Real World Outcomes versus Clinical Trial Results of Durvalumab Maintenance in Veterans with Stage III Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Patient Selection
2.3. Outcomes and Covariates
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
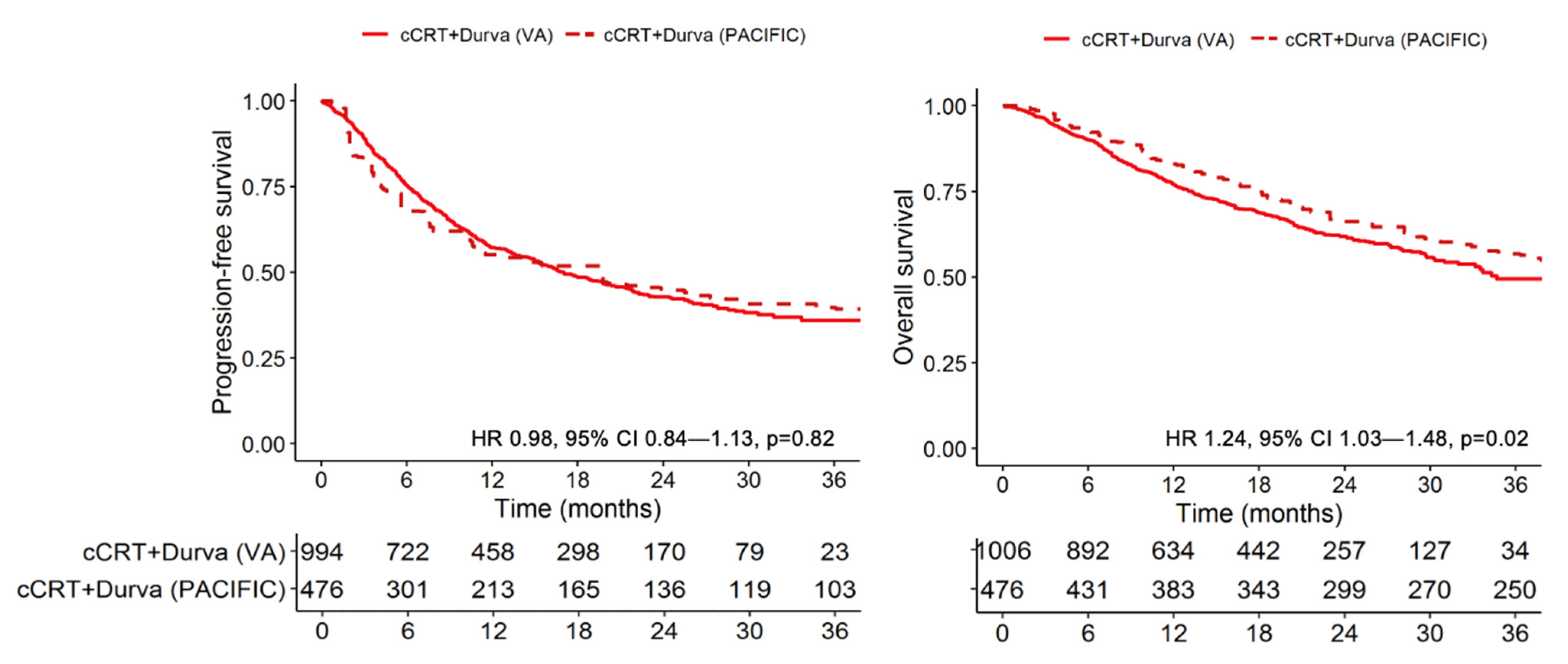
3.2. Progression-Free and Overall Survival
3.3. Efficacy-Effectiveness Factor Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.G.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: An update from the PACIFIC trial. J. Clin. Oncol. 2021, 39, 8511. [Google Scholar] [CrossRef]
- Hui, R.; Ozguroglu, M.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): A randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1670–1680. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Golden, S.E.; Hooker, E.R.; Shull, S.; Howard, M.; Crothers, K.; Thompson, R.F.; Slatore, C.G. Validity of Veterans Health Administration structured data to determine accurate smoking status. Health Inform. J. 2020, 26, 1507–1515. [Google Scholar] [CrossRef] [Green Version]
- Melzer, A.C.; Pinsker, E.A.; Clothier, B.; Noorbaloochi, S.; Burgess, D.J.; Danan, E.R.; Fu, S.S. Validating the use of veterans affairs tobacco health factors for assessing change in smoking status: Accuracy, availability, and approach. BMC Med. Res. Methodol. 2018, 18, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Spigel, D.R.; Garassino, M.C.; Reck, M.; Senan, S.; et al. Four-Year Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-an Update from the PACIFIC Trial. J. Thorac. Oncol. 2021, 16, 860–867. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2021, 21, 111. [Google Scholar] [CrossRef]
- Santana-Davila, R.; Devisetty, K.; Szabo, A.; Sparapani, R.; Arce-Lara, C.; Gore, E.M.; Moran, A.; Williams, C.D.; Kelley, M.J.; Whittle, J. Cisplatin and etoposide versus carboplatin and paclitaxel with concurrent radiotherapy for stage III non-small-cell lung cancer: An analysis of Veterans Health Administration data. J. Clin. Oncol. 2015, 33, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Kehl, K.L.; Greenwald, S.; Chamoun, N.G.; Manberg, P.J.; Schrag, D. Association Between First-Line Immune Checkpoint Inhibition and Survival for Medicare-Insured Patients With Advanced Non-Small Cell Lung Cancer. JAMA Netw. Open 2021, 4, e2111113. [Google Scholar] [CrossRef] [PubMed]
- Cramer-van der Welle, C.M.; Verschueren, M.V.; Tonn, M.; Peters, B.J.M.; Schramel, F.; Klungel, O.H.; Groen, H.J.M.; van de Garde, E.M.W.; Santeon, N.S.G. Real-world outcomes versus clinical trial results of immunotherapy in stage IV non-small cell lung cancer (NSCLC) in the Netherlands. Sci. Rep. 2021, 11, 6306. [Google Scholar] [CrossRef] [PubMed]
