Outcome of SARS-CoV-2-Infected Polish Patients with Chronic Lymphocytic Leukemia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
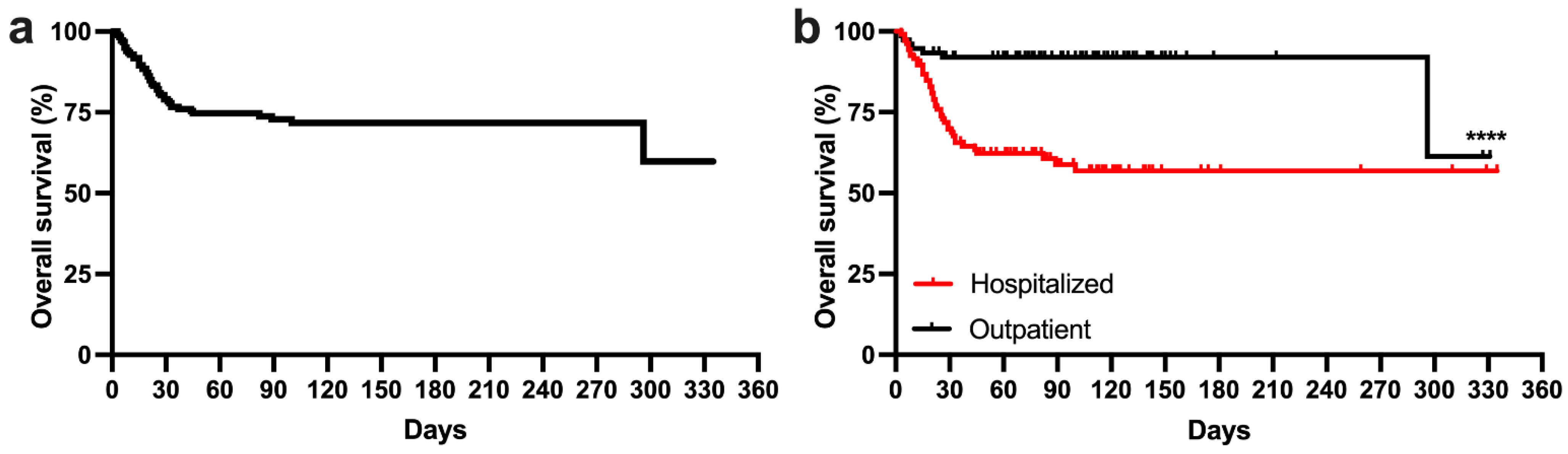
3.2. Survival Analysis of the Whole Study Cohort
3.3. Risk Factors for Hospitalization
3.4. Analysis of the Hospitalized Subgroup
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Harrison, S.L.; Fazio-Eynullayeva, E.; Lane, D.A.; Underhill, P.; Lip, G.Y.H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 2020, 17, e1003321. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Yigenoglu, T.N.; Ata, N.; Altuntas, F.; Bascı, S.; Dal, M.S.; Korkmaz, S.; Namdaroglu, S.; Basturk, A.; Hacıbekiroglu, T.; Dogu, M.H.; et al. The outcome of COVID-19 in patients with hematological malignancy. J. Med. Virol. 2021, 93, 1099–1104. [Google Scholar] [CrossRef]
- Passamonti, F.; Cattaneo, C.; Arcaini, L.; Bruna, R.; Cavo, M.; Merli, F.; Angelucci, E.; Krampera, M.; Cairoli, R.; Della Porta, M.G.; et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: A retrospective, multicentre, cohort study. Lancet Haematol. 2020, 7, e737–e745. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Dohner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. Guidelines for diagnosis, indications for treatment, response assessment and supportive management of chronic lymphocytic leukemia. Blood 2018, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Tadmor, T.; Welslau, M.; Hus, I. A review of the infection pathogenesis and prophylaxis recommendations in patients with chronic lymphocytic leukemia. Expert Rev. Hematol. 2018, 11, 57–70. [Google Scholar] [CrossRef]
- Langerbeins, P.; Eichhorst, B. Immune Dysfunction in Patients with Chronic Lymphocytic Leukemia and Challenges during COVID-19 Pandemic. Acta. Haematol. 2021, 144, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Vijenthira, A.; Gong, I.Y.; Fox, T.A.; Booth, S.; Cook, G.; Fattizzo, B.; Martín-Moro, F.; Razanamahery, J.; Riches, J.C.; Zwicker, J.; et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood 2020, 136, 2881–2892. [Google Scholar] [CrossRef]
- Chatzikonstantinou, T.; Kapetanakis, A.; Scarfò, L.; Karakatsoulis, G.; Allsup, D.; Cabrero, A.A.; Andres, M.; Antic, D.; Baile, M.; Baliakas, P.; et al. COVID-19 severity and mortality in patients with CLL: An update of the international ERIC and Campus CLL study. Leukemia 2021, 35, 3444–3454. [Google Scholar] [CrossRef]
