Cancer-on-a-Chip: Models for Studying Metastasis
Abstract
:Simple Summary
Abstract
1. Introduction
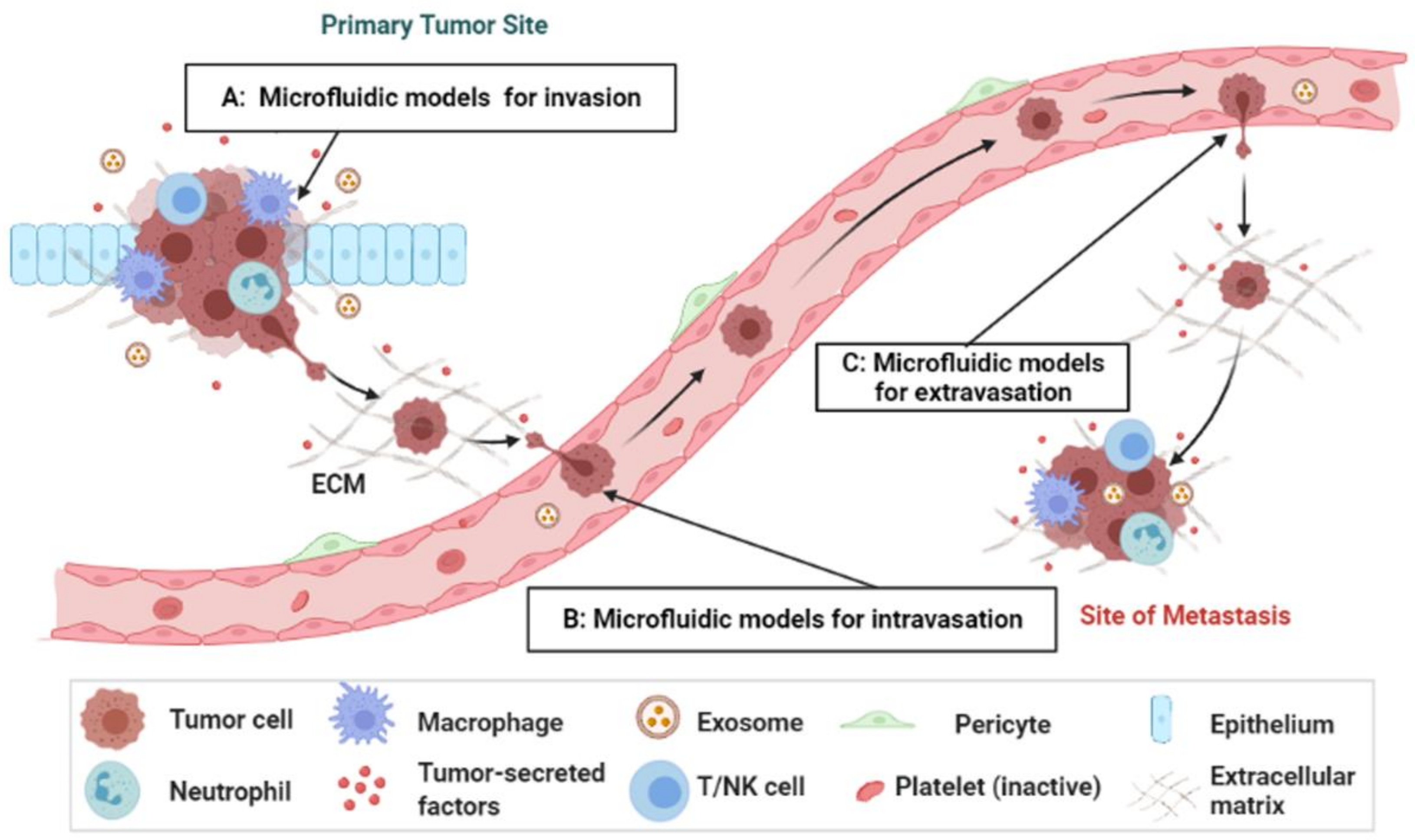
2. Overview of the Metastasis Process
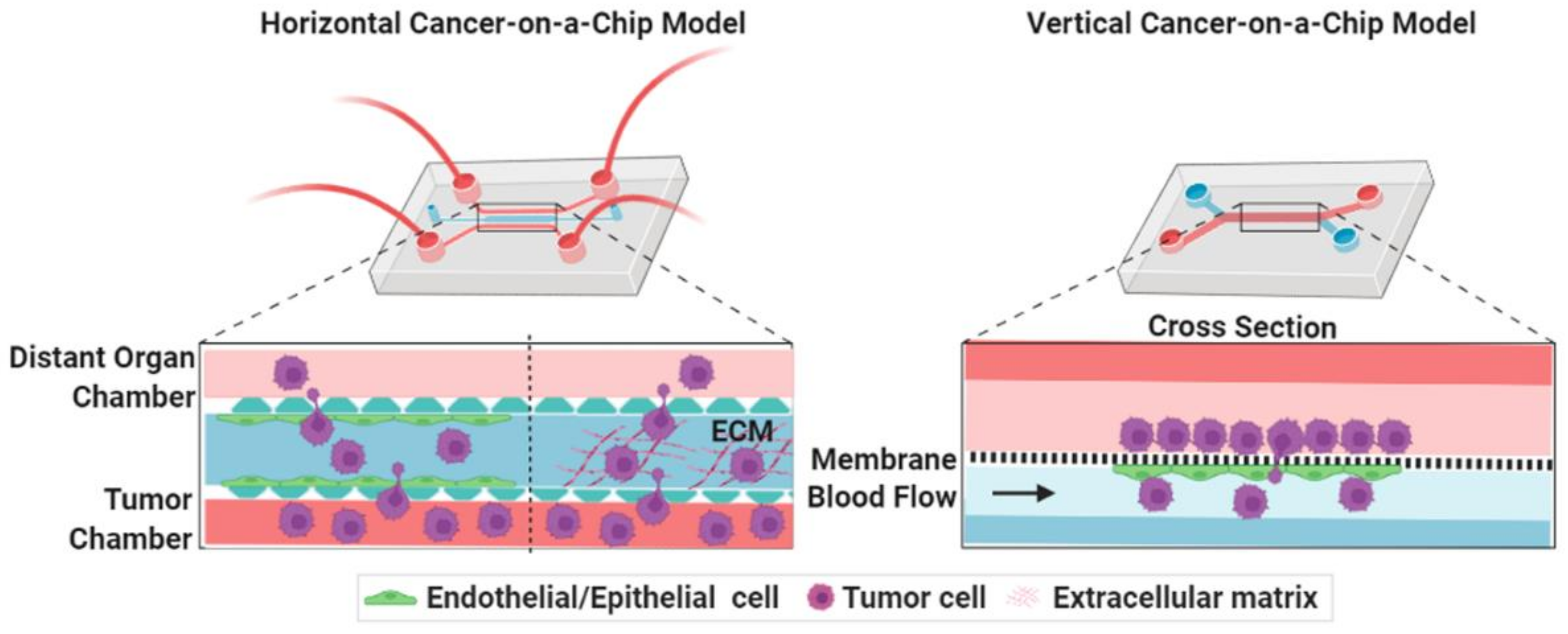
3. Microengineered Metastatic Models
3.1. Cancer-on-a-Chip Models for Studying Cancer Cell Invasion/Intravasation
3.1.1. Influence of Inflammatory Cells
3.1.2. Influence of Cancer-Associated Fibroblasts (CAFs)
3.1.3. Use of Endothelium-Based Models
3.2. Cancer-on-a-Chip Models for Studying Cancer Extravasation
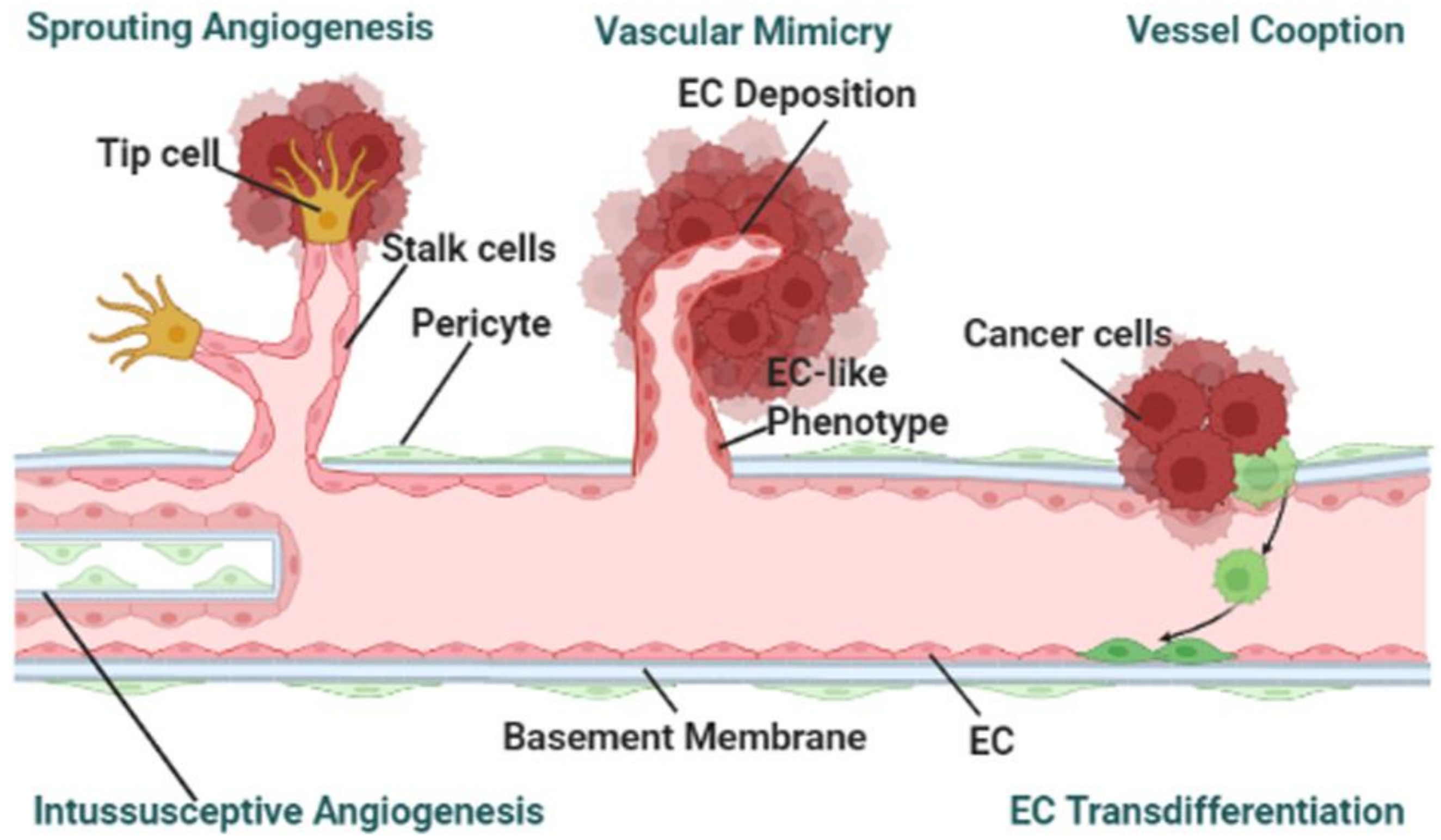
3.3. Cancer-on-a-Chip Models for Studying Tumor-Associated Angiogenesis
4. Microfluidic Modeling: A Focus on Mechanical Factors
4.1. Mechanical Factors in Cancer Cell Migration
4.1.1. Simulating Stiffness in the Microfluidic-Based On-Chip Models
4.1.2. Simulating Fluid Shear Stress in the Microfluidic-Based On-Chip Models
4.1.3. Simulating Cellular Deformability in the Microfluidic-Based On-Chip Models
4.1.4. Simulating Oxygen Gradients and Hypoxia in the Microfluidic-Based On-Chip Models
5. Conclusions, Challenges and Future Perspectives
5.1. Conclusions
5.2. Challenges and Future Perspectives
- The mechanism studies carried out so far focus on either exploring the biochemical or mechanical factors. However, both components are responsible for the progression of the metastatic process. Therefore, microfluidic models should be developed in such a way that both of these factors can be studied simultaneously. This could also be done utilizing existing chip models by carrying out studies where biochemical and mechanical cues are introduced in a stepwise manner.
