Systemic Effects Reflected in Specific Biomarker Patterns Are Instrumental for the Paradigm Change in Prostate Cancer Management: A Strategic Paper
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Prostate Cancer as a Socio-Economic Burden in Focus of the Paradigm Change in Healthcare
1.2. PCa Is a Systemic Multi-Factorial Disease
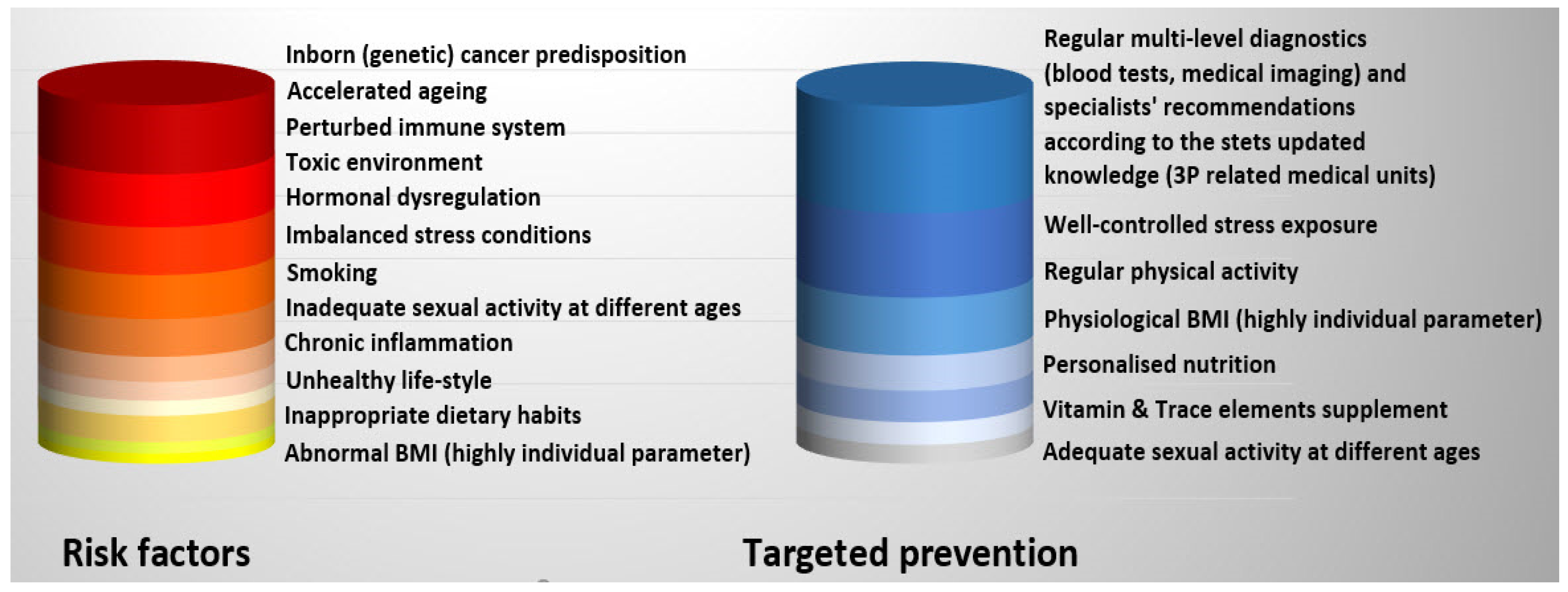
1.3. Non-Modifiable Risk Factors of PCa
1.4. Modifiable PCa Risks
2. PCa Relevant Systemic Effects Reflected in Blood Patterns
2.1. Stress Reactions and Imbalanced Antioxidant Defence in the Pathophysiology of PCa
2.2. Sleep and Melatonin Patterns Related to PCa Risks and Prognosis
2.3. Involvement of the Immune System: Blood Patterns Indicative for PCa Prediction and Patient Stratification
2.4. Systemic Ischemia and Interplay between Endothelin-1 and Nitric Oxide Are Crucial for PCa Development and Progression
2.5. Systemic Inflammation
2.6. Systemic Effects of Homocysteine Metabolism Axes on PCa Development and Progression
3. Anti-Cancer Protection Relevant for PCa Development and Progression
3.1. Mitochondrial Dysfunction, Excessive ROS, Oxidative Stress and the Protective Role of Phytochemicals
3.2. Circadian Rhythm Disruption and the Protective Role of Melatonin
3.3. Inflammation and the Protective Role of Anti-Inflammatory Substances
3.4. Hypoxia–Ischemia, Increased Endothelin-1 and the Protective Role of Phytochemicals and Nitric Oxide
3.5. Microbiome Profiling and a Protective Role of Probiotics in PCa Prevention
4. Status Quo and Expert Recommendations in the Framework of 3P Medicine
4.1. PCa Risk Assessment: Application of Specialised Surveys Is of Clinical Relevance for the Disease Prevention and Management
4.2. Liquid Biopsy Biomarker Panels for PCa Prediction, Prognosis and Patient Stratification: Status Quo and Outlook
- there is strong evidence demonstrating that dysregulation of metabolism plays a crucial role in the development and progression of PCa;
- one of the most prominent risk factors is the metabolic syndrome: there is a synergic interplay of visceral obesity, insulin resistance, low HDL (high-density lipoprotein) cholesterol, high triglycerides, elevated C-reactive protein and low adiponectin levels, amongst others in the pathomechanisms of PCa;
- further, there are organ-specific particularities in the metabolism of the prostate to produce the components of prostatic fluid: PSA, spermine, myo-inositol and citrate; to this end, the levels of citrate in the prostate are orders of magnitude higher than anywhere else in the body;
- finally, neoplastic prostate cells lose the capacity to accumulate zinc which is thought to inhibit the ability to accumulate citrate; metabolomic alterations reflecting this unique phenomenon are hypothesised to result in a PCa-specific metabolome profile that might be instrumental for the disease modelling.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries and 25 Major Cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Kucera, R.; Pecen, L.; Topolcan, O.; Dahal, A.R.; Costigliola, V.; Giordano, F.A.; Golubnitschaja, O. Prostate Cancer Management: Long-Term Beliefs, Epidemic Developments in the Early Twenty-First Century and 3PM Dimensional Solutions. EPMA J. 2020, 11, 399–418. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with Overall and Cause-Specific Mortality: A Population-Based Cohort Study of 3·6 Million Adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef] [Green Version]