- Scarfò, L.; Chatzikonstantinou, T.; Rigolin, G.M.; Quaresmini, G.; Motta, M.; Vitale, C.; Garcia-Marco, J.A.; Hernández-Rivas, J.; Mirás, F.; Baile, M.; et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: A joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia 2020, 34, 2354–2363. [Google Scholar] [CrossRef]
- Roeker, L.E.; Eyre, T.A.; Thompson, M.C.; Lamanna, N.; Coltoff, A.R.; Davids, M.S.; Baker, P.O.; Leslie, L.; Rogers, K.A.; Allan, J.N.; et al. COVID-19 in patients with CLL: Improved survival outcomes and update on management strategies. Blood 2021, 138, 1768–1773. [Google Scholar] [CrossRef]
- Mato, A.R.; Roeker, L.E.; Lamanna, N.; Allan, J.N.; Leslie, L.; Pagel, J.M.; Patel, K.; Osterborg, A.; Wojenski, D.; Kamdar, M.; et al. Outcomes of COVID-19 in patients with CLL: A multicenter international experience. Blood 2020, 136, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Paladugu, S.; Donato, A.A. Remdesivir improved time to recovery in adults hospitalized with COVID-19 and lower respiratory tract involvement. Ann. Intern. Med. 2020, 173, Jc4. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19-Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Pula, B.; Iskierka-Jazdzewska, E.; Dlugosz-Danecka, M.; Szymczyk, A.; Hus, M.; Szeremet, A.; Drozd-Sokolowska, J.; Waszczuk-Gajda, A.; Zaucha, J.M.; Holojda, J.; et al. Long-term Efficacy of Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia: Results of the Polish Adult Leukemia Study Group Observational Study. Anticancer. Res. 2020, 40, 4059–4066. [Google Scholar] [CrossRef] [PubMed]
- Blixt, L.; Bogdanovic, G.; Buggert, M.; Gao, Y.; Hober, S.; Healy, K.; Johansson, H.; Kjellander, C.; Mravinacova, S.; Muschiol, S.; et al. Covid-19 in patients with chronic lymphocytic leukemia: Clinical outcome and B- and T-cell immunity during 13 months in consecutive patients. Leukemia 2021, 1–6. [Google Scholar] [CrossRef]
- Masternak, M.; Knap, J.; Giannopoulos, K. The prognostic value of mean platelet volume in cancer patients. Acta. Haematol. Pol. 2019, 50, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Masternak, M.; Puła, B.; Knap, J.; Waszczuk-Gajda, A.; Drozd-Sokołowska, J.; Wdowiak, K.; Grosicki, S.; Kozłowska, I.; Kaźmierczak, M.; Łabędź, A.; et al. Mean Platelet Volume Has Prognostic Value in Chronic Lymphocytic Leukemia. Cancer Manag. Res. 2020, 12, 9977–9985. