; ↑p-ERK1/2; ↑p-p38; ↓GSH; ↓SOD; ↓CAT; ↓GPx; ↓GST;↑[Ca2+] | Poornima et al., 2014 [123] |
| Neferine | A549 (human lung carcinoma) | 0.039–100 μM (72 h) | Reduced cell viability | ↑R123 uptake; ↓P-gp | Kadioglu et al., 2017 [86] |
| Neferine | A549, H1299, LLC-1 (human lung cancer cells) | 10 µM (4 h) | Suppressed cell growth | ↑Autophagy; ↑LC3-II | Law et al., 2019 [87] |
| Neferine Neferine+cisplatin | A549 (human lung cancer cell) | 10 µM (48 h) | Induced cytotoxicity | ↑Autophagy; ↓GSH; ↑ROS; ↑LC3-II; ↓PI3K; ↓Akt; ↓mTOR | Kalai Selvi et al., 2017 [124] |
| Neferine Neferine + cisplatin | A549 (human lung cancer cell) | 10 µM (12–72 h) | Inhibited proliferation and reduced cell viability | ↑Apoptosis; ↑LDH leakage; ↑NO release; sub-G1 cell cycle arrest; ↓Bcl-2; ↑Bax; ↑Bad; ↑cyt c; ↑cleaved caspase-3; ↑cleaved caspase-9; ↑cleaved PARP; ↑p53; ↑ROS; ↓FAK; ↓VGEF; ↓MMP-2 | Sivalingam et al., 2017 [125] |
| Nuciferine | A549 (human lung adenocarcinoma) | 10–50 µM (24 h) | Exhibited antiproliferative activity and suppressed tumor cell invasion and migration | ↑Apoptosis; ↓Bcl-2; ↑Bax; ↓Wnt/β-catenin signaling; ↑Axin; ↓c-myc; ↓cyclin D; ↓VEGF-A | Liu et al., 2015 [126] |
| Nuciferine | A549 and NCI-H1650 (human lung adenocarcinoma) | 0.05–1.0 mg/mL (24 h) | Reduced cell viability and inhibited cell invasion | Not reported | Qi et al., 2016 [96] |
| Nasopharyngeal cancer | |||||
| Alkaloids from seeds | CNE-1 (human nasopharyngeal carcinoma) | 50–200 µg/mL (24 h) | Reduced cell proliferation | ↑Apoptosis; ↑caspase-3; ↑caspase-8; ↑caspase-9; ↑Bax; ↑Fas;↑FasL; ↓Bcl-2; ↓Bcl-xL; ↓NF-κB; ↑IκB-α | Zhao et al., 2016 [128] |
| Neural cancer | |||||
| Neferine | IMR32 (human neuroblastoma) | 1–30 µM (24 h) | Suppressed proliferation and migration | ↑Apoptosis; G2/M phase arrest; caspase-3 cleavage; PARP cleavage; ↑autophagy; ↑LC3-II; ↑Beclin-1; ↓p-FAK; ↓p-S6K1 | Pham et al., 2018 [129] |
| Nuciferine | SY5Y (human neuroblastoma) | 0.05–1.0 mg/mL (24 h) | Reduced cell viability and suppressed cell invasion | ↓PI3K; ↓IL1B; ↓p-Akt | Qi et al., 2016 [96] |
| Nuciferine | U87MG, U251 (human glioblastoma) | 20–180 µM (24–72 h) | Inhibited cell proliferation, colony formation, mobility, invasion, migration, and epithelial-to-mesenchymal transition | ↑Apoptosis; ↑Bax; ↓Bcl-2; ↓HIF1A; ↓VEGFA; ↑G2 cell cycle arrest; ↓Slug; ↓CDC2; ↑cyclin B1; ↓Vimentin; ↓N-cadherin; ↑E-cadherin; ↓SOX2; ↓p-Akt; ↓p-STAT3 | Li et al., 2019 [130] |
| Ovarian cancer | |||||
| Neferine | A2780, HO8910; SKOV3 (human ovarian cancer) | 1–10 µM (24–72 h) | Exhibited cytotoxic and growth-inhibitory effects | G1 cell cycle arrest; ↓cyclin D; ↑p27; ↑p21; ↑apoptosis; ↑autophagy; ↑LC3-II; ↑Atg7; ↓p-p70S6K; ↓p-4EBP1; ↑p-p38 MAPK; ↑p-JNK | Xu et al., 2016 [131] |
| Prostate cancer | |||||
| Polyphenolic seed extract | LNCaP (human prostate adenocarcinoma) | 6.25–50 μg/mL (24 h & 48 h) | Exhibited antiproliferative activity | Antioxidant effects | Shen et al., 2019 [111] |
| Nuciferine, 7-hydroxydihydro-nuciferine, caaverine, liridodenine & anonaine | DU-145 (human prostate cancer) | IC50 = 80.8–218.4 μM (24 h) | Induced cytotoxicity | Not reported | Liu et al., 2014 [105] |
| Neferine | PC3; LNCaP (human prostate cancer); CD 44+ PC3 CSC (cancer stem cells) | 3.12–100 µM (24–72 h) | Reduced cell proliferation and inhibited migration | ↑Apoptosis; ↑G1 phase cell cycle arrest; ↓Bcl-2; ↓CDK4; ↑caspase-3; ↑cleaved PARP; ↑p21; ↑p27; ↑p53; ↓MMP-9; ↓Slug; ↓Snail; ↓SOD1; ↓CAT; ↓GPx | Erdogan & Turkekul, 2020 [132] |
| Neferine | DU145 and LNCaP (human prostate cancer) | 5–20 µM (18 h) | Reduced cell viability | ↑Apoptosis; ↑autophagy; ↓p62; ↑LC3B-II; ↑autophagosome formation; ↑p-JNK | Nazim et al., 2020 [133] |
| Neferine, liensinine, isoliensinine | LNCaP; PC3; DU-145 (human prostate cancer) | 1–100 µM (24 and 48 h) | Induced cytotoxicity and reduced migration in PC3 and DU145 cells | ↑Apoptosis; ↑autophagy; ↑Bax; ↓Bcl-2; ↑cleaved-caspase-9; ↑cleaved-PARP; ↓PARP; ↑LC3B-II; ↑AR; ↑PSA; ↑5-α reductase | Liu et al., 2021 [134] |
| Renal cancer | |||||
| Neferine | Caki-1 (human renal cancer) | 5–25 µM (24 h) | Inhibited cell proliferation | ↑Apoptosis; ↓Bcl-2; ↑Bax; ↓XIAP; ↓sub-G1 cell population; ↓NF-κB-dependent luciferase activity; ↓p65 | Kim et al., 2019 [135] |
| Sarcoma | |||||
| Neferine | U2OS and Saos-2 (human osteosarcoma) | 1–20 µM (24–72 h) | Inhibited cell proliferation | ↑G1 arrest; ↓cyclin E; ↑p21; ↑p38 MAPK; ↑JNK | Zhang et al., 2012 [136] |
| Skin cancer | |||||
| Aqueous rhizome extract | A431 (epidermoid cancer) | 1–1000 µg/mL (24 h) | Inhibited proliferation and migration | ↓MMP-2; ↓MMP-9 | Karki et al., 2008 [78] |
| Methanolic extracts from flower bud and leaves | B16 melanoma 4A5 cells (murine melanoma) | 3–30 μM (72 h) | Inhibited melanogenesis | ↓Tyrosinase; ↓TRP-1; ↓TRP-2 | Nakamura et al., 2013 [66] |
| Procyanidin extract from seedpod | B16 (murine melanoma) | 25–100 μg/mL (1–5 days) | Displayed cytotoxicity and inhibited cell proliferation | ↑Apoptosis; ↑S cell cycle arrest; ↑calcium | Duan et al., 2010 [137] |
| Leaf extract; gallic acid | B16F1 (murine melanoma) | NLE: 0.1–0.5 mg/mL GA: 60–100 µM (24–72 h) | Reduced melanogenesis | ↓Tyrosinase; ↓MITF; TRP-1; ↓p-PKA; ↓p-CREB; ↓melanin | Lai et al., 2020 [138] |
| 7-Hydroxydehydronuciferine | A375.S2 (human melanoma) | 10–100 µM (24 h) | Inhibited cell proliferation and showed cytotoxicity | ↑Apoptosis | Liu et al., 2014 [105] |
| 7-Hydroxydehydronuciferine | A375.S2, A375 and A2058 cells (human melanoma) | 10–100 μM (24 h) | Induced cytotoxicity and reduced migration | ↑Apoptosis; ↑autophagy; G2/M arrest; ↑ATG-5; ↑ATG-12; ↑ATG-16; ↑AVO | Wu et al., 2015 [139] |
| Materials Tested | Animal Tumor Models | Anticancer Effects | Mechanisms | Dose (Route) | Duration | References |
|---|---|---|---|---|---|---|
| Breast cancer | ||||||
| Flavonoid-rich leaf extract | BALB/c athymic nude mice injected with MCF-7 cells | Reduced tumor volume and weight | ↓HER2; p-HER2; ↓Fas | 0.5 & 1% (diet) | 28 days | Yang et al., 2011 [79] |
| Aqueous leaf extract | MDA-MB-231 cells injected in female C57BL/6 nude mice | Inhibited tumor growth | Not reported | 0.5–2 % (s.c.) | 14 days | Chang et al., 2016 [80] |
| Liensinine + doxorubicin | Female nude mice injected with MDA-MB-231 cells | Reduced tumor growth | ↑Apoptosis; ↑cleaved caspase-3; ↓autophagy/mitophagy; ↑auto-phagosome /mitophagosome; ↑colocalization of DNM1L and TOMM20 | 60 mg/kg (i.p.); 2 mg/kg (i.p.) | 30 days | Zhou et al., 2015 [90] |