- Thalgott, M.; Kron, M.; Brath, J.M.; Ankerst, D.P.; Thompson, I.M.; Gschwend, J.E.; Herkommer, K. Men with Family History of Prostate Cancer Have a Higher Risk of Disease Recurrence after Radical Prostatectomy. World J. Urol. 2018, 36, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.; Modlin, C.S. Disparities in Prostate Cancer in African American Men: What Primary Care Physicians Can Do. Cleve Clin. J. Med. 2012, 79, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellami, M.; Gasmi, M.; Denham, J.; Hayes, L.D.; Stratton, D.; Padulo, J.; Bragazzi, N. Effects of Acute and Chronic Exercise on Immunological Parameters in the Elderly Aged: Can Physical Activity Counteract the Effects of Aging? Front. Immunol. 2018, 9, 2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taverna, G.; Seveso, M.; Giusti, G.; Hurle, R.; Graziotti, P.; Stifter, S.; Chiriva-Internati, M.; Grizzi, F. Senescent Remodeling of the Innate and Adaptive Immune System in the Elderly Men with Prostate Cancer. Curr. Gerontol. Geriatr. Res. 2014, 2014, 478126. [Google Scholar] [CrossRef] [Green Version]
- Ihle, C.L.; Owens, P. Integrating the Immune Microenvironment of Prostate Cancer Induced Bone Disease. Mol. Carcinog. 2020, 59, 822–829. [Google Scholar] [CrossRef]
- Hassan, S.; Karpova, Y.; Baiz, D.; Yancey, D.; Pullikuth, A.; Flores, A.; Register, T.; Cline, J.M.; D’Agostino, R.; Danial, N.; et al. Behavioral Stress Accelerates Prostate Cancer Development in Mice. J. Clin. Investig. 2013, 123, 874–886. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.; Srivastava, J.K.; Shankar, E.; Kanwal, R.; Nawab, A.; Sharma, H.; Bhaskaran, N.; Ponsky, L.E.; Fu, P.; MacLennan, G.T.; et al. Oxidative Stress and Antioxidant Status in High-Risk Prostate Cancer Subjects. Diagnostics 2020, 10, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straif, K.; Baan, R.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Altieri, A.; Benbrahim-Tallaa, L.; Cogliano, V.; WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of Shift-Work, Painting, and Fire-Fighting. Lancet Oncol. 2007, 8, 1065–1066. [Google Scholar] [CrossRef]
- Gapstur, S.M.; Diver, W.R.; Stevens, V.L.; Carter, B.D.; Teras, L.R.; Jacobs, E.J. Work Schedule, Sleep Duration, Insomnia, and Risk of Fatal Prostate Cancer. Am. J. Prev. Med. 2014, 46, S26–S33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, D.; Ju, L.; Zhou, F.; Yu, M.; Ma, H.; Zhang, Y.; Liu, T.; Xiao, Y.; Wang, X.; Qian, K. The Inhibitory Effect of Melatonin on Human Prostate Cancer. Cell Commun. Signal. 2021, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Hood, S.P.; Cosma, G.; Foulds, G.A.; Johnson, C.; Reeder, S.; McArdle, S.E.; Khan, M.A.; Pockley, A.G. Identifying Prostate Cancer and Its Clinical Risk in Asymptomatic Men Using Machine Learning of High Dimensional Peripheral Blood Flow Cytometric Natural Killer Cell Subset Phenotyping Data. eLife 2020, 9, e50936. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, R.A.; Hermes, C.L.; Almeida, T.C.; Bochi, G.V.; De Bona, K.S.; Moretto, M.B.; Moresco, R.N. Ischemia-Modified Albumin and Inflammatory Biomarkers in Patients with Prostate Cancer. Clin. Lab. 2014, 60, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Torres Crigna, A.; Link, B.; Samec, M.; Giordano, F.A.; Kubatka, P.; Golubnitschaja, O. Endothelin-1 Axes in the Framework of Predictive, Preventive and Personalised (3P) Medicine. EPMA J. 2021, 12, 265–305. [Google Scholar] [CrossRef]
- Soni, Y.; Softness, K.; Arora, H.; Ramasamy, R. The Yin Yang Role of Nitric Oxide in Prostate Cancer. Am. J. Men’s Health 2020, 14, 1557988320903191. [Google Scholar] [CrossRef]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic Inflammation: Key Player and Biomarker-Set to Predict and Prevent Cancer Development and Progression Based on Individualized Patient Profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef] [Green Version]
- Koklesova, L.; Mazurakova, A.; Samec, M.; Biringer, K.; Samuel, S.M.; Büsselberg, D.; Kubatka, P.; Golubnitschaja, O. Homocysteine Metabolism as the Target for Predictive Medical Approach, Disease Prevention, Prognosis, and Treatments Tailored to the Person. EPMA J. 2021, 12, 477–505. [Google Scholar] [CrossRef]