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, J.; Li, Y.; Liu, F.; Zhou, Q.; Peng, Z. Association between thrombocytopenia and 180-day prognosis of COVID-19 patients in intensive care units: A two-center observational study. PLoS ONE 2021, 16, e0248671. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta. 2020, 506, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Gu, Y.; Yu, H.; Li, Z.; Wang, Y. Thrombocytopenia Is Associated with COVID-19 Severity and Outcome: An Updated Meta-Analysis of 5637 Patients with Multiple Outcomes. Lab. Med. 2021, 52, 10–15. [Google Scholar] [CrossRef]
- Hana, C.; Aboulenain, S.; Dewaswala, N.; Narendran, V. Does Thrombocytopenia Truly Correlate with COVID-19 Severity? Blood 2020, 136, 39–40. [Google Scholar] [CrossRef]
- Siemieniuk, R.A.; Bartoszko, J.J.; Ge, L.; Zeraatkar, D.; Izcovich, A.; Kum, E.; Pardo-Hernandez, H.; Qasim, A.; Martinez, J.P.D.; Rochwerg, B.; et al. Drug treatments for covid-19: Living systematic review and network meta-analysis. BMJ 2020, 370, m2980. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, A.; Scarfò, L.; Reda, G.; Varettoni, M.; Quaglia, F.M.; Marchetti, M.; De Paoli, L.; Re, F.; Pietrasanta, D.; Rigolin, G.M.; et al. Chronic lymphocytic leukemia management in Italy during the COVID-19 pandemic: A Campus CLL report. Blood 2020, 136, 763–766. [Google Scholar] [CrossRef]
- Treon, S.P.; Castillo, J.J.; Skarbnik, A.P.; Soumerai, J.D.; Ghobrial, I.M.; Guerrera, M.L.; Meid, K.; Yang, G. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood 2020, 135, 1912–1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fürstenau, M.; Langerbeins, P.; De Silva, N.; Fink, A.M.; Robrecht, S.; von Tresckow, J.; Simon, F.; Hohloch, K.; Droogendijk, J.; van der Klift, M.; et al. COVID-19 among fit patients with CLL treated with venetoclax-based combinations. Leukemia 2020, 34, 2225–2229. [Google Scholar] [CrossRef]

| All Patients | Hospitalized Patients | |
|---|---|---|
| Age (median; range) | 68 (37–87) | 69 (37–87) |
| Parameter | N (%) | N (%) |
| Sex | ||
| Men | 119 (63.3%) | 70 (63.1%) |
| Women | 69 (36.7%) | 41 (36.9%) |
| Rai stage | ||
| 0 | 13 (6.9%) | 8 (7.2%) |
| 1 | 40 (21.3%) | 23 (20.7%) |
| 2 | 69 (36.7%) | 36 (32.4%) |
| 3 | 28 (14.9%) | 15 (13.5%) |
| 4 | 32 (17%) | 23 (20.7%) |
| NA | 6 (3.2%) | 6 (5.4%) |
| Binet stage | ||
| A | 45 (23.9%) | 19 (17.1%) |
| B | 70 (37.2%) | 40 (36%) |
| C | 29 (15.4%) | 20 (18%) |
| NA | 44 (23.4%) | 32 (28.8%) |
| ECOG | ||
| 2–4 | 46 (24.5%) | 37 (33.3%) |
| 0–1 | 136 (72.3%) | 71 (64%) |
| NA | 6 (3.2%) | 3 (2.7%) |