- La, J.; Cheng, D.; Brophy, M.T.; Do, N.V.; Lee, J.S.H.; Tuck, D.; Fillmore, N.R. Real-World Outcomes for Patients Treated With Immune Checkpoint Inhibitors in the Veterans Affairs System. JCO Clin. Cancer Inf. 2020, 4, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Khozin, S.; Carson, K.R.; Zhi, J.; Tucker, M.; Lee, S.E.; Light, D.E.; Curtis, M.D.; Bralic, M.; Kaganman, I.; Gossai, A.; et al. Real-World Outcomes of Patients with Metastatic Non-Small Cell Lung Cancer Treated with Programmed Cell Death Protein 1 Inhibitors in the Year Following US Regulatory Approval. Oncologist 2019, 24, 648–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amrane, K.; Geier, M.; Corre, R.; Lena, H.; Leveiller, G.; Gadby, F.; Lamy, R.; Bizec, J.L.; Goarant, E.; Robinet, G.; et al. First-line pembrolizumab for non-small cell lung cancer patients with PD-L1 >/=50% in a multicenter real-life cohort: The PEMBREIZH study. Cancer Med. 2020, 9, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, D.; Lam, J.; Betts, K.A.; Yin, L.; Gao, S.; Yuan, Y.; Hartman, J.; Rao, S.; Lubinga, S.; Stenehjem, D. Real-world outcomes of immunotherapy-based regimens in first-line advanced non-small cell lung cancer. Lung Cancer 2021, 156, 41–49. [Google Scholar] [CrossRef]
- Hussaini, S.; Chehade, R.; Boldt, R.G.; Raphael, J.; Blanchette, P.; Maleki Vareki, S.; Fernandes, R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 92, 102134. [Google Scholar] [CrossRef]
- Brown, D.W. Smoking prevalence among US veterans. J. Gen. Intern. Med. 2010, 25, 147–149. [Google Scholar] [CrossRef] [Green Version]
- Odani, S.; Agaku, I.T.; Graffunder, C.M.; Tynan, M.A.; Armour, B.S. Tobacco Product Use Among Military Veterans—United States, 2010–2015. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Mannino, D.M.; Gagnon, R.C.; Petty, T.L.; Lydick, E. Obstructive lung disease and low lung function in adults in the United States: Data from the National Health and Nutrition Examination Survey, 1988–1994. Arch. Intern. Med. 2000, 160, 1683–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, M.; Hertz, R.P. Utilization of Veterans Affairs Medical Care Services by United States Veterans. In Population Studies Outcomes Research; Pfizer Global Pharmaceuticals: Brooklyn, NY, USA, 2003; Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.389.1296&rep=rep1&type=pdf (accessed on 1 December 2021).