- Collin, S.M.; Metcalfe, C.; Refsum, H.; Lewis, S.J.; Zuccolo, L.; Smith, G.D.; Chen, L.; Harris, R.; Davis, M.; Marsden, G.; et al. Circulating Folate, Vitamin B12, Homocysteine, Vitamin B12 Transport Proteins, and Risk of Prostate Cancer: A Case-Control Study, Systematic Review, and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1632–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Chang, W.-S.; Tsai, C.-W.; Bau, D.-T.; Davis, J.W.; Thompson, T.C.; Logothetis, C.J.; Gu, J. Mitochondrial DNA Copy Number in Peripheral Blood Leukocytes Is Associated with Biochemical Recurrence in Prostate Cancer Patients in African Americans. Carcinogenesis 2020, 41, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Samec, M.; Koklesova, L.; Kudela, E.; Kubatka, P.; Golubnitschaja, O. Mitochondriopathies as a Clue to Systemic Disorders—Analytical Tools and Mitigating Measures in Context of Predictive, Preventive, and Personalized (3P) Medicine. Int. J. Mol. Sci. 2021, 22, 2007. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in Cancer: Initiators, Amplifiers or an Achilles’ Heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Cohen, P.; Stern, M.C.; Odedina, F.; Carpten, J.; Reams, R. Mitochondrial Biology and Prostate Cancer Ethnic Disparity. Carcinogenesis 2018, 39, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Paschos, A.; Pandya, R.; Duivenvoorden, W.C.M.; Pinthus, J.H. Oxidative Stress in Prostate Cancer: Changing Research Concepts towards a Novel Paradigm for Prevention and Therapeutics. Prostate Cancer Prostatic Dis. 2013, 16, 217–225. [Google Scholar] [CrossRef]
- Abbasi, A.; Mostafavi-Pour, Z.; Amiri, A.; Keshavarzi, F.; Nejabat, N.; Ramezani, F.; Sardarian, A.; Zal, F. Chemoprevention of Prostate Cancer Cells by Vitamin C plus Quercetin: Role of Nrf2 in Inducing Oxidative Stress. Nutr. Cancer 2020, 2003–2013. [Google Scholar] [CrossRef]
- Mirzaei, S.; Mohammadi, A.T.; Gholami, M.H.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Hushmandi, K.; Makvandi, P.; Samec, M.; Liskova, A.; et al. Nrf2 Signaling Pathway in Cisplatin Chemotherapy: Potential Involvement in Organ Protection and Chemoresistance. Pharm. Res. 2021, 167, 105575. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Oxidative Stress, Diet and Prostate Cancer. World J. Men’s Health 2021, 39, 195–207. [Google Scholar] [CrossRef]
- Oh, B.; Figtree, G.; Costa, D.; Eade, T.; Hruby, G.; Lim, S.; Elfiky, A.; Martine, N.; Rosenthal, D.; Clarke, S.; et al. Oxidative Stress in Prostate Cancer Patients: A Systematic Review of Case Control Studies. Prostate Int. 2016, 4, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Applegate, C.C.; Rowles, J.L.; Ranard, K.M.; Jeon, S.; Erdman, J.W. Soy Consumption and the Risk of Prostate Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Liskova, A.; Mojzis, J.; Adamkov, M.; et al. Anticancer Activities of Thymus Vulgaris L. in Experimental Breast Carcinoma In Vivo and In Vitro. Int. J. Mol. Sci. 2019, 20, 1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubatka, P.; Kello, M.; Kajo, K.; Kruzliak, P.; Výbohová, D.; Mojžiš, J.; Adamkov, M.; Fialová, S.; Veizerová, L.; Zulli, A.; et al. Oregano Demonstrates Distinct Tumour-Suppressive Effects in the Breast Carcinoma Model. Eur. J. Nutr. 2017, 56, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Uramova, S.; Liskova, A.; Sadlonova, V.; Koklesova, L.; et al. Chemopreventive and Therapeutic Efficacy of Cinnamomum zeylanicum L. Bark in Experimental Breast Carcinoma: Mechanistic In Vivo and In Vitro Analyses. Molecules 2020, 25, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapinova, A.; Kubatka, P.; Liskova, A.; Baranenko, D.; Kruzliak, P.; Matta, M.; Büsselberg, D.; Malicherova, B.; Zulli, A.; Kwon, T.K.; et al. Controlling Metastatic Cancer: The Role of Phytochemicals in Cell Signaling. J. Cancer Res. Clin. Oncol. 2019, 145, 1087–1109. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Liskova, A.; Samec, M.; Qaradakhi, T.; Zulli, A.; Smejkal, K.; Kajo, K.; Jakubikova, J.; Behzadi, P.; Pec, M.; et al. Genoprotective Activities of Plant Natural Substances in Cancer and Chemopreventive Strategies in the Context of 3P Medicine. EPMA J. 2020, 11, 261–287. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Kubatka, P.; Uramova, S.; Zubor, P.; Samuel, S.M.; Zulli, A.; Pec, M.; Bielik, T.; Biringer, K.; et al. The Role of Dietary Phytochemicals in the Carcinogenesis via the Modulation of MiRNA Expression. J. Cancer Res. Clin. Oncol. 2019, 145, 1665–1679. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Zhai, K.; Siddiqui, M.; Abdellatif, B.; Liskova, A.; Kubatka, P.; Büsselberg, D. Natural Compounds in Glioblastoma Therapy: Preclinical Insights, Mechanistic Pathways, and Outlook. Cancers 2021, 13, 2317. [Google Scholar] [CrossRef]
- Zhai, K.; Brockmüller, A.; Kubatka, P.; Shakibaei, M.; Büsselberg, D. Curcumin’s Beneficial Effects on Neuroblastoma: Mechanisms, Challenges, and Potential Solutions. Biomolecules 2020, 10, 1469. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Brockmueller, A.; Zhai, K.; Abdellatif, B.; Siddiqui, M.; Biringer, K.; Kudela, E.; Pec, M.; et al. Flavonoids as an Effective Sensitizer for Anti-Cancer Therapy: Insights into Multi-Faceted Mechanisms and Applicability towards Individualized Patient Profiles. EPMA J. 2021, 12, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.; Abdellatif, B.; Zhai, K.; Liskova, A.; Kubatka, P.; Büsselberg, D. Flavonoids Alleviate Peripheral Neuropathy Induced by Anticancer Drugs. Cancers 2021, 13, 1576. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Athirai, T.; Kiruthiga, B.; Senthilkumar, K.; Elumalai, P.; Arunkumar, R.; Arunakaran, J. Chemopreventive Effect of Quercetin in MNU and Testosterone Induced Prostate Cancer of Sprague-Dawley Rats. Nutr. Cancer 2014, 66, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Rowles, J.L.; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W. Increased Dietary and Circulating Lycopene Are Associated with Reduced Prostate Cancer Risk: A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Rowles, J.L.; Ranard, K.M.; Applegate, C.C.; Jeon, S.; An, R.; Erdman, J.W. Processed and Raw Tomato Consumption and Risk of Prostate Cancer: A Systematic Review and Dose-Response Meta-Analysis. Prostate Cancer Prostatic Dis. 2018, 21, 319–336. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Yang, S.; Wang, Y.; Tang, Z.; Fu, X. Co-Administration of Genistein with Doxorubicin-Loaded Polypeptide Nanoparticles Weakens the Metastasis of Malignant Prostate Cancer by Amplifying Oxidative Damage. Biomater. Sci. 2018, 6, 827–835. [Google Scholar] [CrossRef]
- Khandrika, L.; Kumar, B.; Koul, S.; Maroni, P.; Koul, H.K. Role of Oxidative Stress in Prostate Cancer. Cancer Lett. 2009, 282, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Shu, L.; Zhang, C.; Li, W.; Wu, R.; Guo, Y.; Yang, Y.; Kong, A.-N. Histone Methyltransferase Setd7 Regulates Nrf2 Signaling Pathway by Phenethyl Isothiocyanate and Ursolic Acid in Human Prostate Cancer Cells. Mol. Nutr. Food Res. 2018, 62, 1700840. [Google Scholar] [CrossRef]
- Wendeu-Foyet, M.G.; Menegaux, F. Circadian Disruption and Prostate Cancer Risk: An Updated Review of Epidemiological Evidences. Cancer Epidemiol. Biomark. Prev. 2017, 26, 985–991. [Google Scholar] [CrossRef] [Green Version]
- Sulli, G.; Lam, M.T.Y.; Panda, S. Interplay between Circadian Clock and Cancer: New Frontiers for Cancer Treatment. Trends Cancer 2019, 5, 475–494. [Google Scholar] [CrossRef]
- Papantoniou, K.; Castaño-Vinyals, G.; Espinosa, A.; Aragonés, N.; Pérez-Gómez, B.; Burgos, J.; Gómez-Acebo, I.; Llorca, J.; Peiró, R.; Jimenez-Moleón, J.J.; et al. Night Shift Work, Chronotype and Prostate Cancer Risk in the MCC-Spain Case-Control Study. Int. J. Cancer 2015, 137, 1147–1157. [Google Scholar] [CrossRef]