| Colon cancer | ||||||
| Nuciferine | CT29 cells subcutaneously implanted in nude mice | Reduced tumor weight | Not reported | 9.5 mg/kg (i.p.) | 3 times a week for 3 weeks | Qi et al., 2016 [96] |
| Liensinine | HT29 cells injected in female BALB/c nude mice | Suppressed colorectal tumorigenesis, reduced tumor size | ↓Ki-67 | 30 mg/kg (oral) | Every other day for 15 days | Wang et al., 2018 [97] |
| Eye cancer | ||||||
| Neferine | WERI-Rb-1 cells injected in female athymic nude mice | Reduced tumor volume and weight | ↓Ki-67; ↓VEGF; ↓SOD; ↑MDA | 0.5–2 mg/kg (i.p) | Every 3 days for 30 days | Wang et al., 2020 [100] |
| Gallbladder cancer | ||||||
| Liensinine | NOZ cells injected in BALB/c nude mice | Reduced tumor volume and weight | ↓Ki-67 | 2 mg/kg (i.p) | Every 2 days | Shen et al., 2019 [101] |
| Gastric cancer | ||||||
| Liensinine from seeds | SGC7901 cells injected in BALB/c homozygous (nu/nu) nude mice | Reduced tumor size | ↓Ki-67 | 10 µM (i.p.) | Every 2 days for a month | Yang et al., 2019 [106] |
| Head and neck cancers | ||||||
| Neferine | CAL27 cells injected in male BALB/c nude mice | Reduced tumor volume | ↑Apoptosis; ↑autophagy, ↑cleaved caspase-3, ↑cleaved PARP1, ↑LC3; ↑p62 | 10 mg/kg (i.p) | Not reported | Zhu et al., 2021 [107] |
| Liver cancer | ||||||
| Water-soluble polysaccharides from seeds | H22 cells injected in female Kunming mice | Reduced tumor weight | ↑TNF-ɑ; ↑IL-2; ↑SOD; ↓MDA | 50–200 mg/kg (oral) | 14 days | Zheng et al., 2016 [102] |
| Leaf extract | DEN fed male Sprague-Dawley rats | Reduced tumor size | ↓AST; ↓ALT; ↓albumin; ↓total triglyceride; ↓total cholesterol; ↓lipid peroxidation; ↑GSH; ↑GSHPx; ↑SOD; ↑CAT; ↑GST; ↓Rac1; ↓PKCɑ; ↓TNF-ɑ; ↓IL-6 | 0.5–2.0% (p.o.) | 12 weeks | Horng et al., 2017 [119] |
| Leaf extract | 2-AAF-induced male Wistar rats | Inhibited hepatic fibrosis and hepatocarcinogenesis | ↓Triglycerides; ↓total cholesterol; ↓AFP; ↓IL-6; ↓TNF-ɑ; ↓AST; ↓ALT; ↓γGT; ↓GST-Pi; ↓lipid peroxidation; ↓8-OHdG; ↑Nrf2; ↑CAT; ↑GPx; ↑SOD-1 | 0.5–2% in the diet (p.o.) | 6 months | Yang et al., 2019 [120] |
| Neferine+oxaliplatin | HepG2 and Bel-7402 cells injected in male BALB/c mice | Increased tumor volume reducing the effect of oxaliplatin | ↑E-cadherin; ↓Vimentin; ↓Ki-67; | 20 mg/kg/d (i.p.) | 3 weeks | Deng et al., 2017 [116] |
| Isoliensinine | Huh-7 cells injected in male athymic nude mice and H22 cells injected in Kunming mice | Reduced tumor volume | ↑caspase-3; ↓Bcl-2; ↓Bcl-xL; ↓MMP-9; ↓p65 phosphorylation | 3 and 10 mg/kg/d (i.p. and gavage) | 10 days; 3 weeks | Shu et al., 2015 [117] |
| Isoliensinine | Huh-7 cells transfectants injected in male athymic nude mice | Reduced tumor growth | ↑Caspase-3 activity | 10 mg/kg/d (gavage) | 20 days | Shu et al., 2016 [118] |
| Lung cancer | ||||||
| Leaf extract and leaf polyphenol extract | 4T-1 metastatic tumor in the lung of BALB/c mice | Reduced metastasis and tumor weight | ↓PKCɑ activation | 0.25, 1% (p.o.) | 19 days | Wu et al., 2017 [81] |
| Nuciferine | A549 cells injected in BALB/c mice | Reduced tumor size and weight | ↑Apoptosis; ↓Bcl-2; ↑Bax; ↓Wnt/β-catenin; ↑Axin | 50 mg/kg (i.p.) | 3 times a week for 20 days | Liu et al., 2015 [126] |
| Neferine | DEN-induced lung carcinogenesis in albino male Wistar rats | Suppressed tumor growth | ↓ROS; ↓lipid peroxidation; ↓protein carbonyl; ↑GSH; ↑SOD; ↑GPx; ↑GST; ↑CAT; ↓glycoprotein components; ↑ATPase; ↑p53; ↑Bax; ↑caspase-9; ↑caspase-3; ↓Bcl-2; ↓COX-2; ↓NF-κB; ↓CYP2E1; ↓VEGF; ↓PI3K; ↓Akt; ↓mTOR | 10–20 mg/kg (oral) | 20 alternate days | Sivalingam et al., 2019 [127] |
| Neural cancer | ||||||
| Nuciferine | SY5Y cells subcutaneously implanted in nude mice | Reduced tumor weight | Not reported | 9.5 mg/kg (i.p.) | 3 times a week for 3 weeks | Qi et al., 2016 [96] |
| Nuciferine | U251 cells subcutaneously inoculated in BALB/c nude mice | Suppressed tumor weight and size | ↓Ki-67; ↓CDC2; ↓Bcl-2; ↓HIF1A; ↓N-cadherin; ↓VEGFA | 15 mg/kg (i.p.) | Once a day for 2 weeks | Li et al., 2019 [130] |
| Skin cancer | ||||||
| Procyanidin extract from seedpod | B16 cells inoculated into syngeneic C57BL/6 J mice | Suppressed tumor volume and weight | ↓lipid peroxidation levels; ↑SOD; ↑CAT; ↑GSPx; ↑spleen and thymus index | 60–120 mg/kg (i.g.) | Every 2–3 days for 15 days | Duan et al., 2010 [137] |
| Leaf extract | UV-radiation exposed female guinea pigs | Reversed UVB-induced epidermal hyperplasia and hyperpigmentation | ↓MITF; ↓tyrosinase; ↓TRP-1; ↓PKA; ↓ERK; ↓melanin | 1–2% (topical) | 2 weeks | Lai et al., 2020 [138] |
| 7-Hydroxy-dehydronuciferine | A375.S2 cells injected in BALB/c nu/nu female mice | Reduced tumor volume | Not reported | 20 mg/kg (i.p.) | Every 7 days for 28 days | Wu et al., 2015 [139] |
3.2.13. Nasopharyngeal Cancer
3.2.14. Neural Cancer
3.2.15. Ovarian Cancer
3.2.16. Prostate Cancer
3.2.17. Renal Cancer
3.2.18. Sarcoma
3.2.19. Skin Cancer
4. Bioavailability and Pharmacokinetics of N. nucifera Constituents
5. Toxicity Studies on N. nucifera
6. Conclusions, Current Challenges/Limitations and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-AAF | 2-acetylaminofluorene |
| ADM | adriamycin |
| AFP | α-fetoprotein |
| ALT | alanine aminotransferase |
| AMPK | 5′-adenosine monophosphate-activated protein kinase |
| ASK1 | apoptosis signal-regulating kinase 1 |
| AST | aspartate transaminase |
| AVO | acidic vesicular organelles |
| Bax | Bcl-associated X protein |
| Bcl-2 | B cell lymphoma 2 |
| Bcl-xL | B-cell lymphoma-extra-large |
| CAT | catalase |
| CDK | cyclin-dependent kinase |
| c-Myc | cellular myelocytomatosis |
| COX-2 | cyclooxygenase-2 |
| CREB | cAMP response element-binding protein |
| CSC | cancer stem cells |
| CTGF | connective tissue growth factor |
| cyt c | cytochrome c |
| CYP | cytochrome P450 |
| DEN | diethylnitrosamine |
| DHEA | dehydroepiandrosterone |
| DNM1L | dynamin-1-like protein |
| DR | death receptor |
| E2F1 | E2F transcription factor 1 |
| ERK | extracellular signal-regulated kinase |
| FasL | Fas ligand |
| GC-MS | gas chromatography-mass spectrometry |
| GFP | green fluorescent protein |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| GSTπ | glutathione S-transferase pi |
| γGT | γ-glutamyl transferase |
| γ-H2AX | H2A histone family member X |
| HER2 | human epidermal growth factor receptor 2 |
| HIAP | human inhibitor of apoptosis protein |
