High-Definition Ultrasound Characterization of Squamous Carcinoma of the Tongue: A Descriptive Observational Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Healthy Tongue Features
- (a)
- A well-defined continuous hyperechoic band that corresponded to the epithelial layer.
- (b)
- An anechoic line with a thickness similar to the epithelium above, representing the short, blunt rete ridges. This layer consisted of the oral mucosa limit since it is impossible to identify the basement membrane for resolution limits of all ultrasound equipment (even at higher frequencies).
- (c)
- A homogeneous, relatively hypoechoic band of connective tissue, where submucosa merged and intersected with the ventral muscle bundles of the tongue.
- (d)
- An alternation of hypoechogenic, hyperechoic, and anechoic areas configuring striated muscle bundles that recall the histological fascicular features. A very slight acoustic enhancement was present. This layer represented the thickest portion of the ultrasound image, and within this area, it was also possible to observe the vessels of medium and large caliber.
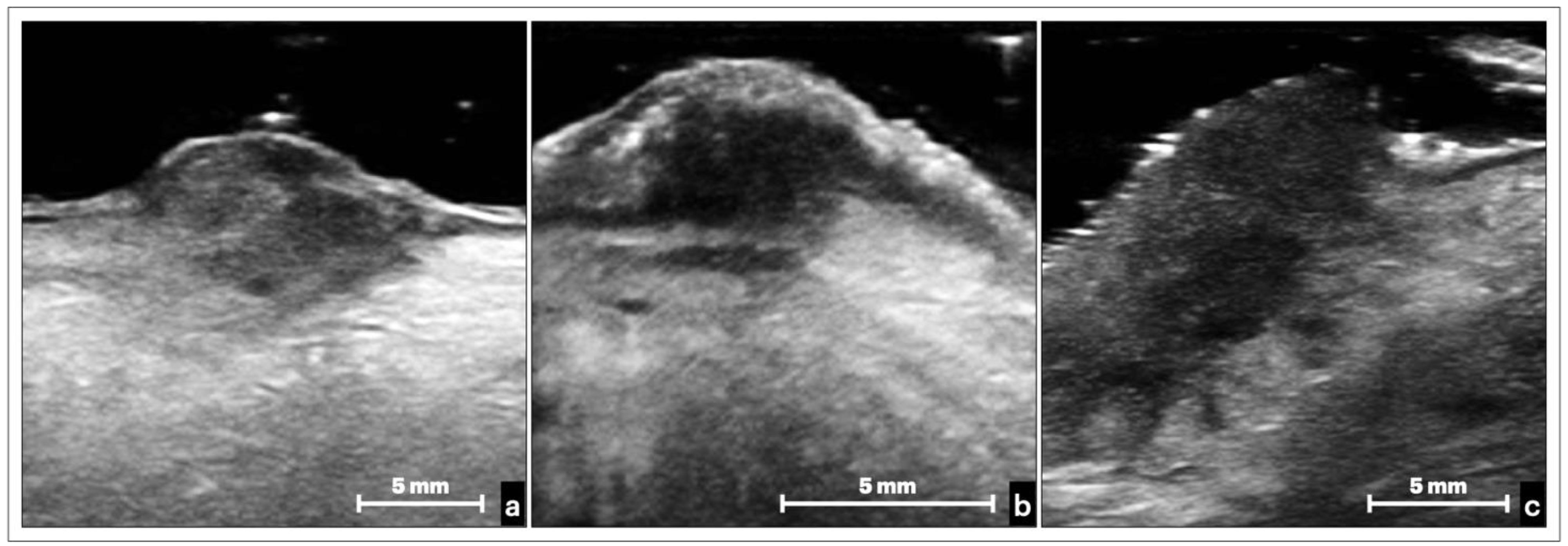
3.2. Ultrasonographic Appearance of Tongue OSCC
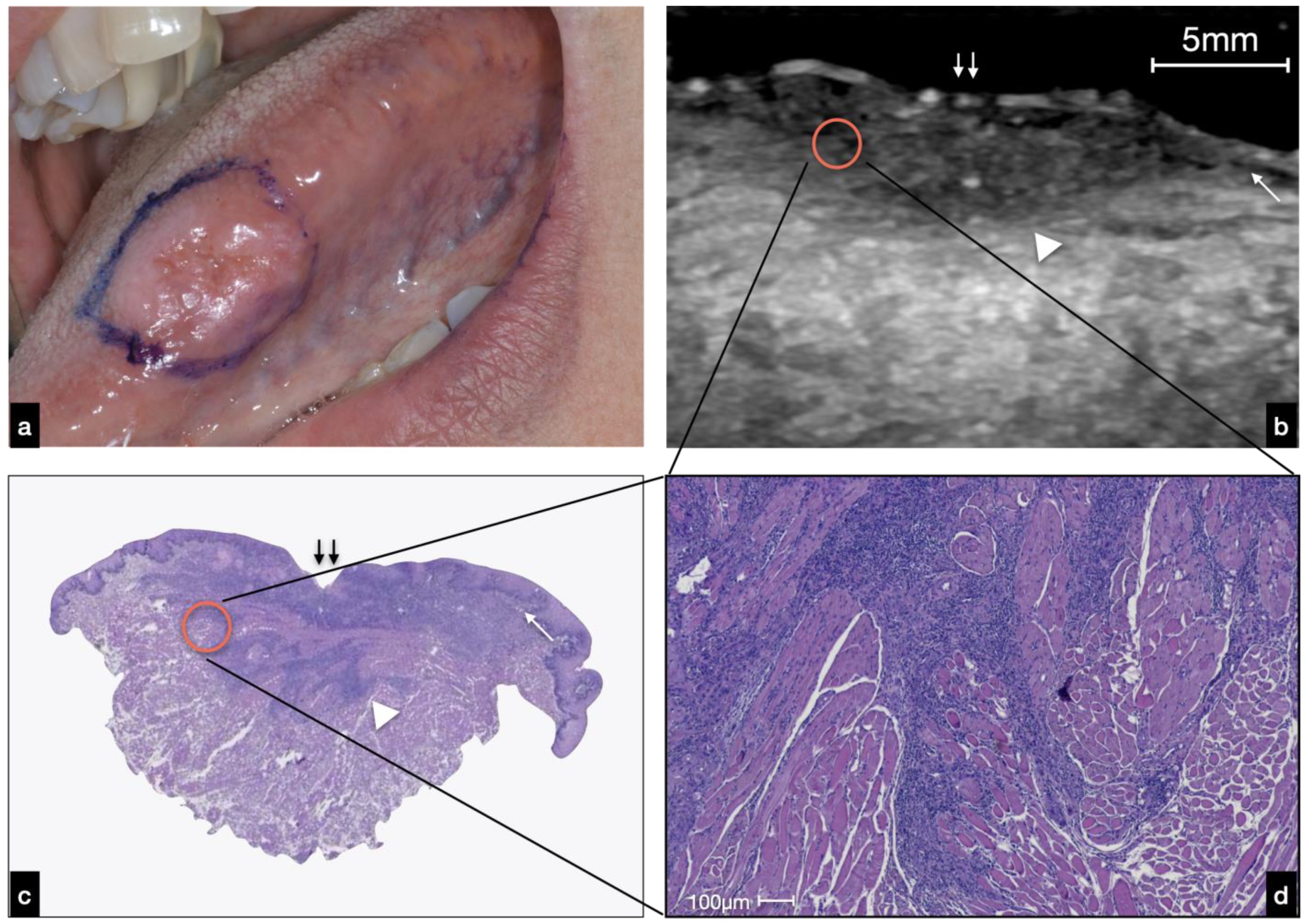
3.2.1. Ulcerated OSCC
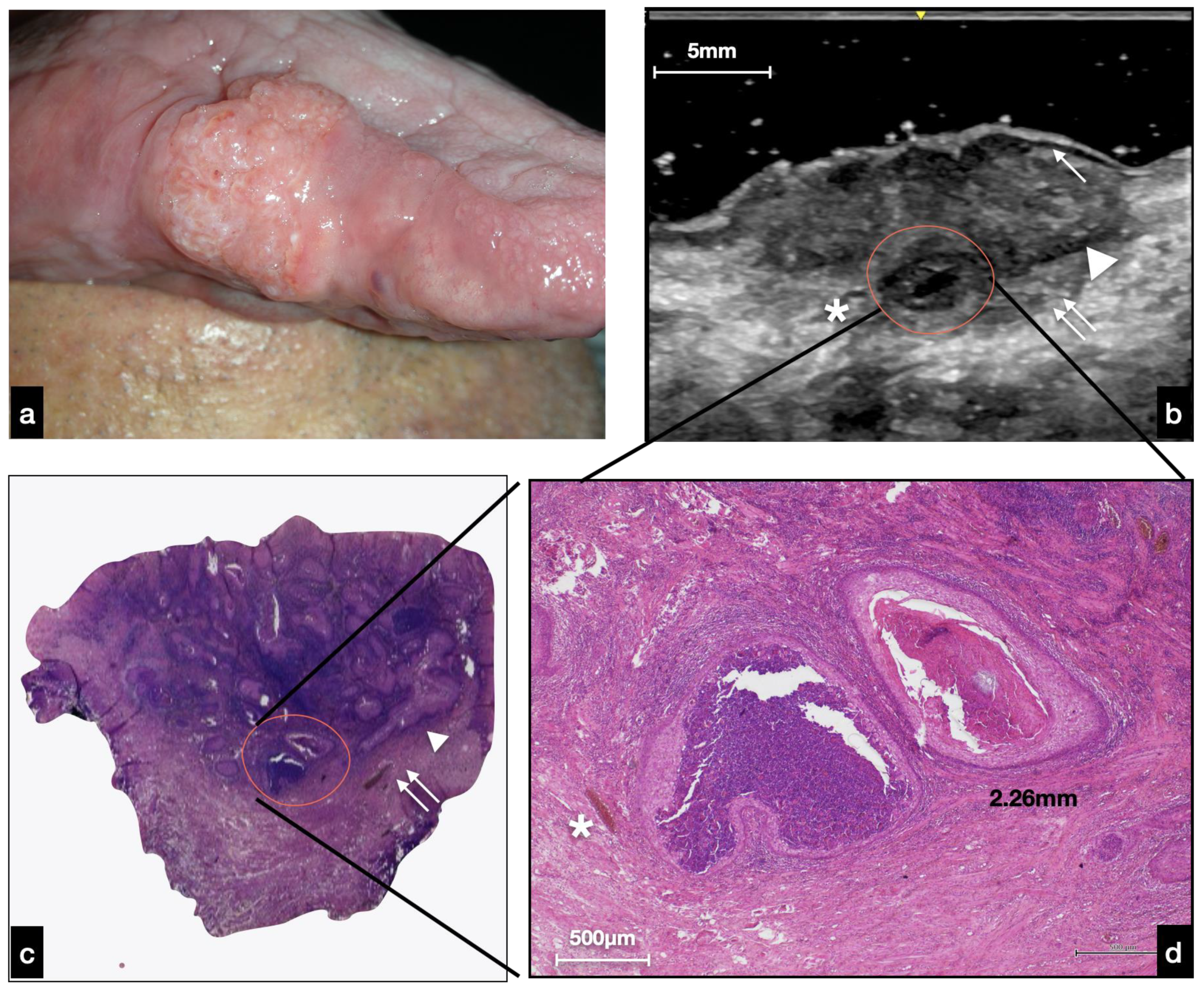