- Most microfluidic-based cancer-on-a-chip models use buffers to simulate the interstitial flow of the blood stream, which are very different from the physiological condition, such as the rheology of the blood. Whole blood viscosity positively correlates with the cancer stage and metastases [70,71,72,73]. Thus, the blood viscosity should be taken into consideration before flowing into the cancer chips. Recently, another study has shown the impact of red blood cell (RBC) dynamics in the rate of tumor oxygenation, where RBC partitioning at the bifurcations of compressed tumor vessels follow a hematocrit-dependent rather than flow rate-independent manner. Compressed vessels bias red blood cell partitioning at bifurcations in a hematocrit-dependent manner: Implications in tumor blood flow. Thus, the inclusion such versatile blood components would be important to analyze the impact of mechanical factors in tumor progression.
- Microfluidic platforms where cells invade free (fluid-filled) space are friendly for downstream analysis. However, in most platforms, cells invade into a 3D hydrogel ECM spacer, which preserves cell-ECM interactions, are difficult for downstream analysis. Thus, the technical difficulty of analyzing samples from microfluidic devices to perform imaging, Western blotting, qPCR, single cell RNA sequencing and high-throughput omics should overcome by engineering the cancer-on-chip systems with various degrees of sophistication.
- PDMS is the most widely used material in the fabrication of microfluidic platform. However, PDMS has several limitations such as: the microstructures are often inaccurate, and it severely absorbs biomolecules. One possible solution could be a microenvironment mimicking material. A bio-based new material—horseradish peroxidase crosslinked silk fibroin has been developed [74]. This new silk fibroin hydrogel has advantages at tuning mechanical properties, such as stiffness. Therefore, utilizing different biocompatible materials and crosslinking agents could provide a solution to the issue of using PDMS.
- Vessel angiogenesis is the marker for forming secondary tumors. The currently used TME mimicking models, other than observed vasculogenic mimicry, have been utilized for studying sprouting angiogenesis [36,37,41,42]. To the best of the authors’ knowledge, no cancer-on-a-chip has been reported to study the other mechanisms: vessel cooption, vascular mimicry, and intussusceptive angiogenesis as shown in Figure 2 [75]. To study the other angiogenesis processes, microfluidic chips will need to be designed in specific ways.
- Preparation and maintenance of 3D culture microfluidic chips are still time-consuming processes. Generally, several hours to days are required for fabrication, modeling, culture, and obtaining the results. The more factors that are introduced, the more precise the control that is needed. Also, to mimic in vivo TME, a lot of time and effort is needed. Implementing digital control systems for this kind of complex processes could ensure precise control and minimize human effort.
- Most studies utilize existing chip models. In some respects, this limits the possibilities that the newer design could offer. Researchers should focus on designing chips that can be used to address multiple factors of the metastasis process.
- Due to the inclusion of artificial ECM components or lack of primary features, present platforms are restrained from working with primary cells or tissues. Some recent studies have involved the patient-derived samples replicating personal physiologically relevant TME [24]. In the future, more attention should be paid towards studying primary cells including cancer cells, CAFs, and models involving immune cells for tailored TME studies.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, Y.-H.V.; Middleton, K.; You, L.; Sun, Y. A review of microfluidic approaches for investigating cancer extravasation during metastasis. Microsyst. Nanoeng. 2018, 4, 17104. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Kim, J.; Song, H.; Sohn, K.Y.; Jeon, M.; Han, K.-H. Microfluidic technologies for circulating tumor cell isolation. Analyst 2018, 143, 2936–2970. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wan, Y.; Hao, S.; Nisic, M.; Harouaka, R.A.; Chen, Y.; Zou, X.; Zheng, S.-Y. Nucleus of Circulating Tumor Cell Determines Its Translocation Through Biomimetic Microconstrictions and Its Physical Enrichment by Microfiltration. Small 2018, 14, 1802899. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Hajal, C.; Benjamin, D.C.; Yu, C.; Azizgolshani, H.; Hynes, R.O.; Kamm, R.D. Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proc. Natl. Acad. Sci. USA 2018, 115, 7022–7027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fetah, K.L.; DiPardo, B.J.; Kongadzem, E.M.; Tomlinson, J.S.; Elzagheid, A.; Elmusrati, M.; Khademhosseini, A.; Ashammakhi, N. Cancer Modeling-on-a-Chip with Future Artificial Intelligence Integration. Small 2019, 15, 1901985. [Google Scholar] [CrossRef] [PubMed]
- Hachey, S.J.; Hughes, C.C.W. Applications of tumor chip technology. Lab Chip 2018, 18, 2893–2912. [Google Scholar] [CrossRef] [PubMed]