| HPLC | high-performance liquid chromatography |
| IκBα | inhibitor of κBαi |
| IL-1β | interleukin-1β |
| iNOS | inducible nitric oxide synthase |
| i.p. | intraperitoneal |
| JNK | c-Jun N-terminal kinase |
| LC3 | light-chain 3 |
| MAPK | mitogen activated protein kinase |
| MDA | malondialdehyde |
| MDR-1 | multidrug resistance 1 |
| MEK | mitogen-activated extracellular signal-regulated kinase |
| MITF | microphthalmia-associated transcription factor |
| MMP | matrix metalloproteinase |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor-κB |
| NLE | N. nucifera leaf extract |
| NLPE | N. nucifera leaf polyphenol extract |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| PARP | poly (ADP-ribose) polymerase |
| P-gp | P-glycoprotein |
| PI3K | phosphoinositide-3-kinase |
| PKA | protein kinase A |
| PKCα | protein kinase Cα |
| PP2A | protein phosphatase 2A |
| PRISMA | preferred reporting items for systematic reviews and meta-analysis |
| PTEN | phosphatase and tensin homolog |
| R123 | rhodamine 123 |
| ROS | reactive oxygen species |
| RAB7A | Ras-related protein Rab-7a |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| Ryr | ryanodine receptor |
| SOD | superoxide dismutase |
| TIMP-2 | tissue inhibitor of metalloproteinase-2 |
| TLR4 | Toll-like receptor 4 |
| TNF-α | tumor necrosis factor-α |
| TOMM20 | translocase of outer mitochondrial membrane 20 |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand |
| TRIM44 | tripartite motif-containing 44 |
| TRP | tyrosine related protein |
| Ulk-1-PERK | Unc-51-like autophagy activating kinase-1-protein kinase RNA-like ER kinase |
| UVB | ultra-violet B |
| VEGF | vascular endothelial growth factor |
| WADA | Word Anti-Doping Agency |
| XIAP | X-linked inhibitor of apoptosis |
| ZFX | zinc finger X-chromosomal protein |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Tseng, M. Diet, cancer and public health nutrition. Public Health Nutr. 2009, 12, 737–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittelman, S.D. The Role of Diet in Cancer Prevention and Chemotherapy Efficacy. Annu. Rev. Nutr. 2020, 40, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Wang, L.; Goh, B.C.; Ahn, K.S.; Bishayee, A.; Sethi, G. Modulation of diverse oncogenic transcription factors by thymoquinone, an essential oil compound isolated from the seeds of Nigella sativa Linn. Pharmacol. Res. 2018, 129, 357–364. [Google Scholar] [CrossRef]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, V.; Tuli, H.S.; Tania, M.; Srivastava, S.; Ritzer, E.E.; Pandey, A.; Aggarwal, D.; Barwal, T.S.; Jain, A.; Kaur, G.; et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. Semin. Cancer Biol. 2020; in press. [Google Scholar] [CrossRef]
- De Greef, D.; Barton, E.M.; Sandberg, E.N.; Croley, C.R.; Pumarol, J.; Wong, T.L.; Das, N.; Bishayee, A. Anticancer potential of garlic and its bioactive constituents: A systematic and comprehensive review. Semin. Cancer Biol. 2021, 73, 219–264. [Google Scholar] [CrossRef] [PubMed]
- Mirza, B.; Croley, C.R.; Ahmad, M.; Pumarol, J.; Das, N.; Sethi, G.; Bishayee, A. Mango (Mangifera indica L.): A magnificent plant with cancer preventive and anticancer therapeutic potential. Crit. Rev. Food Sci. Nutr. 2021, 61, 2125–2151. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. A Systematic Review of the Preventive and Therapeutic Effects of Naringin against Human Malignancies. Front. Pharmacol. 2021, 12, 639840. [Google Scholar] [CrossRef]
- Jamieson, S.; E Wallace, C.; Das, N.; Bhattacharyya, P.; Bishayee, A. Guava (Psidium guajava L.): A glorious plant with cancer preventive and therapeutic potential. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A Broccoli Bioactive Phytocompound with Cancer Preventive Potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef]
- Mondal, A.; Banerjee, S.; Bose, S.; Das, P.P.; Sandberg, E.N.; Atanasov, A.G.; Bishayee, A. Cancer Preventive and Therapeutic Potential of Banana and Its Bioactive Constituents: A Systematic, Comprehensive, and Mechanistic Review. Front. Oncol. 2021, 11, 697143. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.L.; Strandberg, K.R.; Croley, C.R.; Fraser, S.E.; Venkata, K.C.N.; Fimognari, C.; Sethi, G.; Bishayee, A. Pomegranate bioactive constituents target multiple oncogenic and oncosuppressive signaling for cancer prevention and intervention. Semin. Cancer Biol. 2021, 73, 265–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, X. Colored Illustration of Lotus Cultivars in China; China Forestry Publishing House: Beijing, China, 2005. [Google Scholar]
- Ming, R.; VanBuren, R.; Liu, Y.; Yang, M.; Han, Y.; Li, L.-T.; Zhang, Q.; Kim, M.-J.; Schatz, M.C.; Campbell, M.; et al. Genome of the long-living sacred lotus (Nelumbo nucifera Gaertn.). Genome Biol. 2013, 14, R41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Han, Y.; Xu, L.; Zhao, J.; Liu, Y. Comparative analysis of genetic diversity of lotus (Nelumbo) using SSR and SRAP markers. Sci. Hortic. 2012, 142, 185–195. [Google Scholar] [CrossRef]
- Deng, J.; Chen, S.; Yin, X.; Wang, K.; Liu, Y.; Li, S.; Yang, P. Systematic qualitative and quantitative assessment of anthocyanins, flavones and flavonols in the petals of 108 lotus (Nelumbo nucifera) cultivars. Food Chem. 2013, 139, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.A. Ethno-medicinal uses and pharmacological activities of lotus (Nelumbo nucifera). J. Med. Plants Stud. 2014, 2, 42–46. [Google Scholar]
- Zhang, X.; Chen, L.; Wang, Q. New Lotus Flower Cultivars in China; China Forestry Publishing House: Beijing, China, 2011. [Google Scholar]
- Zhang, Y.; Lu, X.; Zeng, S.; Huang, X.; Guo, Z.; Zheng, Y.; Tian, Y.; Zheng, B. Nutritional composition, physiological functions and processing of lotus (Nelumbo nucifera Gaertn.) seeds: A review. Phytochem. Rev. 2015, 14, 321–334. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Pinthong, D.; Hano, C. Flavonoids from Nelumbo nucifera Gaertn., a Medicinal Plant: Uses in Traditional Medicine, Phytochemistry and Pharmacological Activities. Medicines 2018, 5, 127. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Zhang, C.; Cao, D.; Damaris, R.N.; Yang, P. The Latest Studies on Lotus (Nelumbo nucifera)-an Emerging Horticultural Model Plant. Int. J. Mol. Sci. 2019, 20, 3680. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.B. Cultivation of lotus (Nelumbo nucifera Gaertn. ssp. nucifera) and its utilization in China. Genet. Resour. Crop Evol. 2009, 56, 323–330. [Google Scholar] [CrossRef]