| WBC [×109/L] | ||
| ≤25 | 132 (70.2%) | 77 (69.4%) |
| >25 | 56 (29.8%) | 34 (30.6%) |
| Hemoglobin [g/dL] | ||
| ≤10 | 44 (23.4%) | 39 (35.1%) |
| >10 | 143 (76%) | 71 (64%) |
| NA | 1 (0.5%) | 1 (0.9%) |
| Platelets [×109/L] | ||
| ≤100 | 51 (27.1%) | 39 (35.1%) |
| >100 | 137 (72.9%) | 72 (64.9%) |
| Lactate dehydrogenase | ||
| Elevated | 71 (37.8%) | 50 (45%) |
| Normal range | 90 (47.9%) | 43 (38.7%) |
| NA | 27 (14.4%) | 18 (16.2%) |
| BMI | ||
| <18.5 | 1 (0.5%) | 1 (0.9%) |
| 18.5–25 | 48 (25.5%) | 26 (23.4%) |
| 25–30 | 87 (46.3%) | 50 (45%) |
| >30 | 36 (19.1%) | 22 (19.8%) |
| NA | 16 (8.5%) | 12 (10.8%) |
| Creatinine [mg/dL] | ||
| >1.3 | 19 (10.1%) | 14 (12.6%) |
| ≤1.3 | 163 (86.7%) | 95 (85.6%) |
| NA | 6 (3.2%) | 2 (1.8%) |
| Deletion 17p | ||
| Yes | 23 (12.2%) | 71 (64%) |
| No | 126 (67%) | 14 (12.6%) |
| NA | 39 (20.7%) | 26 (23.4%) |
| TP53 mutation | ||
| Yes | 14 (7.4%) | 59 (53.1%) |
| No | 106 (56.4%) | 10 (9%) |
| NA | 68 (36.2%) | 42 (37.8%) |
| Deletion 11q23 | ||
| Yes | 21 (11.2%) | 56 (50.5%) |
| No | 100 (53.2%) | 14 (12.6%) |
| NA | 67 (35.6%) | 41 (36.9%) |
| CLL treatment status | ||
| Treatment-naive | 29 (15.4%) | 15 (13.5%) |
| During treatment | 117 (62.2%) | 74 (66.7%) |
| After treatment | 41 (21.8%) | 22 (19.8%) |
| NA | 1 (0.5%) | 0 (0.0%) |
| Lines of previous therapy | ||
| ≥4 | 23 (12.2%) | 17 (15.3%) |
| 0–3 | 164 (87.2%) | 94 (84.7%) |
| NA | 1 (0.5%) | 0 (0.0%) |
| BTKi treatment | ||
| No | 137 (72.9%) | 88 (79.3%) |
| Yes | 51 (27.1%) | 23 (20.7%) |
| Venetoclax treatment | ||
| Yes | 50 (26.6%) | 23 (20.7%) |
| No | 138 (73.4%) | 88 (79.3%) |
| Anti-CD20 treatment | ||
| Yes | 46 (24.5%) | 34 (30.6%) |
| No | 142 (75.5%) | 77 (69.4%) |
| No | Yes | ||||
|---|---|---|---|---|---|
| Gender | N | (%) | N | (%) | p-value |
| Men | 43 | 61.43% | 27 | 38.57% | 0.99 |
| Women | 25 | 60.98% | 16 | 39.02% | |
| Age | |||||
| >65 | 39 | 56.52% | 30 | 43.48% | 0.23 |
| ≤65 | 29 | 69.05% | 13 | 30.95% | |
| Rai Stage | |||||
| 0 | 3 | 37.50% | 5 | 62.50% | 0.43 |
| 1 | 16 | 69.57% | 7 | 30.43% | |
| 2 | 21 | 58.33% | 15 | 41.67% | |
| 3 | 11 | 73.33% | 4 | 26.67% | |
| 4 | 13 | 56.52% | 10 | 43.48% | |
| Binet stage | |||||
| A | 12 | 63.16% | 7 | 36.84% | 0.87 |
| B | 24 | 60.00% | 16 | 40.00% | |
| C | 11 | 55.00% | 9 | 45.00% | |
| ECOG | |||||
| 2–4 | 47 | 66.20% | 24 | 33.80% | 0.15 |
| 0–1 | 19 | 51.35% | 18 | 48.65% | |
| WBC [×109/L] | |||||
| ≤25 | 48 | 62.34% | 29 | 37.66% | 0.83 |