- Sleeboom, J.J.F.; Amirabadi, H.E.; Nair, P.; Sahlgren, C.M.; Den Toonder, J.M.J. Metastasis in context: Modeling the tumor microenvironment with cancer-on-a-chip approaches. Dis. Model. Mech. 2018, 11, 033100. [Google Scholar] [CrossRef] [Green Version]
- Portillo-Lara, R.; Annabi, N. Microengineered cancer-on-a-chip platforms to study the metastatic microenvironment. Lab Chip 2016, 16, 4063–4081. [Google Scholar] [CrossRef] [Green Version]
- Mansoorifar, A.; Gordon, R.; Bergan, R.C.; Bertassoni, L.E. Bone-on-a-Chip: Microfluidic Technologies and Microphysiologic Models of Bone Tissue. Adv. Funct. Mater. 2021, 31, 2006796. [Google Scholar] [CrossRef]
- Lin, Z.; Luo, G.; Du, W.; Kong, T.; Liu, C.; Liu, Z. Recent Advances in Microfluidic Platforms Applied in Cancer Metastasis: Circulating Tumor Cells′(CTCs) Isolation and Tumor-On-A-Chip. Small 2020, 16, 1903899. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A Perspective on Cancer Cell Metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Goh, A.; Yeh, C.-C.; Lei, K.F. Visualization and Quantification of 3D Tumor Cell Migration under Extracellular Stimulation. ACS Appl. Bio Mater. 2020, 3, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Rothbauer, M.; Zirath, H.; Ertl, P. Recent advances in microfluidic technologies for cell-to-cell interaction studies. Lab Chip 2018, 18, 249–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, Y.; Li, K.; Yang, M.; Tan, Y. Fluid Shear Stress Induces EMT of Circulating Tumor Cells via JNK Signaling in Favor of Their Survival during Hematogenous Dissemination. Int. J. Mol. Sci. 2020, 21, 8115. [Google Scholar] [CrossRef] [PubMed]
- Rostami, P.; Kashaninejad, N.; Moshksayan, K.; Saidi, M.S.; Firoozabadi, B.; Nguyen, N.-T. Novel approaches in cancer management with circulating tumor cell clusters. J. Sci. Adv. Mater. Devices 2019, 4, 1–18. [Google Scholar] [CrossRef]
- Zhou, J.; Kulasinghe, A.; Bogseth, A.; O’Byrne, K.; Punyadeera, C.; Papautsky, I. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst. Nanoeng. 2019, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, M.; Shaikh, A.; Lo, H.C.; Arpino, G.; De Placido, S.; Zhang, X.H.; Cristofanilli, M.; Schiff, R.; Trivedi, M.V. Perspective on Circulating Tumor Cell Clusters: Why It Takes a Village to Metastasize. Cancer Res. 2018, 78, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Qiao, H.; Tang, T. Engineering 3D approaches to model the dynamic microenvironments of cancer bone metastasis. Bone Res. 2018, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Mi, S.; Liu, Z.; Du, Z.; Yi, X.; Sun, W. Three-dimensional microfluidic tumor–macrophage system for breast cancer cell invasion. Biotechnol. Bioeng. 2019, 116, 1731–1741. [Google Scholar] [CrossRef]
- Guo, Z.; Song, J.; Hao, J.; Zhao, H.; Du, X.; Li, E.; Kuang, Y.; Yang, F.; Wang, W.; Deng, J.; et al. M2 macrophages promote NSCLC metastasis by upregulating CRYAB. Cell Death Dis. 2019, 10, 377–387. [Google Scholar] [CrossRef]
- Carroll, M.J.; Fogg, K.C.; Patel, H.A.; Krause, H.B.; Mancha, A.-S.; Patankar, M.S.; Weisman, P.S.; Barroilhet, L.; Kreeger, P.K. Alternatively activated macrophages upregulate mesothelial expression of P-selectin to enhance adhesion of ovarian cancer cells. Cancer Res. 2018, 78, 3560–3573. [Google Scholar] [CrossRef] [Green Version]
- Ayuso, J.M.; Rehman, S.; Virumbrales-Munoz, M.; McMinn, P.H.; Geiger, P.; Fitzgerald, C.; Heaster, T.; Skala, M.C.; Beebe, D.J. Microfluidic tumor-on-a-chip model to evaluate the role of tumor environmental stress on NK cell exhaustion. Sci. Adv. 2021, 7, eabc2331. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Choi, J.-H.; Kim, K.-J.; Shin, M.; Choi, J.-W. Microfluidic System to Analyze the Effects of Interleukin 6 on Lymphatic Breast Cancer Metastasis. Front. Bioeng. Biotechnol. 2021, 8, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.D.; Kratz, A.; Park, J.G.; Barrientos, E.S.; Saini, H.; Nguyen, T.; Pockaj, B.; Mouneimne, G.; LaBaer, J.; Nikkhah, M. A Human Organotypic Microfluidic Tumor Model Permits Investigation of the Interplay between Patient-Derived Fibroblasts and Breast Cancer Cells. Cancer Res. 2019, 79, 3139–3151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugo-Cintrón, K.M.; Gong, M.M.; Ayuso, J.M.; Tomko, L.A.; Beebe, D.J.; Virumbrales-Muñoz, M.; Ponik, S.M. Breast Fibroblasts and ECM Components Modulate Breast Cancer Cell Migration through the Secretion of MMPs in a 3D Microfluidic Co-Culture Model. Cancers 2020, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Zheng, X.; Zhao, L.; Zhang, X. Recapitulating and Deciphering Tumor Microenvironment by Using 3D Printed Plastic Brick–Like Microfluidic Cell Patterning. Adv. Health Mater. 2020, 9, 1901713. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, Y.; Liu, Y.; Zhang, M.; Zhang, X. Microfluidic Control of Tumor and Stromal Cell Spheroids Pairing and Merging for Three-Dimensional Metastasis Study. Anal. Chem. 2020, 92, 7638–7645. [Google Scholar] [CrossRef]
- Kim, K.; Sohn, Y.J.; Lee, R.; Yoo, H.J.; Kang, J.Y.; Choi, N.; Na, D.; Yeon, J.H. Cancer-Associated Fibroblasts Differentiated by Exosomes Isolated from Cancer Cells Promote Cancer Cell Invasion. Int. J. Mol. Sci. 2020, 21, 8153. [Google Scholar] [CrossRef]