- Zhu, F. Structures, properties, and applications of lotus starches. Food Hydrocoll. 2017, 63, 332–348. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, M.; Guo, M. Research advances in traditional and modern use of Nelumbo nucifera: Phytochemicals, health promoting activities and beyond. Crit. Rev. Food Sci. Nutr. 2019, 59, S189–S209. [Google Scholar] [CrossRef] [PubMed]
- Duke, J.A. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Mukherjee, P.K.; Mukherjee, D.; Maji, A.K.; Rai, S.; Heinrich, M. The sacred lotus (Nelumbo nucifera)-phytochemical and therapeutic profile. J. Pharm. Pharmacol. 2009, 61, 407–422. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Wu, J.; Li, J.; Xiao, M.; Yang, Y.; Liu, Z.; Cheng, Y. Plumula Nelumbinis: A review of traditional uses, phytochemistry, pharmacology, pharmacokinetics and safety. J. Ethnopharmacol. 2021, 266, 113429. [Google Scholar] [CrossRef]
- Paudel, K.R.; Panth, N. Phytochemical Profile and Biological Activity of Nelumbo nucifera. Evid.-Based Complement. Altern. Med. 2015, 2015, 789124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, B.R.; Gautam, L.N.S.; Adhikari, D.; Karki, R. A Comprehensive Review on Chemical Profiling of Nelumbo nucifera: Potential for Drug Development. Phytother. Res. 2016, 31, 3–26. [Google Scholar] [CrossRef]
- Asokan, S.M.; Mariappan, R.; Muthusamy, S.; Velmurugan, B.K. Pharmacological benefits of neferine—A comprehensive review. Life Sci. 2018, 199, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Limwachiranon, J.; Huang, H.; Shi, Z.; Li, L.; Luo, Z. Lotus Flavonoids and Phenolic Acids: Health Promotion and Safe Consumption Dosages. Compr. Rev. Food Sci. Food Saf. 2018, 17, 458–471. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, Y.; Ma, D.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; Christian, M.; He, Z. Alkaloids from lotus (Nelumbo nucifera): Recent advances in biosynthesis, pharmacokinetics, bioactivity, safety, and industrial applications. Crit. Rev. Food Sci. Nutr. 2021, 30, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Manogaran, P.; Beeraka, N.M.; Padma, V.V. The Cytoprotective and Anti-cancer Potential of Bisbenzylisoquinoline Alkaloids from Nelumbo nucifera. Curr. Top. Med. Chem. 2019, 19, 2940–2957. [Google Scholar] [CrossRef] [PubMed]
- Priya, L.B.; Huang, C.; Hu, R.; Balasubramanian, B.; Baskaran, R. An updated review on pharmacological properties of neferine—A bisbenzylisoquinoline alkaloid from Nelumbo nucifera. J. Food Biochem. 2021, 45, e13986. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y.-P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L.M.; Morris-Natschke, S.L.; et al. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure–activity correlations with related alkaloids. Bioorg. Med. Chem. 2005, 13, 443–448. [Google Scholar] [CrossRef]
- Huang, B.; Ban, X.; He, J.; Tong, J.; Tian, J.; Wang, Y. Comparative Analysis of Essential Oil Components and Antioxidant Activity of Extracts of Nelumbo nucifera from Various Areas of China. J. Agric. Food Chem. 2010, 58, 441–448. [Google Scholar] [CrossRef]
- Chen, S.; Wu, B.-H.; Fang, J.-B.; Liu, Y.-L.; Zhang, H.-H.; Fang, L.-C.; Guan, L.; Li, S.-H. Analysis of flavonoids from lotus (Nelumbo nucifera) leaves using high performance liquid chromatography/photodiode array detector tandem electrospray ionization mass spectrometry and an extraction method optimized by orthogonal design. J. Chromatogr. A 2012, 1227, 145–153. [Google Scholar] [CrossRef]
- Noysang, C.; Boonmatit, N. Preliminary Phytochemicals and Pharmacologic Activities Assessment of White and Pink Nelumbo nucifera Gaertn. Flowers. Appl. Mech. Mater. 2019, 891, 41–51. [Google Scholar] [CrossRef]
- Zhenjia, Z.; Minglin, W.; Daijie, W.; Wenjuan, D.; Xiao, W.; Chengchao, Z. Preparative separation of alkaloids from Nelumbo nucifera leaves by conventional and pH-zone-refining counter-current chromatography. J. Chromatogr. B 2010, 878, 1647–1651. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, E.S.; Lee, C.; Kim, S.; Cho, S.-H.; Hwang, B.Y.; Lee, M.K. Chemical constituents from Nelumbo nucifera leaves and their anti-obesity effects. Bioorg. Med. Chem. Lett. 2013, 23, 3604–3608. [Google Scholar] [CrossRef]
- Ma, C.; Wang, J.; Chu, H.; Zhang, X.; Wang, Z.; Wang, H.; Li, G. Purification and Characterization of Aporphine Alkaloids from Leaves of Nelumbo nucifera Gaertn and Their Effects on Glucose Consumption in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2014, 15, 3481–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.-H.; He, X.-X.; You, C.; Tao, X.; Wang, L.-S.; Zhang, M.-D.; Zhou, Y.-F.; Chang, Q. Pharmacokinetics of Nuciferine and N-Nornuciferine, Two Major Alkaloids From Nelumbo nucifera Leaves, in Rat Plasma and the Brain. Front. Pharmacol. 2018, 9, 902. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Fang, L.; Xi, H.; Guan, L.; Fang, J.; Liu, Y.; Wu, B.; Li, S. Simultaneous qualitative assessment and quantitative analysis of flavonoids in various tissues of lotus (Nelumbo nucifera) using high performance liquid chromatography coupled with triple quad mass spectrometry. Anal. Chim. Acta 2012, 724, 127–135. [Google Scholar] [CrossRef]
- Xiao, J.; Tian, B.; Xie, B.; Yang, E.; Shi, J.; Sun, Z. Supercritical fluid extraction and identification of isoquinoline alkaloids from leaves of Nelumbo nucifera Gaertn. Eur. Food Res. Technol. 2010, 231, 407–414. [Google Scholar] [CrossRef]
- Do, T.C.M.V.; Nguyen, T.D.; Tran, H.; Stuppner, H.; Ganzera, M. Analysis of alkaloids in Lotus (Nelumbo nucifera Gaertn.) leaves by non-aqueous capillary electrophoresis using ultraviolet and mass spectrometric detection. J. Chromatogr. A 2013, 1302, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Kihyun, K.; Chang, S.W.; Shiyong, R.; Sangun, C.; Kangro, L. Phytochemical constituents of Nelumbo nucifera. Nat. Prod. Sci. 2009, 15, 90–95. [Google Scholar]
- Lin, H.-Y.; Kuo, Y.-H.; Lin, Y.-L.; Chiang, W. Antioxidative Effect and Active Components from Leaves of Lotus (Nelumbo nucifera). J. Agric. Food Chem. 2009, 57, 6623–6629. [Google Scholar] [CrossRef]
- Yajima, H. Prevention of diet-induced obesity by dietary polyphenols derived from Nelumbo nucifera and black tea. In Polyphenols in Human Health and Disease; Academic Press: London, UK, 2014; pp. 135–142. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Q.; Lin, X.; Chen, Y.; Qian, L.; Li, K.; Lu, X.; Zheng, B.; Chen, L. Chemical composition of essential oil from Folium nelumbinis and its antioxidant activity. BioRxiv 2018, 419945. [Google Scholar] [CrossRef]