- Markt, S.C.; Valdimarsdottir, U.A.; Shui, I.M.; Sigurdardottir, L.G.; Rider, J.R.; Tamimi, R.M.; Batista, J.L.; Haneuse, S.; Flynn-Evans, E.; Lockley, S.W.; et al. Circadian Clock Genes and Risk of Fatal Prostate Cancer. Cancer Causes Control 2015, 26, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashrafizadeh, M.; Najafi, M.; Kavyiani, N.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Anti-Inflammatory Activity of Melatonin: A Focus on the Role of NLRP3 Inflammasome. Inflammation 2021, 44, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J.M. Melatonin and the immune system therapeutic potential in cancer, viral diseases, and immunodeficiency states. In The Pineal Gland and Cancer: Neuroimmunoendocrine Mechanisms in Malignancy; Bartsch, C., Bartsch, H., Blask, D.E., Cardinali, D.P., Hrushesky, W.J.M., Mecke, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 384–394. ISBN 978-3-642-59512-7. [Google Scholar]
- Gatti, G.; Lucini, V.; Dugnani, S.; Calastretti, A.; Spadoni, G.; Bedini, A.; Rivara, S.; Mor, M.; Canti, G.; Scaglione, F.; et al. Antiproliferative and Pro-Apoptotic Activity of Melatonin Analogues on Melanoma and Breast Cancer Cells. Oncotarget 2017, 8, 68338–68353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Yang, H.-L.; Gu, C.-J.; Liu, Y.-K.; Shao, J.; Zhu, R.; He, Y.-Y.; Zhu, X.-Y.; Li, M.-Q. Melatonin Restricts the Viability and Angiogenesis of Vascular Endothelial Cells by Suppressing HIF-1α/ROS/VEGF. Int. J. Mol. Med. 2019, 43, 945–955. [Google Scholar] [CrossRef] [Green Version]
- Kubatka, P.; Zubor, P.; Busselberg, D.; Kwon, T.K.; Adamek, M.; Petrovic, D.; Opatrilova, R.; Gazdikova, K.; Caprnda, M.; Rodrigo, L.; et al. Melatonin and Breast Cancer: Evidences from Preclinical and Human Studies. Crit. Rev. Oncol. Hematol. 2018, 122, 133–143. [Google Scholar] [CrossRef]
- Bojková, B.; Kubatka, P.; Qaradakhi, T.; Zulli, A.; Kajo, K. Melatonin May Increase Anticancer Potential of Pleiotropic Drugs. Int. J. Mol. Sci. 2018, 19, 3910. [Google Scholar] [CrossRef] [Green Version]
- Orendas, P.; Kubatka, P.; Kajo, K.; Stollarova, N.; Kassayova, M.; Bojkova, B.; Pec, M.; Nosal, V.; Kiskova, T.; Zihlavnikova, K.; et al. Melatonin Enhanced Bexarotene Efficacy in Experimental Mammary Carcinogenesis. Neoplasma 2012, 59, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Kubatka, P.; Bojková, B.; Kassayová, M.; Orendáš, P.; Kajo, K.; Výbohová, D.; Kružliak, P.; Adamicová, K.; Péč, M.; Stollárová, N.; et al. Combination of Pitavastatin and Melatonin Shows Partial Antineoplastic Effects in a Rat Breast Carcinoma Model. Acta Histochem. 2014, 116, 1454–1461. [Google Scholar] [CrossRef]
- Orendáš, P.; Kubatka, P.; Bojková, B.; Kassayová, M.; Kajo, K.; Výbohová, D.; Kružliak, P.; Péč, M.; Adamkov, M.; Kapinová, A.; et al. Melatonin Potentiates the Anti-Tumour Effect of Pravastatin in Rat Mammary Gland Carcinoma Model. Int. J. Exp. Pathol. 2014, 95, 401–410. [Google Scholar] [CrossRef]
- Bojková, B.; Kajo, K.; Kubatka, P.; Solár, P.; Péč, M.; Adamkov, M. Metformin and Melatonin Improve Histopathological Outcome of NMU-Induced Mammary Tumors in Rats. Pathol. Res. Pract. 2019, 215, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.-Y.; Huang, S.-P.; Bao, B.-Y.; Wu, M.-T. Urinary Melatonin-Sulfate/Cortisol Ratio and the Presence of Prostate Cancer: A Case-Control Study. Sci. Rep. 2016, 6, 29606. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardottir, L.G.; Markt, S.C.; Rider, J.R.; Haneuse, S.; Fall, K.; Schernhammer, E.S.; Tamimi, R.M.; Flynn-Evans, E.; Batista, J.L.; Launer, L.; et al. Urinary Melatonin Levels, Sleep Disruption, and Risk of Prostate Cancer in Elderly Men. Eur. Urol. 2015, 67, 191–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.-W.; Tai, H.-C.; Tang, C.-H.; Lin, L.-W.; Lin, T.-H.; Chang, A.-C.; Chen, P.-C.; Chen, Y.-H.; Wang, P.-C.; Lai, Y.-W.; et al. Melatonin Impedes Prostate Cancer Metastasis by Suppressing MMP-13 Expression. J. Cell Physiol. 2021, 236, 3979–3990. [Google Scholar] [CrossRef] [PubMed]
- Zharinov, G.M.; Bogomolov, O.A.; Chepurnaya, I.V.; Neklasova, N.Y.; Anisimov, V.N. Melatonin Increases Overall Survival of Prostate Cancer Patients with Poor Prognosis after Combined Hormone Radiation Treatment. Oncotarget 2020, 11, 3723–3729. [Google Scholar] [CrossRef]