3.2.2. Nodular OSCC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| US | ultrasound |
| OSCC | oral squamous cell carcinoma |
| SCC | squamous cell carcinoma |
| BCC | squamous cell basalioma |
| BM | basal membrane |
| V | vessel |
References
- Boccellino, M.; Di Stasio, D.; Dipalma, G.; Cantore, S.; Ambrosio, P.; Coppola, M.; Quagliuolo, L.; Scarano, A.; Malcangi, G.; Borsani, E.; et al. Steroids and Growth Factors in Oral Squamous Cell Carcinoma: Useful Source of Dental-Derived Stem Cells to Develop a Steroidogenic Model in New Clinical Strategies. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8730–8740. [Google Scholar] [CrossRef]
- Gentile, E.; Maio, C.; Romano, A.; Laino, L.; Lucchese, A. The Potential Role of in vivo Optical Coherence Tomography for Evaluating Oral Soft Tissue: A Systematic Review. J. Oral Pathol. Med. 2017, 46, 864–876. [Google Scholar] [CrossRef]
- Lucchese, A.; Gentile, E.; Romano, A.; Maio, C.; Laino, L.; Serpico, R. The Potential Role of in vivo Reflectance Confocal Microscopy for Evaluating Oral Cavity Lesions: A Systematic Review. J. Oral Pathol. Med. 2016, 45, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Di Stasio, D.; Petruzzi, M.; Serpico, R.; Lucchese, A. In Vivo Reflectance Confocal Microscopy of Oral Lichen Planus. Int. J. Dermatol. 2019, 58, 940–945. [Google Scholar] [CrossRef]