- Itoh, A.; Saitoh, T.; Tani, K.; Uchigaki, M.; Sugimoto, Y.; Yamada, J.; Nakajima, H.; Ohshiro, H.; Sun, S.; Tanahashi, T. Bisbenzylisoquinoline Alkaloids from Nelumbo nucifera. Chem. Pharm. Bull. 2011, 59, 947–951. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Yang, G.; Li, H.; Zhang, G.; Guo, Z. Characterization of the Chemical Composition of Lotus Plumule Oil. J. Agric. Food Chem. 2006, 54, 7672–7677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Jiang, M.; Ying, X.; Cui, Q.; Han, Y.; Hou, Y.; Gao, J.; Bai, G.; Luo, G. Identification and Comparison of Anti-Inflammatory Ingredients from Different Organs of Lotus Nelumbo by UPLC/Q-TOF and PCA Coupled with a NF-κB Reporter Gene Assay. PLoS ONE 2013, 8, e81971. [Google Scholar] [CrossRef]
- Duan, X.H.; Jiang, J.Q. A new benzylisoquinoline alkaloid from stems of Nelumbo nucifera. Chin. Chem. Lett. 2008, 19, 308–310. [Google Scholar] [CrossRef]
- Indrayan, A.; Sharma, S.; Durgapal, D.; Kumar, N.; Kumar, M. Determination of nutritive value and analysis of mineral el-ements for some medicinally valued plants from Uttaranchal. Curr. Sci. 2005, 89, 1252–1255. [Google Scholar]
- Das, S.; Ray, B.; Ghosal, P.K. Structural studies of a polysaccharide from the seeds of Nelumbo nucifera. Carbohydr. Res. 1992, 224, 331–335. [Google Scholar] [CrossRef]
- Jiang, Y.; Ng, T.B.; Liu, Z.; Wang, C.; Li, N.; Qiao, W.; Liua, F. Immunoregulatory and anti-HIV-1 enzyme activities of antioxidant components from lotus (Nelumbo nucifera Gaertn.) rhizome. Biosci. Rep. 2011, 31, 381–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, P.K.; Singh, D. A new lipid and other constituents from the rhizomes of Nelumbo nucifera. J. Asian Nat. Prod. Res. 2009, 11, 583–587. [Google Scholar] [CrossRef]
- Kredy, H.M.; Huang, D.; Xie, B.; He, H.; Yang, E.; Tian, B.; Xiao, D. Flavonols of lotus (Nelumbo nucifera, Gaertn.) seed epicarp and their antioxidant potential. Eur. Food Res. Technol. 2010, 231, 387–394. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, S.-S.; Ibrahim, S.; Li, E.-H.; Yang, H.; Huang, W. Identification and antioxidant properties of polyphenols in lotus seed epicarp at different ripening stages. Food Chem. 2015, 185, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Shad, M.A. Phytochemical composition and antioxidant properties of rhizomes of Nilumbo nucifera. J. Med. Plants Res. 2012, 6, 972–980. [Google Scholar] [CrossRef]
- Nakamura, S.; Nakashima, S.; Tanabe, G.; Oda, Y.; Yokota, N.; Fujimoto, K.; Matsumoto, T.; Sakuma, R.; Ohta, T.; Ogawa, K.; et al. Alkaloid constituents from flower buds and leaves of sacred lotus (Nelumbo nucifera, Nymphaeaceae) with melanogenesis inhibitory activity in B16 melanoma cells. Bioorg. Med. Chem. 2013, 21, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Maneenet, J.; Omar, A.M.; Sun, S.; Kim, M.J.; Daodee, S.; Monthakantirat, O.; Boonyarat, C.; Chulikhit, Y.; Awale, S. Benzylisoquinoline alkaloids from Nelumbo nucifera Gaertn. petals with antiausterity activities against the HeLa human cervical cancer cell line. Z. Nat. C 2021, 76, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-H.; Yang, J.-X.; Xiao, C.-H.; Mao, T.-Y.; Zhang, J.; Zhang, H.-Y. Differences in flavonoid pathway metabolites and transcripts affect yellow petal colouration in the aquatic plant Nelumbo nucifera. BMC Plant Biol. 2019, 19, 277. [Google Scholar] [CrossRef]
- Rho, T.; Yoon, K.D. Chemical Constituents of Nelumbo nucifera Seeds. Nat. Prod. Sci. 2017, 23, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.M.; Yang, W.L.; Yang, S.C.; Chen, C.Y. Chemical constituents from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Chem. Nat. Compd. 2011, 47, 316–318. [Google Scholar] [CrossRef]
- Agnihotri, V.; ElSohly, H.N.; Khan, S.I.; Jacob, M.R.; Joshi, V.C.; Smillie, T.; Khan, I.A.; Walker, L.A. Constituents of Nelumbo nucifera leaves and their antimalarial and antifungal activity. Phytochem. Lett. 2008, 1, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Kato, E.; Inagaki, Y.; Kawabata, J. Higenamine 4′-O-β-d-glucoside in the lotus plumule induces glucose uptake of L6 cells through β2-adrenergic receptor. Bioorg. Med. Chem. 2015, 23, 3317–3321. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, T.; Kitagawa, N.; Tanabe, G.; Ninomiya, K.; Okugawa, S.; Motai, C.; Kamei, I.; Yoshikawa, M.; Lee, I.-J.; Muraoka, O. Quantitative Determination of Alkaloids in Lotus Flower (Flower Buds of Nelumbo nucifera) and Their Melanogenesis Inhibitory Activity. Molecules 2016, 21, 930. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.L.; Liu, B.; Shi, R.B.; Tu, G.Z. Flavonoid chemical compositions of Folium nelumbinis, Beijing Zhongyiyao Daxue Xuebao. J. Beijing Univ. Trad. Chin. Med. 2008, 31, 116–118. [Google Scholar]
- Liu, H.; Liu, J.; Zhang, J.; Qi, Y.; Jia, X.; Zhang, B.; Xiao, P. Simultaneous Quantitative and Chemical Fingerprint Analysis of Receptaculum Nelumbinis Based on HPLC–DAD-MS Combined with Chemometrics. J. Chromatogr. Sci. 2016, 54, 618–624. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Karki, R.; Rhyu, D.Y.; Kim, D.W. Effect of Nelumbo nucifera on proliferation, migration and expression of MMP-2 and MMP-9 of rSMC, A431 and MDA-MB-231. Korean J. Plant Res. 2008, 21, 96–102. [Google Scholar]
- Yang, M.-Y.; Chang, Y.-C.; Chan, K.-C.; Lee, Y.-J.; Wang, C.-J. Flavonoid-enriched extracts from Nelumbo nucifera leaves inhibits proliferation of breast cancer in vitro and in vivo. Eur. J. Integr. Med. 2011, 3, e153–e163. [Google Scholar] [CrossRef]
- Chang, C.-H.; Ou, T.-T.; Yang, M.-Y.; Huang, C.-C.; Wang, C.-J. Nelumbo nucifera Gaertn leaves extract inhibits the angiogenesis and metastasis of breast cancer cells by downregulation connective tissue growth factor (CTGF) mediated PI3K/AKT/ERK signaling. J. Ethnopharmacol. 2016, 188, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Yang, M.-Y.; Lee, Y.-J.; Wang, C.-J. Nelumbo nucifera leaf polyphenol extract inhibits breast cancer cells metastasis in vitro and in vivo through PKCα targeting. J. Funct. Foods 2017, 37, 480–490. [Google Scholar] [CrossRef]
- Arjun, P.; Priya, S.; Krishnamoorthy, M.; Balasubramanian, K. Phytochemical analysis and anticancer activity of Nelumbo nucifera extracts. J. Acad. Ind. Res. 2012, 1, 81–85. [Google Scholar]