- Tewari, A.K.; Stockert, J.A.; Yadav, S.S.; Yadav, K.K.; Khan, I. Inflammation and Prostate Cancer. Adv. Exp. Med. Biol. 2018, 1095, 41–65. [Google Scholar] [CrossRef]
- Gurel, B.; Lucia, M.S.; Thompson, I.M.; Goodman, P.J.; Tangen, C.M.; Kristal, A.R.; Parnes, H.L.; Hoque, A.; Lippman, S.M.; Sutcliffe, S.; et al. Chronic Inflammation in Benign Prostate Tissue Is Associated with High-Grade Prostate Cancer in the Placebo Arm of the Prostate Cancer Prevention Trial. Cancer Epidemiol. Biomark. Prev. 2014, 23, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, T.; Livas, L.; Kyprianou, N. Inflammation in Prostate Cancer Progression and Therapeutic Targeting. Transl. Urol. 2015, 4, 455–463. [Google Scholar] [CrossRef]
- Taverna, G.; Pedretti, E.; Di Caro, G.; Borroni, E.M.; Marchesi, F.; Grizzi, F. Inflammation and Prostate Cancer: Friends or Foe? Inflamm. Res. 2015, 64, 275–286. [Google Scholar] [CrossRef]
- Rani, A.; Dasgupta, P.; Murphy, J.J. Prostate Cancer: The Role of Inflammation and Chemokines. Am. J. Pathol. 2019, 189, 2119–2137. [Google Scholar] [CrossRef] [Green Version]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liskova, A.; Kubatka, P.; Samec, M.; Zubor, P.; Mlyncek, M.; Bielik, T.; Samuel, S.M.; Zulli, A.; Kwon, T.K.; Büsselberg, D. Dietary Phytochemicals Targeting Cancer Stem Cells. Molecules 2019, 24, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liskova, A.; Koklesova, L.; Samec, M.; Smejkal, K.; Samuel, S.M.; Varghese, E.; Abotaleb, M.; Biringer, K.; Kudela, E.; Danko, J.; et al. Flavonoids in Cancer Metastasis. Cancers 2020, 12, 1498. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Koklesova, L.; Samec, M.; Varghese, E.; Abotaleb, M.; Samuel, S.M.; Smejkal, K.; Biringer, K.; Petras, M.; Blahutova, D.; et al. Implications of Flavonoids as Potential Modulators of Cancer Neovascularity. J. Cancer Res. Clin. Oncol. 2020, 146, 3079–3096. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Liskova, A.; Samec, M.; Buhrmann, C.; Samuel, S.M.; Varghese, E.; Ashrafizadeh, M.; Najafi, M.; Shakibaei, M.; Büsselberg, D.; et al. Carotenoids in Cancer Apoptosis-The Road from Bench to Bedside and Back. Cancers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Kapinova, A.; Stefanicka, P.; Kubatka, P.; Zubor, P.; Uramova, S.; Kello, M.; Mojzis, J.; Blahutova, D.; Qaradakhi, T.; Zulli, A.; et al. Are Plant-Based Functional Foods Better Choice against Cancer than Single Phytochemicals? A Critical Review of Current Breast Cancer Research. Biomed. Pharmacother. 2017, 96, 1465–1477. [Google Scholar] [CrossRef]
- Jiang, L.-N.; Liu, Y.-B.; Li, B.-H. Lycopene Exerts Anti-Inflammatory Effect to Inhibit Prostate Cancer Progression. Asian J. 2019, 21, 80–85. [Google Scholar] [CrossRef]
- Vogt, T.M.; Mayne, S.T.; Graubard, B.I.; Swanson, C.A.; Sowell, A.L.; Schoenberg, J.B.; Swanson, G.M.; Greenberg, R.S.; Hoover, R.N.; Hayes, R.B.; et al. Serum Lycopene, Other Serum Carotenoids, and Risk of Prostate Cancer in US Blacks and Whites. Am. J. Epidemiol. 2002, 155, 1023–1032. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.-Y.; Hung, J.-C.; Heber, D.; Go, V.L.W.; Reuter, V.E.; Cordon-Cardo, C.; Scher, H.I.; Marshall, J.R.; Zhang, Z.-F. Inverse Associations between Plasma Lycopene and Other Carotenoids and Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2001, 10, 749–756. [Google Scholar]
- Kirsh, V.A.; Mayne, S.T.; Peters, U.; Chatterjee, N.; Leitzmann, M.F.; Dixon, L.B.; Urban, D.A.; Crawford, E.D.; Hayes, R.B. A Prospective Study of Lycopene and Tomato Product Intake and Risk of Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2006, 15, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Peters, U.; Leitzmann, M.F.; Chatterjee, N.; Wang, Y.; Albanes, D.; Gelmann, E.P.; Friesen, M.D.; Riboli, E.; Hayes, R.B. Serum Lycopene, Other Carotenoids, and Prostate Cancer Risk: A Nested Case-Control Study in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol. Biomark. Prev. 2007, 16, 962–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oczkowski, M.; Dziendzikowska, K.; Pasternak-Winiarska, A.; Włodarek, D.; Gromadzka-Ostrowska, J. Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients 2021, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, N.; Yuan, C.; Hamada, T.; Cao, Y.; Babic, A.; Morales-Oyarvide, V.; Kraft, P.; Ng, K.; Giovannucci, E.; Ogino, S.; et al. Regular Use of Aspirin or Non-Aspirin Nonsteroidal Anti-Inflammatory Drugs Is Not Associated with Risk of Incident Pancreatic Cancer in Two Large Cohort Studies. Gastroenterology 2018, 154, 1380–1390.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinas, C.A.; Kwon, E.M.; FitzGerald, L.M.; Feng, Z.; Nelson, P.S.; Ostrander, E.A.; Peters, U.; Stanford, J.L. Use of Aspirin and Other Nonsteroidal Antiinflammatory Medications in Relation to Prostate Cancer Risk. Am. J. Epidemiol. 2010, 172, 578–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurwitz, L.M.; Joshu, C.E.; Barber, J.R.; Prizment, A.E.; Vitolins, M.Z.; Jones, M.R.; Folsom, A.R.; Han, M.; Platz, E.A. Aspirin and Non-Aspirin NSAID Use and Prostate Cancer Incidence, Mortality, and Case-Fatality in the Atherosclerosis Risk in Communities Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doat, S.; Cénée, S.; Trétarre, B.; Rebillard, X.; Lamy, P.-J.; Bringer, J.-P.; Iborra, F.; Murez, T.; Sanchez, M.; Menegaux, F. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Prostate Cancer Risk: Results from the EPICAP Study. Cancer Med. 2017, 6, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.K.; Daugherty, S.E.; Liao, L.M.; Freedman, N.D.; Abnet, C.C.; Pfeiffer, R.; Cook, M.B. Do Aspirin and Other NSAIDs Confer a Survival Benefit in Men Diagnosed with Prostate Cancer? A Pooled Analysis of NIH-AARP and PLCO Cohorts. Cancer Prev. Res. 2017, 10, 410–420. [Google Scholar] [CrossRef] [Green Version]
- Vishnupriya, P.; Aparna, A.; Vijaya Padma, V. Lipoxygenase (LOX) Pathway: A Promising Target to Combat Cancer. Curr. Pharm. Des. 2021, 27, 3349–3369. [Google Scholar] [CrossRef]
- Goftari, S.N.; Sadeghian, H.; Bahrami, A.R.; Maleki, F.; Matin, M.M. Stylosin and Some of Its Synthetic Derivatives Induce Apoptosis in Prostate Cancer Cells as 15-Lipoxygenase Enzyme Inhibitors. Naunyn Schmiedebergs Arch. Pharm. 2019, 392, 1491–1502. [Google Scholar] [CrossRef]
- Yarla, N.S.; Azad, R.; Basha, M.; Rajack, A.; Kaladhar, D.S.V.G.K.; Allam, B.K.; Pragada, R.R.; Singh, K.N.; Sunanda, K.K.; Pallu, R.; et al. 5-Lipoxygenase and Cyclooxygenase Inhibitory Dammarane Triterpenoid 1 from Borassus Flabellifer Seed Coat Inhibits Tumor Necrosis Factor-α Secretion in LPSInduced THP-1 Human Monocytes and Induces Apoptosis in MIA PaCa-2 Pancreatic Cancer Cells. Anticancer Agents Med. Chem. 2015, 15, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ahmad, A.; Kong, D.; Ali, S.; Azmi, A.S.; Li, Y.; Banerjee, S.; Padhye, S.; Sarkar, F.H. Hypoxia Induced Aggressiveness of Prostate Cancer Cells Is Linked with Deregulated Expression of VEGF, IL-6 and MiRNAs That Are Attenuated by CDF. PLoS ONE 2012, 7, e0043726. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.; Amir, S.; Golan, M.; Ben-Neriah, Y.; Mabjeesh, N.J. β-TrCP Upregulates HIF-1 in Prostate Cancer Cells. Prostate 2019, 79, 403–413. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, D.; Liu, J.; Axcrona, K.; Kvalheim, G.; Stokke, T.; Nesland, J.M.; Suo, Z. Prostate Cancer Cell Lines under Hypoxia Exhibit Greater Stem-Like Properties. PLoS ONE 2011, 6, e0029170. [Google Scholar] [CrossRef] [Green Version]
- Marignol, L.; Rivera-Figueroa, K.; Lynch, T.; Hollywood, D. Hypoxia, Notch Signalling, and Prostate Cancer. Nat. Rev. Urol. 2013, 10, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Whyteside, A.R.W.R.; Hinsley, E.E.H.E.; Lambert, L.A.L.A.; McDermott, P.J.M.J.; Turner, A.J.T.J. ECE-1 Influences Prostate Cancer Cell Invasion via ET-1-Mediated FAK Phosphorylation and ET-1-Independent MechanismsThis Article Is One of a Selection of Papers Published in the Two-Part Special Issue Entitled 20 Years of Endothelin Research. Can. J. Physiol. Pharmacol. 2010, 88, 850–854. [Google Scholar] [CrossRef]