- Lucchese, A.; Scivetti, M.; Pilolli, G.P.; Favia, G. Analysis of Ghost Cells in Calcifying Cystic Odontogenic Tumors by Confocal Laser Scanning Microscopy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 104, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.L.; Copel, J.A. Point-of-Care Ultrasonography. N. Engl. J. Med. 2011, 364, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, A.; Brown, J.; Rudralingam, M. The Use of Intraoral Ultrasound in the Characterization of Minor Salivary Gland Malignancy: Report of Two Cases. Dentomaxillofac. Radiol. 2016, 45, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Salmon, B.; Le Denmat, D. Intraoral Ultrasonography: Development of a Specific High-Frequency Probe and Clinical Pilot Study. Clin. Oral Investig. 2012, 16, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Angelelli, G.; Moschetta, M.; Limongelli, L.; Albergo, A.; Lacalendola, E.; Brindicci, F.; Favia, G.; Maiorano, E. Endocavitary Sonography of Early Oral Cavity Malignant Tumors. Head Neck 2017, 39, 1349–1356. [Google Scholar] [CrossRef]
- Di Stasio, D.; Lauritano, D.; Paparella, R.; Franco, R.; Montella, M.; Serpico, R.; Lucchese, A. Ultrasound Imaging of Oral Fibroma: A Case Report. J. Biol. Regul. Homeost. Agents 2017, 31, 23–26. [Google Scholar]