- Jing, B.; Luo, Y.; Lin, B.; Li, J.; Wang, Z.A.; Du, Y. Establishment and application of a dynamic tumor-vessel microsystem for studying different stages of tumor metastasis and evaluating anti-tumor drugs. RSC Adv. 2019, 9, 17137–17147. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.-R.; Ospina-Muñoz, N.; Noe-Kim, V.; Yang, Y.; Elzey, B.D.; Konieczny, S.F.; Han, B. Subtype-specific characterization of breast cancer invasion using a microfluidic tumor platform. PLoS ONE 2020, 15, e0234012. [Google Scholar] [CrossRef]
- Saha, B.; Mathur, T.; Tronolone, J.J.; Chokshi, M.; Lokhande, G.K.; Selahi, A.; Gaharwar, A.K.; Afshar-Kharghan, V.; Sood, A.K.; Bao, G.; et al. Human tumor microenvironment chip evaluates the consequences of platelet extravasation and combinatorial antitumor-antiplatelet therapy in ovarian cancer. Sci. Adv. 2021, 7, eabg5283. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, M.; Bersini, S.; Valtorta, S.; Proietto, M.; Crippa, M.; Boussommier-Calleja, A.; Labelle, M.; Moresco, R.M.; Vanoni, M.; Kamm, R.D. The driving role of the Cdk5/Tln1/FAKS732 axis in cancer cell extravasation dissected by human vascularized microfluidic models. Biomaterials 2021, 276, 120975. [Google Scholar] [CrossRef] [PubMed]
- Beyer, S.; Blocki, A.; Cheung, M.C.Y.; Wan, Z.H.Y.; Mehrjou, B.; Kamm, R.D. Lectin Staining of Microvascular Glycocalyx in Microfluidic Cancer Cell Extravasation Assays. Life 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Offeddu, G.S.; Hajal, C.; Foley, C.R.; Wan, Z.; Ibrahim, L.; Coughlin, M.F.; Kamm, R.D. The cancer glycocalyx mediates intravascular adhesion and extravasation during metastatic dissemination. Commun. Biol. 2021, 4, 255. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Miermont, A.; Lim, C.T.; Kamm, R.D. A 3D microvascular network model to study the impact of hypoxia on the extravasation potential of breast cell lines. Sci. Rep. 2018, 8, 17949. [Google Scholar] [CrossRef]
- Van Duinen, V.; Zhu, D.; Ramakers, C.; Van Zonneveld, A.J.; Vulto, P.; Hankemeier, T. Perfused 3D angiogenic sprouting in a high-throughput in vitro platform. Angiogenesis 2019, 22, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Li, S.; Du, K.; Li, P.; Qiu, B.; Ding, W. On-Chip Replication of Extremely Early-Stage Tumor Behavior. ACS Appl. Mater. Interfaces 2021, 13, 19768–19777. [Google Scholar] [CrossRef]
- Abe, Y.; Watanabe, M.; Chung, S.; Kamm, R.D.; Tanishita, K.; Sudo, R. Balance of interstitial flow magnitude and vascular endothelial growth factor concentration modulates three-dimensional microvascular network formation. APL Bioeng. 2019, 3, 036102. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Lim, J.; Jusoh, N.; Lee, J.; Park, T.-E.; Kim, Y.; Kim, J.; Jeon, N.L. 3D Microfluidic Bone Tumor Microenvironment Comprised of Hydroxyapatite/Fibrin Composite. Front. Bioeng. Biotechnol. 2019, 7, 168. [Google Scholar] [CrossRef] [Green Version]
- Sewell-Loftin, M.K.; Katz, J.B.; George, S.C.; Longmore, G.D. Micro-strains in the extracellular matrix induce angiogenesis. Lab Chip 2020, 20, 2776–2787. [Google Scholar] [CrossRef]
- Kwak, T.J.; Lee, E. In vitro modeling of solid tumor interactions with perfused blood vessels. Sci. Rep. 2020, 10, 20142. [Google Scholar] [CrossRef] [PubMed]
- Gadde, M.; Phillips, C.; Ghousifam, N.; Sorace, A.G.; Wong, E.; Krishnamurthy, S.; Syed, A.; Rahal, O.; Yankeelov, T.E.; Woodward, W.A.; et al. In vitro vascularized tumor platform for modeling tumor-vasculature interactions of inflammatory breast cancer. Biotechnol. Bioeng. 2020, 117, 3572–3590. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [PubMed]
- Hajal, C.; Ibrahim, L.; Serrano, J.C.; Offeddu, G.S.; Kamm, R.D. The effects of luminal and trans-endothelial fluid flows on the extravasation and tissue invasion of tumor cells in a 3D in vitro microvascular platform. Biomaterials 2021, 265, 120470. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Q.; Pei, J. Microfluidic-Based 3D Engineered Microvascular Networks and Their Applications in Vascularized Microtumor Models. Micromachines 2018, 9, 493. [Google Scholar] [CrossRef] [Green Version]
- Bittner, K.R.; Jiménez, J.M.; Peyton, S.R. Vascularized Biomaterials to Study Cancer Metastasis. Adv. Health Mater. 2020, 9, e1901459. [Google Scholar] [CrossRef]
- Vasudevan, J.; Lim, C.T.; Fernandez, J.G. Cell Migration and Breast Cancer Metastasis in Biomimetic Extracellular Matrices with Independently Tunable Stiffness. Adv. Funct. Mater. 2020, 30, 2005383. [Google Scholar] [CrossRef]
- Amos, S.E.; Choi, Y.S. The Cancer Microenvironment: Mechanical Challenges of the Metastatic Cascade. Front. Bioeng. Biotechnol. 2021, 9, 625859. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell. Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Wan, L.; Neumann, C.A.; LeDuc, P.R. Tumor-on-a-chip for integrating a 3D tumor microenvironment: Chemical and mechanical factors. Lab Chip 2020, 20, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Broders-Bondon, F.; Ho-Bouldoires, T.H.N.; Fernandez-Sanchez, M.-E.; Farge, E. Mechanotransduction in tumor progression: The dark side of the force. J. Cell Biol. 2018, 217, 1571–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, P.Y.; Brenot, A.; King, A.C.; Longmore, G.D.; George, S.C. Randomly Distributed K14+ Breast Tumor Cells Polarize to the Leading Edge and Guide Collective Migration in Response to Chemical and Mechanical Environmental Cues. Cancer Res. 2019, 79, 1899–1912. [Google Scholar] [CrossRef] [Green Version]