- Krubha, A.; Vasan, P.T. Phytochemical Analysis and Anticancer Activity of Nelumbo nucifera Floral Receptacle Extracts in MCF-7 Cell Line. J. Acad. Ind. Res. 2016, 4, 251–256. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wu, T.; Li, B.; Liu, T.; Wang, R.; Liu, Q.; Liu, Z.; Gong, Y.; Shao, C. Isoliensinine induces apoptosis in triple-negative human breast cancer cells through ROS generation and p38 MAPK/JNK activation. Sci. Rep. 2015, 5, 12579. [Google Scholar] [CrossRef]
- Yang, D.; Zou, X.; Yi, R.; Liu, W.; Peng, D.; Zhao, X. Neferine increase in vitro anticancer effect of dehydroepiandrosterone on MCF-7 human breast cancer cells. Appl. Biol. Chem. 2016, 59, 585–596. [Google Scholar] [CrossRef]
- Kadioglu, O.; Law, B.Y.K.; Mok, S.W.F.; Xu, S.-W.; Efferth, T.; Wong, V.K.W. Mode of Action Analyses of Neferine, a Bisbenzylisoquinoline Alkaloid of Lotus (Nelumbo nucifera) against Multidrug-Resistant Tumor Cells. Front. Pharmacol. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, B.Y.K.; Michelangeli, F.; Qu, Y.Q.; Xu, S.-W.; Han, Y.; Mok, S.W.F.; de Seabra Rodrigues Dias, I.R.; Javed, M.-H.; Chan, W.-K.; Xue, W.-W.; et al. Neferine induces autophagy-dependent cell death in apoptosis-resistant cancers via ryanodine receptor and Ca2+-dependent mechanism. Sci. Rep. 2019, 9, 20034. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Wang, T.; Shao, H.; Gao, J.; Wang, Y.; Ge, Y. Neferine inhibits MDA-MB-231 cells growth and metastasis by regulating miR-374a/FGFR-2. Chem. Interact. 2019, 309, 108716. [Google Scholar] [CrossRef]
- Kang, E.J.; Lee, S.K.; Park, K.-K.; Son, S.H.; Kim, K.R.; Chung, W.-Y. Liensinine and Nuciferine, Bioactive Components of Nelumbo nucifera, Inhibit the Growth of Breast Cancer Cells and Breast Cancer-Associated Bone Loss. Evid.-Based Complement. Altern. Med. 2017, 2017, 1583185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Li, G.; Zheng, Y.; Shen, H.-M.; Hu, X.; Ming, Q.-L.; Huang, C.; Li, P.; Gao, N. A novel autophagy/mitophagy inhibitor liensinine sensitizes breast cancer cells to chemotherapy through DNM1L-mediated mitochondrial fission. Autophagy 2015, 11, 1259–1279. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Xu, H.; Chen, H.; Sun, B.; Huang, H.; Fan, H.; Zheng, J. Seco-neferine A–F, three new pairs of benzyltetrahydroisoquinoline alkaloid epimers from Plumula Nelumbinis and their activity. Fitoterapia 2021, 153, 104994. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Cheng, Y.; Zhou, Z.-W.; Long, H.-Z.; Luo, H.-Y.; Wen, D.-D.; Cheng, L.; Gao, L.-C. Isoliensinine induces cervical cancer cell cycle arrest and apoptosis by inhibiting the AKT/GSK3α pathway. Oncol. Lett. 2022, 23, 8. [Google Scholar] [CrossRef]
- Dasari, S.; Bakthavachalam, V.; Chinnapaka, S.; Venkatesan, R.; Samy, A.L.P.A.; Munirathinam, G. Neferine, an alkaloid from lotus seed embryo targets HeLa and SiHa cervical cancer cells via pro-oxidant anticancer mechanism. Phytother. Res. 2020, 34, 2366–2384. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, X.; Wang, C.; Peng, D.; Zhu, K.; Song, J.-L. Anticancer activity of Nelumbo nucifera stamen extract in human colon cancer HCT-116 cells in vitro. Oncol. Lett. 2017, 13, 1470–1478. [Google Scholar] [CrossRef] [Green Version]
- Prasath, M.; Narasimha, M.B.; Chih-Yang, H.; Viswanadha, V.P. Neferine and isoliensinine from Nelumbo nucifera induced reactive oxygen species (ROS)-mediated apoptosis in colorectal cancer HCT-15 cells. Afr. J. Pharm. Pharmacol. 2019, 13, 90–99. [Google Scholar] [CrossRef]
- Qi, Q.; Li, R.; Li, H.-Y.; Cao, Y.-B.; Bai, M.; Fan, X.-J.; Wang, S.-Y.; Zhang, B.; Li, S. Identification of the anti-tumor activity and mechanisms of nuciferine through a network pharmacology approach. Acta Pharmacol. Sin. 2016, 37, 963–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, Y.-J.; Huang, X.-H.; Zheng, C.-C.; Yin, X.-F.; Li, B.; He, Q.-Y. Liensinine perchlorate inhibits colorectal cancer tumorigenesis by inducing mitochondrial dysfunction and apoptosis. Food Funct. 2018, 9, 5536–5546. [Google Scholar] [CrossRef]
- Guon, T.E.; Chung, H.S. Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncol. Lett. 2016, 11, 2463–2470. [Google Scholar] [CrossRef] [Green Version]
- An, K.; Zhang, Y.; Liu, Y.; Yan, S.; Hou, Z.; Cao, M.; Liu, G.; Dong, C.; Gao, J.; Liu, G. Neferine induces apoptosis by modulating the ROS-mediated JNK pathway in esophageal squamous cell carcinoma. Oncol. Rep. 2020, 44, 1116–1126. [Google Scholar] [CrossRef]
- Wang, J.; Dong, Y.; Li, Q. Neferine induces mitochondrial dysfunction to exert anti-proliferative and anti-invasive activities on retinoblastoma. Exp. Biol. Med. 2020, 245, 1385–1394. [Google Scholar] [CrossRef]
- Shen, Y.; Bian, R.; Li, Y.; Gao, Y.; Liu, Y.; Xu, Y.; Song, X.; Zhang, Y. Liensinine induces gallbladder cancer apoptosis and G2/M arrest by inhibiting ZFX-induced PI3K/AKT pathway. Acta Biochim. Biophys. Sin. 2019, 51, 607–614. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Q.; Zhuang, W.; Lu, X.; Miron, A.; Chai, T.-T.; Zheng, B.; Xiao, J. Cytotoxic, Antitumor and Immunomodulatory Effects of the Water-Soluble Polysaccharides from Lotus (Nelumbo nucifera Gaertn.) Seeds. Molecules 2016, 21, 1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Li, Y.; Cao, P.; Xie, Z.; Qin, Z. Synergistic effect of hyperthermia and neferine on reverse multidrug resistance in adriamycin-resistant SGC7901/ADM gastric cancer cells. J. Huazhong Univ. Sci. Technol. 2011, 31, 488–496. [Google Scholar] [CrossRef]
- Xue, F.; Liu, Z.; Xu, J.; Xu, X.; Chen, X.; Tian, F. Neferine inhibits growth and migration of gastrointestinal stromal tumor cell line GIST-T1 by up-regulation of miR-449a. Biomed. Pharmacother. 2019, 109, 1951–1959. [Google Scholar] [CrossRef]
- Liu, C.-M.; Kao, C.-L.; Wu, H.-M.; Li, W.-J.; Huang, C.-T.; Li, H.-T.; Chen, C.-Y. Antioxidant and Anticancer Aporphine Alkaloids from the Leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules 2014, 19, 17829–17838. [Google Scholar] [CrossRef]
- Yang, J.-H.; Yu, K.; Si, X.-K.; Li, S.; Cao, Y.-J.; Li, W.; Zhang, J.-X. Liensinine inhibited gastric cancer cell growth through ROS generation and the PI3K/AKT pathway. J. Cancer 2019, 10, 6431–6438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.; Li, X.; Tang, X.; Jiang, J.; Han, Y.; Li, Y.; Ma, C.; Liu, Z.; He, Y. Neferine promotes the apoptosis of HNSCC through the accumulation of p62/SQSTM1 caused by autophagic flux inhibition. Int. J. Mol. Med. 2021, 48, 124. [Google Scholar] [CrossRef] [PubMed]