- Cui, P.; Liu, Z.; Wang, G.; Ma, J.; Qian, Y.; Zhang, F.; Han, C.; Long, Y.; Li, Y.; Zheng, X.; et al. Risk factors for pneumonitis in patients treated with anti-programmed death-1 therapy: A case-control study. Cancer Med. 2018, 7, 4115–4120. [Google Scholar] [CrossRef] [PubMed]
- Toh, C.K.; Wong, E.H.; Lim, W.T.; Leong, S.S.; Fong, K.W.; Wee, J.; Tan, E.H. The impact of smoking status on the behavior and survival outcome of patients with advanced non-small cell lung cancer: A retrospective analysis. Chest 2004, 126, 1750–1756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, M.; Giobbie-Hurder, A.; Hatabu, H.; Ramaiya, N.H.; Hodi, F.S. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1607–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Fu, Y.; Zhu, B.; Zhang, B.; Wang, J. Pneumonitis Induced by Immune Checkpoint Inhibitors: From Clinical Data to Translational Investigation. Front. Oncol. 2020, 10, 1785. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keppler, J.S. Federal regulations and reimbursement for PET. J. Nucl. Med. Technol. 2001, 29, 173–179. [Google Scholar]
- Bietendorf, J. FDG PET reimbursement. J. Nucl. Med. Technol. 2004, 32, 33–38. [Google Scholar]
- Morgensztern, D.; Ng, S.H.; Gao, F.; Govindan, R. Trends in stage distribution for patients with non-small cell lung cancer: A National Cancer Database survey. J. Thorac. Oncol. 2010, 5, 29–33. [Google Scholar] [CrossRef] [Green Version]

| Variable | Cohort 1 (cCRT Plus Durvalumab) | Cohort 2 (cCRT Alone) | p-Value * | Durvalumab Group (PACIFIC) [1] | Placebo Group (PACIFIC) [1] | |
|---|---|---|---|---|---|---|
| N | 1006 | 989 | 476 | 237 | ||
| Age, median in years (IQR) | 69 (64–72) | 68 (64–71) | 0.009 | 64 | 64 | |
| Race, n (%) | African American | 221 (22.0) | 161 (16.3) | 0.001 | 120 (25.2) | 72 (30.4) |
| Caucasian | 745 (74.1) | 767 (77.6) | 337 (70.8) | 157 (66.2) | ||
| Other/unknown | 40 (3.98) | 61 (6.17) | 120 (25.2) | 72 (30.4) | ||
| Sex, n (%) | Female | 47 (4.67) | 26 (2.63) | 0.015 | 142 (29.8) | 71 (30.0) |
| Male | 959 (95.3) | 963 (97.4) | 334 (70.2) | 166 (70.0) | ||
| CCI, n (%) | 0–2 | 148 (14.7) | 241 (24.4) | <0.001 | ||
| 3–5 | 342 (34.0) | 363 (36.7) | ||||
| 6–8 | 137 (13.6) | 127 (12.8) | ||||
| 9+ | 379 (37.7) | 258 (26.1) | ||||
| Smoking, n (%) | Current | 435 (43.2) | 432 (43.7) | 0.001 | 79 (16.6) | 38 (16.0) |
| Former | 402 (40.0) | 334 (33.8) | 354 (74.4) | 178 (75.1) | ||
| Never | 87 (8.65) | 98 (9.91) | 43 (9.0) | 21 (8.9) | ||
| Unknown | 82 (8.15) | 125 (12.6) | - | - | ||
| Stage, n (%) | IIIA | 559 (55.6) | 667 (67.4) | <0.001 | 252 (52.9) | 125 (52.7) |
| IIIB | 352 (35.0) | 322 (32.6) | 212 (44.5) | 107 (45.1) | ||
| IIIC | 66 (6.56) | -- | - | - | ||
| III NOS | 29 (2.88) | -- | 12 (2.5) | 5 (2.1) | ||
| Concurrent chemotherapy, n (%) | Carboplatin/paclitaxel | 711 (70.7) | 700 (70.8) | <0.001 | ||
| Cisplatin/etoposide | 62 (6.16) | 92 (9.30) | ||||
| Platinum/pemetrexed | 106 (10.5) | 6 (0.61) | ||||
| Other/unknown | 127 (12.6) | 191 (19.3) | ||||
| Histology | Adenocarcinoma | 490 (48.7) | 340 (34.4) | <0.001 | 252 (52.9) | 135 (57.0) |