- Nam, H.; Funamoto, K.; Jeon, J.S. Cancer cell migration and cancer drug screening in oxygen tension gradient chip. Biomicrofluidics 2020, 14, 044107. [Google Scholar] [CrossRef] [PubMed]
- Azadi, S.; Shadpour, M.T.; Warkiani, M.E. Characterizing the effect of substrate stiffness on the extravasation potential of breast cancer cells using a 3D microfluidic model. Biotechnol. Bioeng. 2021, 118, 823–835. [Google Scholar] [CrossRef]
- Nakamura, M.; Ono, D.; Sugita, S. Mechanophenotyping of B16 Melanoma Cell Variants for the Assessment of the Efficacy of (-)-Epigallocatechin Gallate Treatment Using a Tapered Microfluidic Device. Micromachines 2019, 10, 207. [Google Scholar] [CrossRef] [Green Version]
- Mani, V.; Lyu, Z.; Kumar, V.; Ercal, B.; Chen, H.; Malhotra, S.V.; Demirci, U. Epithelial-to-Mesenchymal Transition (EMT) and Drug Response in Dynamic Bioengineered Lung Cancer Microenvironment. Adv. Biosyst. 2019, 3, e180022. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Thompson, C.L.; Duffy, M.P.; Lunetto, S.; Nolan, J.; Pearce, O.M.; Jacobs, C.R.; Knight, M.M. Mechanical Stimulation Modulates Osteocyte Regulation of Cancer Cell Phenotype. Cancers 2021, 13, 2906. [Google Scholar] [CrossRef]
- Mei, X.; Middleton, K.; Shim, D.; Wan, Q.; Xu, L.; Ma, Y.-H.V.; Devadas, D.; Walji, N.; Wang, L.; Young, E.W.K.; et al. Microfluidic platform for studying osteocyte mechanoregulation of breast cancer bone metastasis. Integr. Biol. 2019, 11, 119–129. [Google Scholar] [CrossRef]
- Marrella, A.; Fedi, A.; Varani, G.; Vaccari, I.; Fato, M.; Firpo, G.; Guida, P.; Aceto, N.; Scaglione, S. High blood flow shear stress values are associated with circulating tumor cells cluster disaggregation in a multi-channel microfluidic device. PLoS ONE 2021, 16, e0245536. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mao, S.; Khan, M.; Zhang, Q.; Huang, Q.; Feng, S.; Lin, J.-M. Responses of Cellular Adhesion Strength and Stiffness to Fluid Shear Stress during Tumor Cell Rolling Motion. ACS Sensors 2019, 4, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Du, P.; Xiao, X.; Liu, Y.; Peng, Y.; Yang, C.; Yue, T. Microfluidic-Based Mechanical Phenotyping of Androgen-Sensitive and Non-sensitive Prostate Cancer Cells Lines. Micromachines 2019, 10, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armistead, F.J.; De Pablo, J.G.; Gadêlha, H.; Peyman, S.A.; Evans, S.D. Physical Biomarkers of Disease Progression: On-Chip Monitoring of Changes in Mechanobiology of Colorectal Cancer Cells. Sci. Rep. 2020, 10, 3254. [Google Scholar] [CrossRef]
- Li, F.; Cima, I.; Vo, J.H.; Tan, M.-H.; Ohl, C.D. Single Cell Hydrodynamic Stretching and Microsieve Filtration Reveal Genetic, Phenotypic and Treatment-Related Links to Cellular Deformability. Micromachines 2020, 11, 486. [Google Scholar] [CrossRef]
- Cognart, H.A.; Viovy, J.-L.; Villard, C. Fluid shear stress coupled with narrow constrictions induce cell type-dependent morphological and molecular changes in SK-BR-3 and MDA-MB-231 cells. Sci. Rep. 2020, 10, 6386. [Google Scholar] [CrossRef] [Green Version]
- Ledvina, V.; Klepárník, K.; Legartová, S.; Bártová, E. A device for investigation of natural cell mobility and deformability. Electrophoresis 2020, 41, 1238–1244. [Google Scholar] [CrossRef]
- Sleeboom, J.J.F.; den Toonder, J.M.J.; Sahlgren, C.M. MDA-MB-231 Breast Cancer Cells and Their CSC Population Migrate Towards Low Oxygen in a Microfluidic Gradient Device. Int. J. Mol. Sci. 2018, 19, 3047. [Google Scholar] [CrossRef] [Green Version]
- Refet-Mollof, E.; Najyb, O.; Chermat, R.; Glory, A.; Lafontaine, J.; Wong, P.; Gervais, T. Hypoxic Jumbo Spheroids On-A-Chip (HOnAChip): Insights into Treatment Efficacy. Cancers 2021, 13, 4046. [Google Scholar] [CrossRef]
- Han, J.W.; Sung, P.S.; Jang, J.W.; Choi, J.Y.; Yoon, S.K. Whole blood viscosity is associated with extrahepatic metastases and survival in patients with hepatocellular carcinoma. PLoS ONE 2021, 16, e0260311. [Google Scholar] [CrossRef]
- Tikhomirova, I.; Petrochenko, E.; Malysheva, Y.; Ryabov, M.; Kislov, N. Interrelation of blood coagulation and hemorheology in cancer. Clin. Hemorheol. Microcirc. 2017, 64, 635–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Tempelhoff, G.F.; Nieman, F.; Heilmann, L.; Hommel, G. Association between blood rheology, thrombosis and cancer survival in patients with gynecologic malignancy. Clin. Hemorheol. Microcirc. 2000, 22, 107–130. [Google Scholar] [PubMed]
- Hu, D.-E.; Ruan, J.-C.; Wang, P.-Q. Hemorheological changes in cancer. Clin. Hemorheol. Microcirc. 1988, 8, 945–956. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Maia, F.R.; Vieira, S.; Reis, R.L.; Oliveira, J.M. Tuning enzymatically crosslinked silk fibroin hydrogel properties for the development of a colorectal cancer extravasation 3D model on a chip. Glob. Chall. 2018, 2, 1700100. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
| Models | Cues | Cancer Type | Microfluidic Features | General Outcomes | Ref. |
|---|---|---|---|---|---|