- Shintani, S.; Yoshihama, Y.; Ueyama, Y.; Terakado, N.; Kamei, S.; Fijimoto, Y.; Hasegawa, Y.; Matsuura, H.; Matsumura, T. The Usefulness of Intraoral Ultrasonography in the Evaluation of Oral Cancer. Int. J. Oral Maxillofac. Surg. 2001, 30, 139–143. [Google Scholar] [CrossRef]
- Lam, M.; Chaudhari, A.J.; Sun, Y.; Zhou, F.; Dobbie, A.; Gandour-Edwards, R.F.; Tinling, S.P.; Farwell, D.G.; Monsky, W.L.; Shung, K.K.; et al. Ultrasound Backscatter Microscopy for Imaging of Oral Carcinoma. J. Ultrasound Med. 2013, 32, 1789–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein Nulent, T.J.W.; Noorlag, R.; Van Cann, E.M.; Pameijer, F.A.; Willems, S.M.; Yesuratnam, A.; Rosenberg, A.J.W.P.; de Bree, R.; van Es, R.J.J. Intraoral Ultrasonography to Measure Tumor Thickness of Oral Cancer: A Systematic Review and Meta-Analysis. Oral Oncol. 2018, 77, 29–36. [Google Scholar] [CrossRef]
- Iida, Y.; Kamijo, T.; Kusafuka, K.; Omae, K.; Nishiya, Y.; Hamaguchi, N.; Morita, K.; Onitsuka, T. Depth of Invasion in Superficial Oral Tongue Carcinoma Quantified Using Intraoral Ultrasonography. Laryngoscope 2018, 128, 2778–2782. [Google Scholar] [CrossRef] [PubMed]
- Horos Project. Available online: https://horosproject.org/ (accessed on 16 December 2021).