| >25 | 20 | 58.82% | 14 | 41.18% | |
| Hemoglobin [g/dL] | |||||
| ≤10 | 18 | 46.15% | 21 | 53.85% | 0.02 |
| >10 | 49 | 69.01% | 22 | 30.99% | |
| Platelets [×109/L] | |||||
| ≤100 | 18 | 46.15% | 21 | 53.85% | 0.02 |
| >100 | 50 | 69.44% | 22 | 30.56% | |
| Lactate dehydrogenase | |||||
| Elevated | 26 | 52.00% | 24 | 48.00% | 0.21 |
| Normal range | 28 | 65.12% | 15 | 34.88% | |
| BMI | |||||
| <18.5 | 1 | 100.00% | 0 | 0.00% | 0.74 |
| 18.6–24.9 | 17 | 65.38% | 9 | 34.62% | |
| 25–30 | 31 | 62.00% | 19 | 38.00% | |
| >30 | 12 | 54.55% | 10 | 45.45% | |
| Creatinine [mg/dL] | |||||
| >1.3 | 8 | 57.14% | 6 | 42.86% | 0.6 |
| ≤1.3 | 59 | 62.11% | 36 | 37.89% | |
| Deletion 17p | |||||
| Yes | 10 | 71.43% | 4 | 28.57% | 0.55 |
| No | 43 | 60.56% | 28 | 39.44% | |
| Deletion 11q23 | |||||
| Yes | 9 | 64.29% | 5 | 35.71% | 0.99 |
| No | 35 | 62.50% | 21 | 37.50% | |
| TP53 mutation | |||||
| Yes | 5 | 17.86% | 23 | 82.14% | 0.73 |
| No | 5 | 50.00% | 5 | 50.00% | |
| CLL treatment status | |||||
| Treatment naive | 8 | 53.33% | 7 | 46.67% | 0.79 |
| After | 14 | 63.64% | 8 | 36.36% | |
| During | 46 | 62.16% | 28 | 37.84% | |
| Lines of previous therapy | |||||
| ≥4 | 10 | 58.82% | 7 | 41.18% | 0.99 |
| 0–3 | 58 | 61.70% | 36 | 38.30% | |
| BTKi treatment | |||||
| No | 52 | 61.18% | 33 | 38.82% | 0.99 |
| Yes | 55 | 62.50% | 33 | 37.50% | |
| Venetoclax treatment | |||||
| Yes | 13 | 56.52% | 10 | 43.48% | 0.64 |
| No | 112 | 73.68% | 40 | 26.32% | |
| Anti-CD20 treatment | |||||
| Yes | 20 | 58.82% | 14 | 41.18% | 0.83 |
| No | 48 | 62.34% | 29 | 37.66% | |
| No. of Patients | OS [Days] | 95% CI | HR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Men | 70 | nr | 45–nr | 1.02 | 0.55–1.91 | 0.76 |
| Women | 41 | nr | 33–nr | |||
| Age | ||||||
| >65 | 69 | nr | 29–nr | 1.71 | 0.92–3.18 | 0.14 |
| ≤65 | 42 | nr | 89–nr | |||
| Rai Stage | ||||||
| 0 | 8 | 29 | 15–nr | 0.35 | ||
| 1 | 23 | nr | 21–nr | |||
| 2 | 36 | nr | 33–nr | |||
| 3 | 15 | nr | 44–nr | |||
| 4 | 23 | nr | 23–nr | |||
| Binet stage | ||||||
| A | 19 | nr | 20–nr | 0.89 | ||
| B | 40 | nr | 82–nr | |||
| C | 20 | nr | 19–nr | |||
| ECOG | ||||||
| 2–4 | 37 | nr | 33–nr | 1.55 | 0.8–3.0 | 0.59 |
| 0–1 | 71 | nr | 89–nr | |||
| WBC [×109/L] | ||||||
| ≤25 | 77 | nr | 31–nr | 0.97 | 0.5–1.85 | 0.92 |
| >25 | 34 | nr | 31–nr | |||
| Hemoglobin [g/dL] | ||||||
| ≤10 | 39 | 44 | 29–nr | 2.0 | 1.05–3.8 | 0.04 |
| >10 | 71 | nr | nr | |||