| Invasion/Intravasation | Inflammatory Cells | Breast cancer | A juxtaposed dual-layer cell-loaded hydrogels biomimetic microfluidic system | Tumor-associated macrophages (TAM) phenotyping was maintained by breast cancer cells while breast cancer cells promoted the differentiation of U937 cells into TAM. | [19] |
| Lung cancer | A microfluidic-based co-culture device with two polydimethylsiloxane (PDMS) layers sandwiching a transwell membrane segmented into two chambers | M2 macrophages upregulated CRYAB expression and activated the ERK1/2/Fra-1/slug signaling pathway to promote epithelial-to-mesenchymal transition (EMT) and malignancy of lung cancer cells. | [20] | ||
| Ovarian Cancer | Micro-culture device with a PDMS ring | Activated macrophages secreted MIP-1β that activated CCR5/PI3K signaling in mesothelial cells and induced P-selectin expression on the cell surface to promote ovarian cancer adhesion. | [21] | ||
| Breast cancer | A central microchamber where cancer cells with/without NK cells can be grown that is connected to a vessel at one corner of the chip | The process of natural killer (NK) cell exhaustion was shown in response to tumor microenvironmental stresses. | [22] | ||
| Breast Cancer | A three-channel microfluidic system mimicking the lymph vessel-tissue-blood vessel structure | Vascular endothelial growth factor (VEGF) secreted by HLECs upon IL-6 stimulation caused the HUVECs to grow inside the cancer cell clusters that are located near the lymphatic channel. | [23] | ||
| CAFs | Breast Cancer | 3D co-culture organotypic invasion model for crosstalk of fibroblasts and cancer cells | Cancer-Associated Fibroblasts (CAFs) enhanced invasion by inducing gene expression of glycoprotein non-metastatic B. | [24] | |
| Breast cancer | LumeNEXT microfluidic model | Both metalloproteinases (MMP) and fibronectin were essential for the invasion of MDA-MB-231 cells. | [25] | ||
| Breast cancer | A 3D-printed brick like cell patterning microfluidic platform | The tumor cells and fibroblasts crucially impacted each other. | [26] | ||
| Colon and breast cancer | A microwell array-based microfluidic platform | Tumor spheroids could envelop fibroblast spheroids completely that helped the colon cancer cells to invade at short time. | [27] | ||
| Melanoma, squamous and breast cancer | 3D five-channel model that allows co-culturing CAFs and cancer cells with real-time monitoring of invasion process | The invasive area of cancer cells into the ECM in the presence of exosomes-induced CAFs was higher than exosome non-treated CAFs. | [28] | ||
| Endothelium based models | Breast and liver cancer | A vascular cavity with fluid flow in the laminated microfluidic chip | MDA-MB-231 breast cancer cells invaded paracellularly by disrupting the intercellular endothelial junction, whereas HepG2 liver cancer cells invaded through the transcellular process. | [29] | |
| Breast cancer | Invasive ductal carcinoma-on-chip by viscous fingering | MCF-7 cell line was non-invasive and non-tumorigenic unless supplemented with estrogen whereas TNB subtypes invaded into the surrounding matrix. | [30] | ||
| Ovarian cancer | Ovarian TME organ-on-chip platform | Platelets promote ovarian cancer invasion by interactions between glycoprotein Ⅵ and tumor galectin-3 under shear. | [31] | ||
| Extravasation | Breast cancer and fibrosarcoma | Cdk5 affects vascular adhesion, structure of Tln1 and FAK supports invadopodia formation while FAKS732 phosphorylation participates in trans-endothelial migration. | [32] | ||
| Breast Cancer | The glycocalyx defects during extravasation from perfusable endothelial lumens was visualized. | [33] | |||
| Breast Cancer | Microfluidic chip with three to five microchannels that can form microvascular networks | Trans-endothelial migration and invasion of cancer cells occur through binding CD44 to the sub-endothelial ECM components. | [34] | ||
| Melanoma | Lipopolysaccharide (LPS)-stimulated neutrophils aggregate under flow, and arrest by mechanical trapping and interactions with endothelial ICAM-1 into the vascularized channels. | [4] | |||
| Breast Cancer | Induced by hypoxia, both HIF-α protein level and rate of cancer cells extravasation increased. | [35] | |||
| Angiogenesis | Three-lane microfluidic titer plates | A combination of VEGF-165, PMA, and S1P was the foremost optimum cocktail to trigger vigorous and directional angiogenesis. | [36] | ||
| Cervix cancer | A tumor spheroid-based microfluidic device | At an extremely early stage of cancer, endotheliocytes and fibroblasts accelerated the proliferation and migration of HeLa cells in chips while vasculogenic mimicry was observed. | [37] | ||
| MVN-chip | Effect of interstitial flow in vascular sprouting cannot be substituted by increasing vascular endothelial growth factor. | [38] | |||
| Colorectal and gastric cancer | 3D microfluidic bone model with hydroxyapatite acid stimulating mechanical properties of bone | During angiogenesis in the HA/fibrin composite, the number of blood vessel sprouts decreased as the HA concentration increased. | [39] | ||
| Multi-tissue chamber model with one central chamber adjacent to other chambers and two lateral media lines | Increased levels of CAF mechanical activity contributed to increased angiogenesis. | [40] | |||
| Breast cancer | Microfluidic organ-on-a-chip models of solid tumors | Culturing MDA-MB-231/HUVECs in a HLFs-laden, fibrin-based ECM promoted angiogenesis and tumor cell migration. | [41] | ||