- EI-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. WHO Classification of Head and Neck Tumours; IARC Publications: Lyon, France, 2017; ISBN 978-92-832-2438-9. [Google Scholar]
- Belfiore, M.P.; Reginelli, A.; Russo, A.; Russo, G.M.; Rocco, M.P.; Moscarella, E.; Ferrante, M.; Sica, A.; Grassi, R.; Cappabianca, S. Usefulness of High-Frequency Ultrasonography in the Diagnosis of Melanoma: Mini Review. Front. Oncol. 2021, 11, 673026. [Google Scholar] [CrossRef]
- Remonti, L.R.; Kramer, C.K.; Leitão, C.B.; Pinto, L.C.F.; Gross, J.L. Thyroid Ultrasound Features and Risk of Carcinoma: A Systematic Review and Meta-Analysis of Observational Studies. Thyroid. Off. J. Am. Thyroid Assoc. 2015, 25, 538–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.J.; Chang, J.L. Practical Salivary Ultrasound Imaging Tips and Pearls. Otolaryngol. Clin. N. Am. 2021, 54, 471–487. [Google Scholar] [CrossRef]
- Tadayyon, H.; Sannachi, L.; Gangeh, M.; Sadeghi-Naini, A.; Tran, W.; Trudeau, M.E.; Pritchard, K.; Ghandi, S.; Verma, S.; Czarnota, G.J. Quantitative Ultrasound Assessment of Breast Tumor Response to Chemotherapy Using a Multi-Parameter Approach. Oncotarget 2016, 7, 45094–45111. [Google Scholar] [CrossRef] [Green Version]
- Cozzolino, I.; Ronchi, A.; Messina, G.; Montella, M.; Morgillo, F.; Vicidomini, G.; Tirino, V.; Grimaldi, A.; Marino, F.Z.; Santini, M.; et al. Adequacy of Cytologic Samples by Ultrasound-Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology of Peripheral Pulmonary Nodules for Morphologic Diagnosis and Molecular Evaluations: Comparison With Computed Tomography-Guided Percutaneous Tran. Arch. Pathol. Lab. Med. 2020, 144, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in Diagnosis of Pancreatic Cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.; Wortsman, X. Dermatology Ultrasound. Imaging Technique, Tips and Tricks, High-Resolution Anatomy. Ultrasound Q. 2020, 36, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Bamber, J.; Chuchu, N.; Bayliss, S.E.; Takwoingi, Y.; Davenport, C.; Godfrey, K.; O’sullivan, C.; Matin, R.N.; Deeks, J.J.; et al. High-Frequency Ultrasound for Diagnosing Skin Cancer in Adults. Cochrane Database Syst. Rev. 2018, 2018, CD013188. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.; Roldán, F.A.; Varelli, C.; Bard, R.; Corvino, A.; Wortsman, X. Skin Cancer: Findings and Role of High-Resolution Ultrasound. J. Ultrasound 2019, 22, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.Q.; Wang, L.F.; Li, X.L.; Wang, Q.; Li, M.X.; Ma, Y.Y.; Xiang, L.H.; Guo, L.H.; Xu, H.X. High-Frequency Ultrasound in the Diagnosis of the Spectrum of Cutaneous Squamous Cell Carcinoma: Noninvasively Distinguishing Actinic Keratosis, Bowen’s Disease, and Invasive Squamous Cell Carcinoma. Ski. Res. Technol. 2021, 27, 831–840. [Google Scholar] [CrossRef]