| Squamous cell carcinoma | 485 (48.2) | 522 (52.8) | 224 (47.1) | 102 (43.0) | ||
| Other | 31 (3.08) | 127 (12.8) | - | - | ||
| Time from RT end to durvalumab start, median in days (IQR) | 42 (29–63) | -- |
| PFS | OS | ||||
|---|---|---|---|---|---|
| Variable | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Cohort | Cohort 2 (pre-durvalumab) | Ref | Ref | Ref | Ref |
| Cohort 1 (durvalumab) | 0.62 (0.55–0.70) | <0.001 | 0.57 (0.50–0.66) | <0.001 | |
| Age (per 10 years) | 1.12 (1.03–1.22) | 0.009 | 1.20 (1.10–1.32) | <0.001 | |
| Male | 1.36 (0.95–1.94) | 0.09 | 1.33 (0.92–1.93) | 0.13 | |
| Race | African American | Ref | Ref | Ref | Ref |
| Caucasian | 1.03 (0.89–1.19) | 0.69 | 1.16 (0.98–1.36) | 0.08 | |
| Other/unknown | 1.05 (0.81–1.37) | 0.71 | 1.05 (0.78–1.41) | 0.74 | |
| Smoking | Current | Ref | Ref | Ref | Ref |
| Former | 1.01 (0.89–1.15) | 0.85 | 1.04 (0.91–1.19) | 0.58 | |
| Never | 1.03 (0.85–1.25) | 0.79 | 1.01 (0.82–1.24) | 0.94 | |
| Unknown | 1.07 (0.89–1.30) | 0.47 | 1.10 (0.90–1.34) | 0.34 | |
| Stage | IIIA | Ref | Ref | Ref | Ref |
| IIIB | 1.23 (1.09–1.38) | <0.001 | 1.21 (1.07–1.37) | 0.003 | |
| IIIC | 1.49 (1.07–2.07) | 0.019 | 1.23 (0.81–1.86) | 0.32 | |
| III NOS | 0.89 (0.51–1.57) | 0.69 | 0.95 (0.48–1.85) | 0.87 | |
| Chemotherapy | Other/unknown | Ref | Ref | Ref | Ref |
| Carboplatin/paclitaxel | 1.01 (0.89–1.14) | 0.93 | 0.96 (0.85–1.10) | 0.58 | |
| Histology | Adenocarcinoma | Ref | Ref | Ref | Ref |
| Squamous cell carcinoma | 0.86 (0.77–0.97) | 0.01 | 0.97 (0.85–1.10) | 0.59 | |
| Other | 1.02 (0.83–1.25) | 0.87 | 1.07 (0.86–1.32) | 0.55 | |
| CCI | 0–2 | Ref | Ref | Ref | Ref |
| 3–5 | 1.18 (1.01–1.38) | 0.04 | 1.22 (1.03–1.43) | 0.02 | |
| 6–8 | 1.30 (1.07–1.58) | 0.008 | 1.12 (0.91–1.38) | 0.30 | |
| 9+ | 1.20 (1.02–1.41) | 0.03 | 1.26 (1.06–1.50) | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sankar, K.; Bryant, A.K.; Strohbehn, G.W.; Zhao, L.; Elliott, D.; Moghanaki, D.; Kelley, M.J.; Ramnath, N.; Green, M.D. Real World Outcomes versus Clinical Trial Results of Durvalumab Maintenance in Veterans with Stage III Non-Small Cell Lung Cancer. Cancers 2022, 14, 614. https://doi.org/10.3390/cancers14030614
Sankar K, Bryant AK, Strohbehn GW, Zhao L, Elliott D, Moghanaki D, Kelley MJ, Ramnath N, Green MD. Real World Outcomes versus Clinical Trial Results of Durvalumab Maintenance in Veterans with Stage III Non-Small Cell Lung Cancer. Cancers. 2022; 14(3):614. https://doi.org/10.3390/cancers14030614
Chicago/Turabian StyleSankar, Kamya, Alex K. Bryant, Garth W. Strohbehn, Lili Zhao, David Elliott, Drew Moghanaki, Michael J. Kelley, Nithya Ramnath, and Michael D. Green. 2022. "Real World Outcomes versus Clinical Trial Results of Durvalumab Maintenance in Veterans with Stage III Non-Small Cell Lung Cancer" Cancers 14, no. 3: 614. https://doi.org/10.3390/cancers14030614
APA StyleSankar, K., Bryant, A. K., Strohbehn, G. W., Zhao, L., Elliott, D., Moghanaki, D., Kelley, M. J., Ramnath, N., & Green, M. D. (2022). Real World Outcomes versus Clinical Trial Results of Durvalumab Maintenance in Veterans with Stage III Non-Small Cell Lung Cancer. Cancers, 14(3), 614. https://doi.org/10.3390/cancers14030614