| Inflammatory breast cancer (IBC) | 3D in vitro vascularized microfluidic based inflammatory breast cancer model | IBC platforms increased collagen ECM porosity and expressed higher levels of VEGF than non-IBC and control. | [42] |
| Mechanical Factors | Cancer Type | Microfluidic Features | General Outcomes | Ref. |
|---|---|---|---|---|
| Stiffness | Breast cancer | Microfluidic devices contained three independently addressable parallel channels | Cancer cells increase their extravasation capability in regard to the substrate stiffening which could be connected with the expression level of metalloproteinase-9 (MMP-9) | [56] |
| Melanoma | A linearly tapered microflow channel | Test cancer cells’ shape recovery time after compressed compared to relative invasive ability. | [57] | |
| Shear stress | Breast cancer | microvascular network (MVN)-chip | The luminal flow increased the intravasation, where trans-endothelial flow increased migratory speeds of extravasation. | [44] |
| Breast and liver cancer | A vascular cavity with fluid flow in the laminated microfluidic chip | Cancer cells migrate in the direction of “blood flow”. | [29] | |
| Lung cancer | A multichannel microfluidic model | Flow induced EMT by decreasing E-cadherin expression but increasing N-cadherin and vimentin expressions. | [58] | |
| Breast and prostate cancer | Microfluidic organ-chip using two overlapped microchannels separated by a membrane | Mechanical stimulation by constant high flow rate in comparison to standard flow rate reversed the inhibition metastatic effects. | [59] | |
| Breast cancer | A novel microfluidic cancer extravasation tissue platform | The shear stress caused by interstitial fluid flow from bone loading exercise prevents bone metastasis. | [60] | |
| Breast cancer | A novel multichannel microfluidic device simultaneously reproduces different hemodynamic wall shear stress | Increasing shear stress was associated with disaggregation of cell clusters while low shear stress was associated with the opposite effect. | [61] | |
| Breast cancer | Three parallel tissue chambers surrounded by two parallel microfluidic lines | Pre-existing K14+ leader cells travel through the organoid to “polarize” to the front rim in regard to SDF1 gradient, and interstitial flow. | [54] | |
| Brain cancer | A straight microfluidic channel combined with a live single-cell extraction and atomic force microscopy | The cells unexposed to fluid shear stress exhibited greater nuclear stiffness than cortex stiffness, while after fluid shear stress exposure the cortex hardened, and nucleus softened. | [62] | |
| Cellular deformability | Prostate and colorectal cancer | Microfluidic hydrodynamic stretching with high-speed capturing camera | Shear stress-induced deformation as potential biomarkers of early detection or metastatic progression. | [63,64] |
| Colorectal and breast cancer | A stretchable hydrodynamic microfluidic system with microfilter | The mesenchymal-like cells had higher deformability than the epithelial-like cells. | [65] | |
| Breast cancer | Five types of geometric microfluidic models | Highly metastatic cancer cell lines have higher plasticity and deformability under shear flow and mechanical stress. | [66] | |
| Breast cancer | Microconstriction array mimicking capillaries and endothelial junctions in a microfluidic device with real-time monitoring | The deformability and size of the nucleus determine the cell’s translocation through the microconstrictions. | [3] | |
| Cervix cancer | Microfluidic arrays of circular cross-section micropillars with decreasing spacing | Except for mitosis, there were no difference in migration velocities among cell cycle phases. | [67] | |
| Oxygen gradient and hypoxia | Breast cancer | The microfluidic chip with simulated oxygen gradient. | No changes in the migration pattern of breast cancer stem cells (CSCs) were observed from the average cancer cell population. | [68] |
| Breast cancer | A microfluidic device with five channels including two gas channels, two media channels, and a gel channel | Cancer cells direct toward higher oxygen tension and resist cell death against anticancer drug. | [55] | |
| Sarcoma | Hypoxic jumbo spheroids on-a-chip | Validate the establishment of the device and jumbo spheroids. | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Karim, M.; Hasan, M.M.; Hooper, J.; Wahab, R.; Roy, S.; Al-Hilal, T.A. Cancer-on-a-Chip: Models for Studying Metastasis. Cancers 2022, 14, 648. https://doi.org/10.3390/cancers14030648
Zhang X, Karim M, Hasan MM, Hooper J, Wahab R, Roy S, Al-Hilal TA. Cancer-on-a-Chip: Models for Studying Metastasis. Cancers. 2022; 14(3):648. https://doi.org/10.3390/cancers14030648
Chicago/Turabian StyleZhang, Xiaojun, Mazharul Karim, Md Mahedi Hasan, Jacob Hooper, Riajul Wahab, Sourav Roy, and Taslim A. Al-Hilal. 2022. "Cancer-on-a-Chip: Models for Studying Metastasis" Cancers 14, no. 3: 648. https://doi.org/10.3390/cancers14030648
APA StyleZhang, X., Karim, M., Hasan, M. M., Hooper, J., Wahab, R., Roy, S., & Al-Hilal, T. A. (2022). Cancer-on-a-Chip: Models for Studying Metastasis. Cancers, 14(3), 648. https://doi.org/10.3390/cancers14030648