- Byun, D.-S. Kaempferol Isolated from Nelumbo nucifera Stamens Negatively Regulates FcεRI Expression in Human Basophilic KU812F Cells. J. Microbiol. Biotechnol. 2009, 19, 155–160. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, X.-P.; Yang, Z.-S.; Xiao, Y.-H.; Min, H.; Li, Y.-J. Neferine increases STI571 chemosensitivity via inhibition of P-gp expression in STI571-resistant K562 cells. Leuk. Lymphoma 2011, 52, 694–700. [Google Scholar] [CrossRef]
- Li, Q.-H.; Sui, L.-P.; Zhao, Y.-H.; Chen, B.-G.; Li, J.; Ma, Z.-H.; Hu, Z.-H.; Tang, Y.-L.; Guo, Y.-X. Tripartite Motif-Containing 44 is Involved in the Tumorigenesis of Laryngeal Squamous Cell Carcinoma, and its Expression is Downregulated by Nuciferine. Tohoku J. Exp. Med. 2021, 254, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Guan, Y.; Song, X.; He, J.; Xie, Z.; Zhang, Y.; Zhang, H.; Tang, D. Polyphenols extract from lotus seedpod ( Nelumbo nucifera Gaertn.): Phenolic compositions, antioxidant, and antiproliferative activities. Food Sci. Nutr. 2019, 7, 3062–3070. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Xu, H.; Luo, X.; Zhang, H.; He, Y.; Sun, G.; Sun, X. Procyanidins from Nelumbo nucifera Gaertn. Seedpod induce autophagy mediated by reactive oxygen species generation in human hepatoma G2 cells. Biomed. Pharmacother. 2016, 79, 135–152. [Google Scholar] [CrossRef]
- Xiao-Hong, A.; Xiao-Qing, T.; Yan-Ping, L.; Hua-Qing, L.; Lin, D. Effect of Neferine on Adriamycin-resistance of Thermotolerant Hepatocarcinoma Cell Line HepG2/thermotolerance. Chin. J Cancer 2007, 26, 357–360. [Google Scholar]
- Yoon, J.-S.; Kim, H.-M.; Yadunandam, A.K.; Kim, N.-H.; Jung, H.-A.; Choi, J.-S.; Kim, C.-Y.; Kim, G.-D. Neferine isolated from Nelumbo nucifera enhances anti-cancer activities in Hep3B cells: Molecular mechanisms of cell cycle arrest, ER stress induced apoptosis and anti-angiogenic response. Phytomedicine 2013, 20, 1013–1022. [Google Scholar] [CrossRef]
- Poornima, P.; Quency, R.S.; Padma, V.V. Neferine induces reactive oxygen species mediated intrinsic pathway of apoptosis in HepG2 cells. Food Chem. 2013, 136, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Zeng, S.; Ma, J.; Zhang, Y.; Qu, Y.; Han, Y.; Yin, L.; Cai, C.; Guo, C.; Shen, H. The anti-tumor activities of Neferine on cell invasion and oxaliplatin sensitivity regulated by EMT via Snail signaling in hepatocellular carcinoma. Sci. Rep. 2017, 7, 41616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, G.; Yue, L.; Zhao, W.; Xu, C.; Yang, J.; Wang, S.; Yang, X. Isoliensinine, a Bioactive Alkaloid Derived from Embryos of Nelumbo nucifera, Induces Hepatocellular Carcinoma Cell Apoptosis through Suppression of NF-κB Signaling. J. Agric. Food Chem. 2015, 63, 8793–8803. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Zhang, L.; Jiang, S.; Cheng, Z.; Wang, G.; Huang, X.; Yang, X. Isoliensinine induces dephosphorylation of NF-κB p65 subunit at Ser536 via a PP2A-dependent mechanism in hepatocellular carcinoma cells: Roles of impairing PP2A/I2PP2A interaction. Oncotarget 2016, 7, 40285–40296. [Google Scholar] [CrossRef] [Green Version]
- Horng, C.-T.; Huang, C.-W.; Yang, M.-Y.; Chen, T.-H.; Chang, Y.-C.; Wang, C.-J. Nelumbo nucifera leaf extract treatment attenuated preneoplastic lesions and oxidative stress in the livers of diethylnitrosamine-treated rats. Environ. Toxicol. 2017, 32, 2327–2340. [Google Scholar] [CrossRef]
- Yang, M.-Y.; Hung, T.-W.; Wang, C.-J.; Tseng, T.-H. Inhibitory Effect of Nelumbo nucifera Leaf Extract on 2-Acetylaminofluorene-induced Hepatocarcinogenesis Through Enhancing Antioxidative Potential and Alleviating Inflammation in Rats. Antioxidants 2019, 8, 329. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.; Yang, I.; Kim, S.; Lee, C. Lotus ( Nelumbo nucifera ) seedpod extract inhibits cell proliferation and induces apoptosis in non-small cell lung cancer cells via downregulation of Axl. J. Food Biochem. 2021, 45, e13601. [Google Scholar] [CrossRef]
- Poornima, P.; Weng, C.F.; Padma, V.V. Neferine from Nelumbo nucifera induces autophagy through the inhibition of PI3K/Akt/mTOR pathway and ROS hyper generation in A549 cells. Food Chem. 2013, 141, 3598–3605. [Google Scholar] [CrossRef]
- Poornima, P.; Weng, C.F.; Padma, V.V. Neferine, an alkaloid from lotus seed embryo, inhibits human lung cancer cell growth by MAPK activation and cell cycle arrest. BioFactors 2014, 40, 121–131. [Google Scholar] [CrossRef]
- Selvi, S.K.; Vinoth, A.; Varadharajan, T.; Weng, C.F.; Padma, V.V. Neferine augments therapeutic efficacy of cisplatin through ROS- mediated non-canonical autophagy in human lung adenocarcinoma (A549 cells). Food Chem. Toxicol. 2017, 103, 28–40. [Google Scholar] [CrossRef]
- Sivalingam, K.S.; Paramasivan, P.; Weng, C.F.; Viswanadha, V.P. Neferine Potentiates the Antitumor Effect of Cisplatin in Human Lung Adenocarcinoma Cells via a Mitochondria-Mediated Apoptosis Pathway. J. Cell. Biochem. 2017, 118, 2865–2876. [Google Scholar] [CrossRef]
- Liu, W.; Yi, D.-D.; Guo, J.-L.; Xiang, Z.-X.; Deng, L.-F.; He, L. Nuciferine, extracted from Nelumbo nucifera Gaertn, inhibits tumor-promoting effect of nicotine involving Wnt/β-catenin signaling in non-small cell lung cancer. J. Ethnopharmacol. 2015, 165, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, K.; Amirthalingam, V.; Ganasan, K.; Huang, C.-Y.; Viswanadha, V.P. Neferine suppresses diethylnitrosamine-induced lung carcinogenesis in Wistar rats. Food Chem. Toxicol. 2019, 123, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Feng, X.; Peng, D.; Liu, W.; Sun, P.; Li, G.; Gu, L.; Song, J.-L. Anticancer activities of alkaloids extracted from the Ba lotus seed in human nasopharyngeal carcinoma CNE-1 cells. Exp. Ther. Med. 2016, 12, 3113–3120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, D.-C.; Chang, Y.-C.; Lin, S.-R.; Fuh, Y.-M.; Tsai, M.-J.; Weng, C.-F. FAK and S6K1 Inhibitor, Neferine, Dually Induces Autophagy and Apoptosis in Human Neuroblastoma Cells. Molecules 2018, 23, 3110. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Chen, Y.; An, T.; Liu, P.; Zhu, J.; Yang, H.; Zhang, W.; Dong, T.; Jiang, J.; Zhang, Y.; et al. Nuciferine inhibits the progression of glioblastoma by suppressing the SOX2-AKT/STAT3-Slug signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 139. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhang, X.; Li, Y.; Lu, S.; Lu, S.; Li, J.; Wang, Y.; Tian, X.; Wei, J.-J.; Shao, C.; et al. Neferine induces autophagy of human ovarian cancer cells via p38 MAPK/JNK activation. Tumor Biol. 2016, 37, 8721–8729. [Google Scholar] [CrossRef]