| Platelets [×109/L] | ||||||
| ≤100 | 39 | 31 | 19–nr | 2.37 | 1.2–4.67 | 0.004 |
| >100 | 72 | nr | nr | |||
| Lactate dehydrogenase | ||||||
| Elevated | 50 | 100 | 22–nr | 1.3 | 0.68–2.49 | 0.28 |
| Normal range | 43 | nr | 33–nr | |||
| BMI | ||||||
| <18.5 | 1 | nr | nr | 0.8 | ||
| 18.5–25 | 26 | nr | 44–nr | |||
| 25–30 | 50 | nr | 29–nr | |||
| >30 | 22 | nr | 25–nr | |||
| Creatinine [mg/dL] | ||||||
| >1.3 | 14 | 100 | 26–nr | 1.34 | 0.47–3.83 | 0.32 |
| ≤1.3 | 95 | nr | 82–nr | |||
| Deletion 17p | ||||||
| No | 71 | nr | 45–nr | 1.35 | 0.53–3.48 | 0.59 |
| Yes | 14 | nr | 8–nr | |||
| Deletion 11q23 | ||||||
| Yes | 14 | nr | 15–nr | 1.1 | 0.4–3.04 | 0.92 |
| No | 56 | nr | 82–nr | |||
| TP53 mutation | ||||||
| Yes | 10 | nr | 3–nr | 0.65 | 0.21–2.0 | 0.42 |
| No | 59 | nr | 45–nr | |||
| CLL treatment status | ||||||
| Treatment naive | 15 | 37 | 20–nr | 0.64 | ||
| After | 22 | nr | 33–nr | |||
| During | 74 | nr | 82–nr | |||
| Lines of previous therapy | ||||||
| ≥4 | 17 | nr | 21–nr | 1.01 | 0.45–2.29 | 0.91 |
| 0–3 | 94 | nr | 45–nr | |||
| BTKi treatment | ||||||
| No | 85 | 370 | 45–nr | 1.07 | 0.52–2.21 | 0.99 |
| Yes | 26 | nr | 21–nr | |||
| Venetoclax treatment | ||||||
| Yes | 23 | 100 | 27–nr | 1.2 | 0.57–2.54 | 0.73 |
| No | 88 | nr | 82–nr | |||
| Anti-CD20 treatment | ||||||
| Yes | 32 | nr | 23–nr | 1.34 | 0.68–2.66 | 0.5 |
| No | 77 | nr | 82–nr | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puła, B.; Pruszczyk, K.; Pietrusza, E.; Morawska, M.; Piszczek, W.; Kalicińska, E.; Szeremet, A.; Tryc-Szponder, J.; Wąsik-Szczepanek, E.; Drozd-Sokołowska, J.; et al. Outcome of SARS-CoV-2-Infected Polish Patients with Chronic Lymphocytic Leukemia. Cancers 2022, 14, 558. https://doi.org/10.3390/cancers14030558
Puła B, Pruszczyk K, Pietrusza E, Morawska M, Piszczek W, Kalicińska E, Szeremet A, Tryc-Szponder J, Wąsik-Szczepanek E, Drozd-Sokołowska J, et al. Outcome of SARS-CoV-2-Infected Polish Patients with Chronic Lymphocytic Leukemia. Cancers. 2022; 14(3):558. https://doi.org/10.3390/cancers14030558
Chicago/Turabian StylePuła, Bartosz, Katarzyna Pruszczyk, Ewa Pietrusza, Marta Morawska, Weronika Piszczek, Elżbieta Kalicińska, Agnieszka Szeremet, Jagoda Tryc-Szponder, Ewa Wąsik-Szczepanek, Joanna Drozd-Sokołowska, and et al. 2022. "Outcome of SARS-CoV-2-Infected Polish Patients with Chronic Lymphocytic Leukemia" Cancers 14, no. 3: 558. https://doi.org/10.3390/cancers14030558