- Tarabichi, O.; Bulbul, M.G.; Kanumuri, V.V.; Faquin, W.C.; Juliano, A.F.; Cunnane, M.E.; Varvares, M.A. Utility of Intraoral Ultrasound in Managing Oral Tongue Squamous Cell Carcinoma: Systematic Review. Laryngoscope 2019, 129, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Chammas, M.C.; MacEdo, T.A.A.; Moyses, R.A.; Gerhard, R.; Durazzo, M.D.; Cernea, C.R.; Cerri, G.G. Relationship between the Appearance of Tongue Carcinoma on Intraoral Ultrasonography and Neck Metastasis. Oral Radiol. 2011, 27, 1–7. [Google Scholar] [CrossRef]
- Izzetti, R.; Vitali, S.; Aringhieri, G.; Caramella, D.; Nisi, M.; Oranges, T.; Dini, V.; Graziani, F.; Gabriele, M. The Efficacy of Ultra-High Frequency Ultrasonography in the Diagnosis of Intraoral Lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 401–410. [Google Scholar] [CrossRef]


| Patient | Age | Gender | Clinical Features | Tongue Subsite | Histopathology |
|---|---|---|---|---|---|
| 1 | 73 | F | ulcerated | left margin | OSCC—G2 |
| 2 | 69 | M | nodular | right margin | OSCC—G2 |
| 3 | 36 | M | ulcerated | left margin | OSCC—G2 |
| 4 | 79 | M | ulcerated | dorsum/right margin | OSCC—G2 |
| 5 | 50 | M | ulcerated | right margin | OSCC—G1 |
| 6 | 82 | F | ulcerated | right margin | OSCC—G2 |
| 7 | 79 | M | nodular | ventral/left margin | OSCC—G1 |
| 8 | 79 | F | nodular | left margin | OSCC—G1 |
| 9 | 76 | M | nodular | dorsum/left margin | OSCC—G2 |
| 10 | 47 | M | ulcerated | ventral/right margin | OSCC—G1 |
| 11 | 73 | M | nodular | right margin | OSCC—G1 |
| 12 | 73 | F | nodular | dorsum/right margin | OSCC—G1 |
| 13 | 57 | M | nodular | right margin | OSCC—G1 |
| 14 | 78 | M | ulcerated | left margin | OSCC—G1 |
| 15 | 60 | M | ulcerated | left margin | OSCC—G1 |
| 16 | 68 | M | ulcerated | left margin | OSCC—G1 |
| 17 | 45 | M | nodular | right margin | OSCC—G1 |
| 18 | 61 | F | ulcerated | left margin | OSCC—G2 |
| 19 | 70 | M | nodular | left margin | OSCC—G3 |
| 20 | 51 | F | nodular | right margin | OSCC—G1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stasio, D.; Montella, M.; Romano, A.; Colella, G.; Serpico, R.; Lucchese, A. High-Definition Ultrasound Characterization of Squamous Carcinoma of the Tongue: A Descriptive Observational Study. Cancers 2022, 14, 564. https://doi.org/10.3390/cancers14030564
Di Stasio D, Montella M, Romano A, Colella G, Serpico R, Lucchese A. High-Definition Ultrasound Characterization of Squamous Carcinoma of the Tongue: A Descriptive Observational Study. Cancers. 2022; 14(3):564. https://doi.org/10.3390/cancers14030564
Chicago/Turabian StyleDi Stasio, Dario, Marco Montella, Antonio Romano, Giuseppe Colella, Rosario Serpico, and Alberta Lucchese. 2022. "High-Definition Ultrasound Characterization of Squamous Carcinoma of the Tongue: A Descriptive Observational Study" Cancers 14, no. 3: 564. https://doi.org/10.3390/cancers14030564
APA StyleDi Stasio, D., Montella, M., Romano, A., Colella, G., Serpico, R., & Lucchese, A. (2022). High-Definition Ultrasound Characterization of Squamous Carcinoma of the Tongue: A Descriptive Observational Study. Cancers, 14(3), 564. https://doi.org/10.3390/cancers14030564