- Erdogan, S.; Turkekul, K. Neferine inhibits proliferation and migration of human prostate cancer stem cells through p38 MAPK/JNK activation. J. Food Biochem. 2020, 44, e13253. [Google Scholar] [CrossRef]
- Nazim, U.M.; Yin, H.; Park, S. Neferine treatment enhances the TRAIL-induced apoptosis of human prostate cancer cells via autophagic flux and the JNK pathway. Int. J. Oncol. 2020, 56, 1152–1161. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.M.; Wu, Z.; Pan, B.; An, L.; Zhu, C.; Zhou, J.; Jiang, Y. The antiandrogenic effect of neferine, liensinine and isoliensinine by inhibiting 5-α-reductase and androgen receptor expression via PI3K/AKT signaling pathway in prostate cancer. Pharmazie 2021, 76, 225–231. [Google Scholar] [CrossRef]
- Kim, E.; Sung, E.-G.; Song, I.-H.; Kim, J.-Y.; Sung, H.-J.; Sohn, H.-Y.; Park, J.-Y.; Lee, T.-J. Neferine-induced apoptosis is dependent on the suppression of Bcl-2 expression via downregulation of p65 in renal cancer cells. Acta Biochim. Biophys. Sin. 2019, 51, 734–742. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Xu, B.; Sun, Z.; Gong, Y.; Shao, C. Neferine, an alkaloid ingredient in lotus seed embryo, inhibits proliferation of human osteosarcoma cells by promoting p38 MAPK-mediated p21 stabilization. Eur. J. Pharmacol. 2012, 677, 47–54. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, H.; Xu, F.; Xie, B.; Yang, X.; Wang, Y.; Yan, Y. Inhibition effect of procyanidins from lotus seedpod on mouse B16 melanoma invivo and in vitro. Food Chem. 2010, 122, 84–91. [Google Scholar] [CrossRef]
- Lai, P.-J.; Kao, E.-S.; Chen, S.-R.; Huang, Y.-T.; Wang, C.-J.; Huang, H.-P. Nelumbo nucifera Leaf Extracts Inhibit Melanogenesis in B16 Melanoma Cells and Guinea Pigs through Downregulation of CREB/MITF Activation. J. Food Nutr. Res. 2020, 8, 459–465. [Google Scholar] [CrossRef]
- Wu, P.-F.; Chiu, C.-C.; Chen, C.-Y.; Wang, H.-M.D. 7-Hydroxydehydronuciferine induces human melanoma death via triggering autophagy and apoptosis. Exp. Dermatol. 2015, 24, 930–935. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Wu, J.; Jia, Y.; Wang, W.; Zhang, S.; Wang, J. Improved RP-HPLC method to determine neferine in dog plasma and its application to pharmacokinetics. J. Chromatogr. B 2007, 857, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bai, Y.; Zhao, L.; Hu, T.; Hu, B.; Wang, J.; Xiang, J. Pharmacokinetics and metabolism of neferine in rats after a single oral administration. Biopharm. Drug Dispos. 2007, 28, 361–372. [Google Scholar] [CrossRef]
- Zhou, H.; Li, L.; Jiang, H.; Zeng, S. Identification of Three New N-Demethylated and O-Demethyled Bisbenzylisoquinoline Alkaloid Metabolites of Isoliensinine from Dog Hepatic Microsomes. Molecules 2012, 17, 11712–11720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Hellum, B.H.; Liang, A.; Nilsen, O.G. The In Vitro Inhibition of Human CYP1A2, CYP2D6 and CYP3A4 by Tetrahydropalmatine, Neferine and Berberine. Phytother. Res. 2011, 26, 277–283. [Google Scholar] [CrossRef]
- Ye, L.-H.; He, X.-X.; Kong, L.-T.; Liao, Y.-H.; Pan, R.-L.; Xiao, B.-X.; Liu, X.-M.; Chang, Q. Identification and characterization of potent CYP2D6 inhibitors in lotus leaves. J. Ethnopharmacol. 2014, 153, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Ge, Y.; Chen, X.; Li, J.; Yang, X.; Wang, H.; Gao, X.; Chang, Y.-X. Simultaneous Determination of Five Alkaloids by HPLC-MS/MS Combined With Micro-SPE in Rat Plasma and Its Application to Pharmacokinetics After Oral Administration of Lotus Leaf Extract. Front. Pharmacol. 2019, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wang, X.; Wang, Z.; Wang, Y.; Luan, Z.; Gao, X.; Wang, R. The risk of higenamine adverse analytical findings following oral administration of plumula nelumbinis capsules. Drug Test. Anal. 2019, 11, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-H.; Lin, J.-Y. Purified active lotus plumule (Nelumbo nucifera Gaertn) polysaccharides exert anti-inflammatory activity through decreasing Toll-like receptor-2 and -4 expressions using mouse primary splenocytes. J. Ethnopharmacol. 2013, 147, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Kunanusorn, P.; Panthong, A.; Pittayanurak, P.; Wanauppathamkul, S.; Nathasaen, N.; Reutrakul, V. Acute and subchronic oral toxicity studies of Nelumbo nucifera stamens extract in rats. J. Ethnopharmacol. 2011, 134, 789–795. [Google Scholar] [CrossRef]
- Rajput, M.A.; Alam Khan, R. Phytochemical screening, acute toxicity, anxiolytic and antidepressant activities of the Nelumbo nucifera fruit. Metab. Brain Dis. 2017, 32, 743–749. [Google Scholar] [CrossRef]
- Liu, X.; Qu, W.; Liang, J.Y. Research progress of Nelumbo nucefera gaertn. Strait Pharm. J. 2010, 22, 1–5. [Google Scholar]
- Chung, H.-S.; Lee, H.J.; Shim, I.; Bae, H. Assessment of anti-depressant effect of nelumbinis semen on rats under chronic mild stress and its subchronic oral toxicity in rats and beagle dogs. BMC Complement. Altern. Med. 2012, 12, 68–82. [Google Scholar] [CrossRef] [Green Version]

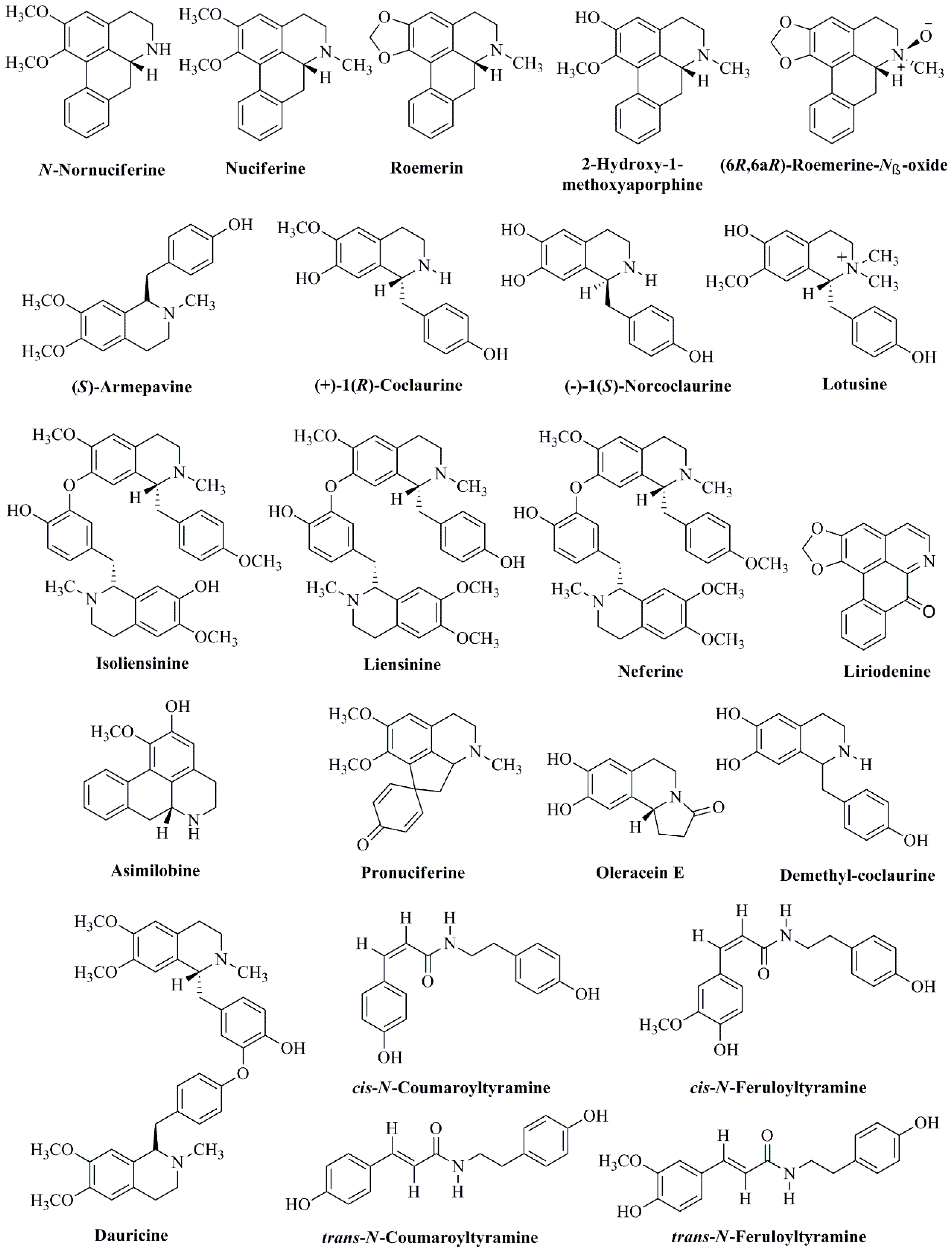

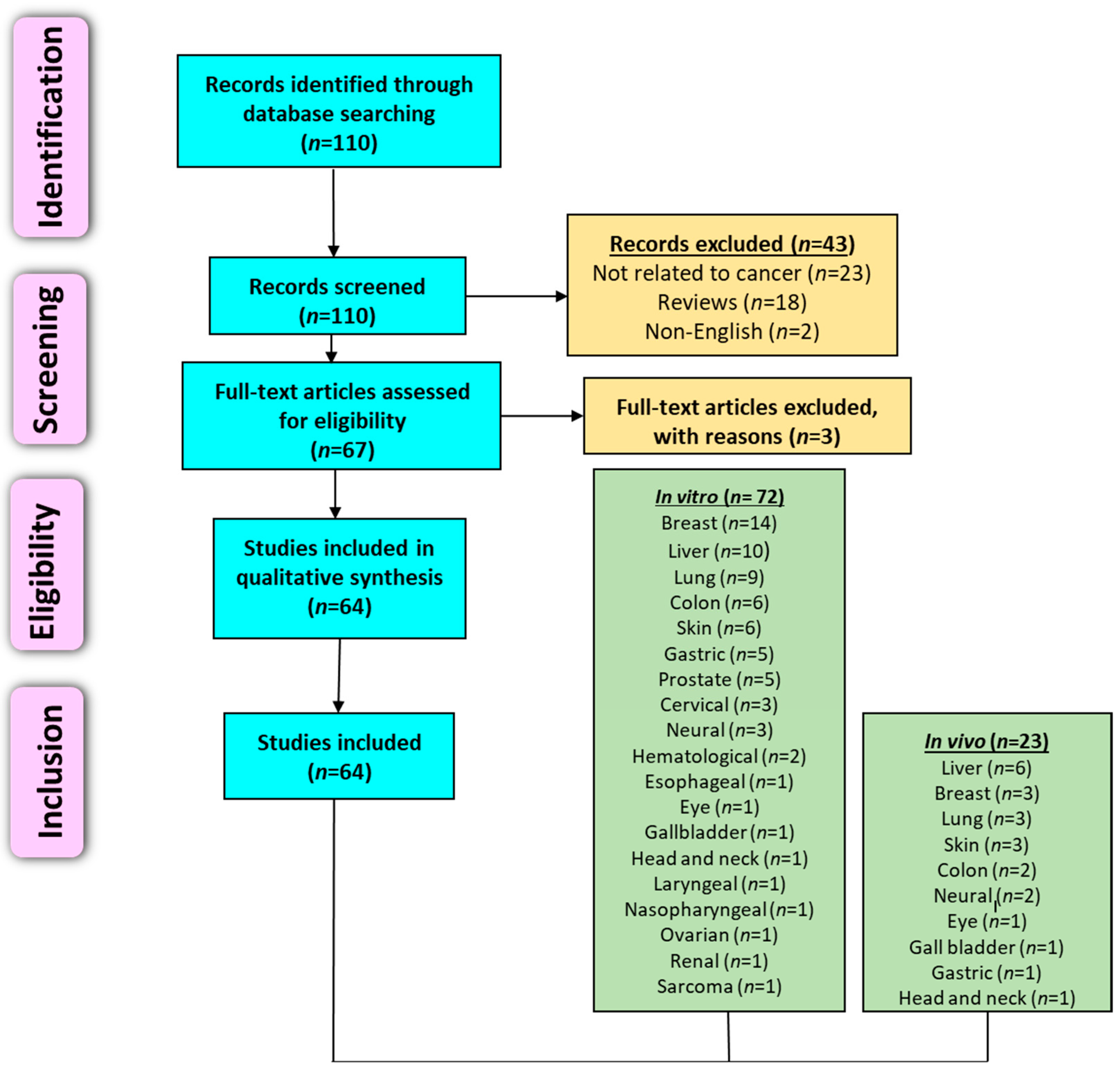
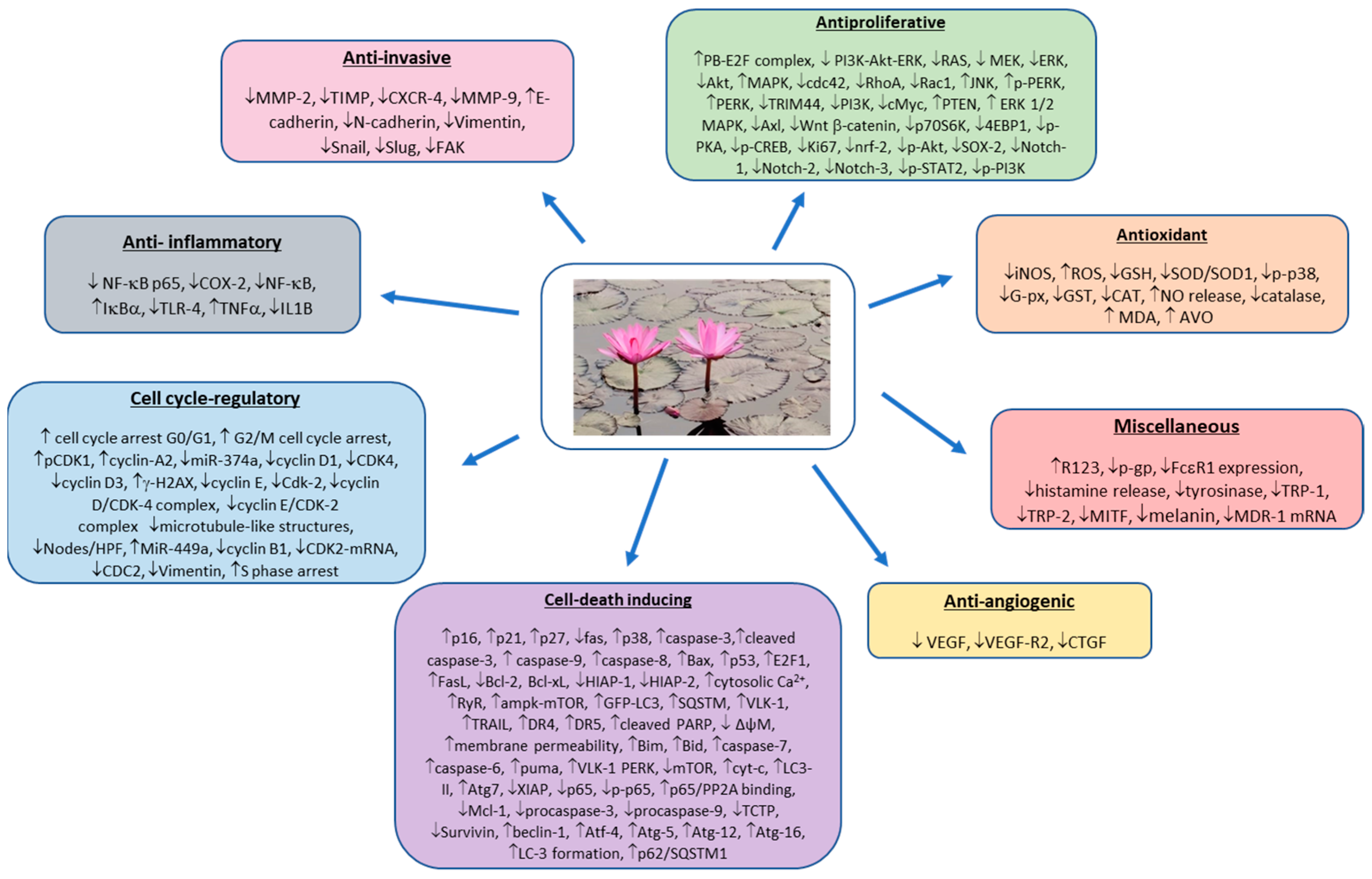
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bishayee, A.; Patel, P.A.; Sharma, P.; Thoutireddy, S.; Das, N. Lotus (Nelumbo nucifera Gaertn.) and Its Bioactive Phytocompounds: A Tribute to Cancer Prevention and Intervention. Cancers 2022, 14, 529. https://doi.org/10.3390/cancers14030529
Bishayee A, Patel PA, Sharma P, Thoutireddy S, Das N. Lotus (Nelumbo nucifera Gaertn.) and Its Bioactive Phytocompounds: A Tribute to Cancer Prevention and Intervention. Cancers. 2022; 14(3):529. https://doi.org/10.3390/cancers14030529
Chicago/Turabian StyleBishayee, Anupam, Palak A. Patel, Priya Sharma, Shivani Thoutireddy, and Niranjan Das. 2022. "Lotus (Nelumbo nucifera Gaertn.) and Its Bioactive Phytocompounds: A Tribute to Cancer Prevention and Intervention" Cancers 14, no. 3: 529. https://doi.org/10.3390/cancers14030529
APA StyleBishayee, A., Patel, P. A., Sharma, P., Thoutireddy, S., & Das, N. (2022). Lotus (Nelumbo nucifera Gaertn.) and Its Bioactive Phytocompounds: A Tribute to Cancer Prevention and Intervention. Cancers, 14(3), 529. https://doi.org/10.3390/cancers14030529