- Wang, R.; Dashwood, R.H. Endothelins and Their Receptors in Cancer: Identification of Therapeutic Targets. Pharm. Res. 2011, 63, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Mirzoeva, S.; Kim, N.D.; Chiu, K.; Franzen, C.A.; Bergan, R.C.; Pelling, J.C. Inhibition of HIF-1 Alpha and VEGF Expression by the Chemopreventive Bioflavonoid Apigenin Is Accompanied by Akt Inhibition in Human Prostate Carcinoma PC3-M Cells. Mol. Carcinog. 2008, 47, 686–700. [Google Scholar] [CrossRef]
- Deep, G.; Kumar, R.; Nambiar, D.K.; Jain, A.K.; Ramteke, A.M.; Serkova, N.J.; Agarwal, C.; Agarwal, R. Silibinin Inhibits Hypoxia-Induced HIF-1α-Mediated Signaling, Angiogenesis and Lipogenesis in Prostate Cancer Cells: In Vitro Evidence and in Vivo Functional Imaging and Metabolomics. Mol. Carcinog. 2017, 56, 833–848. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-H.; Lee, Y.J. Quercetin Suppresses Hypoxia-Induced Accumulation of Hypoxia-Inducible Factor-1alpha (HIF-1alpha) through Inhibiting Protein Synthesis. J. Cell. Biochem. 2008, 105, 546–553. [Google Scholar] [CrossRef]
- Fahey, J.M.; Girotti, A.W. Accelerated Migration and Invasion Of Prostate Cancer Cells After a Photodynamic Therapy-Like Challenge: Role of Nitric OxidE. Nitric Oxide 2015, 49, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Siemens, D.R.; Heaton, J.P.W.; Adams, M.A.; Kawakami, J.; Graham, C.H. Phase II Study of Nitric Oxide Donor for Men with Increasing Prostate-Specific Antigen Level after Surgery or Radiotherapy for Prostate Cancer. Urology 2009, 74, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Panara, K.; Kuchakulla, M.; Kulandavelu, S.; Burnstein, K.L.; Schally, A.V.; Hare, J.M.; Ramasamy, R. Alterations of Tumor Microenvironment by Nitric Oxide Impedes Castration-Resistant Prostate Cancer Growth. Proc. Natl. Acad. Sci. USA 2018, 115, 11298–11303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massari, F.; Mollica, V.; Di Nunno, V.; Gatto, L.; Santoni, M.; Scarpelli, M.; Cimadamore, A.; Lopez-Beltran, A.; Cheng, L.; Battelli, N.; et al. The Human Microbiota and Prostate Cancer: Friend or Foe? Cancers 2019, 11, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, C.M.; Shrestha, E.; Peiffer, L.B.; Sfanos, K.S. The Microbiome in Prostate Inflammation and Prostate Cancer. Prostate Cancer Prostatic Dis. 2018, 21, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.M.; Liss, M.A. The Microbiome and Prostate Cancer Risk. Curr. Urol. Rep. 2019, 20, 66. [Google Scholar] [CrossRef]
- Shrestha, E.; White, J.R.; Yu, S.-H.; Kulac, I.; Ertunc, O.; De Marzo, A.M.; Yegnasubramanian, S.; Mangold, L.A.; Partin, A.W.; Sfanos, K.S. Profiling the Urinary Microbiome in Men with Positive versus Negative Biopsies for Prostate Cancer. J. Urol. 2018, 199, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katongole, P.; Sande, O.J.; Joloba, M.; Reynolds, S.J.; Niyonzima, N. The Human Microbiome and Its Link in Prostate Cancer Risk and Pathogenesis. Infect. Agent Cancer 2020, 15, 53. [Google Scholar] [CrossRef]
- Cavarretta, I.; Ferrarese, R.; Cazzaniga, W.; Saita, D.; Lucianò, R.; Ceresola, E.R.; Locatelli, I.; Visconti, L.; Lavorgna, G.; Briganti, A.; et al. The Microbiome of the Prostate Tumor Microenvironment. Eur. Urol. 2017, 72, 625–631. [Google Scholar] [CrossRef]
- Ma, X.; Chi, C.; Fan, L.; Dong, B.; Shao, X.; Xie, S.; Li, M.; Xue, W. The Microbiome of Prostate Fluid Is Associated With Prostate Cancer. Front. Microbiol. 2019, 10, 1664. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, M.A.P.; Rekha, P.-D. Microbiome—The “Unforeseen Organ”. Nat. Rev. Urol. 2017, 14, 521–522. [Google Scholar] [CrossRef]
- Liss, M.A.; White, J.R.; Goros, M.; Gelfond, J.; Leach, R.; Johnson-Pais, T.; Lai, Z.; Rourke, E.; Basler, J.; Ankerst, D.; et al. Metabolic Biosynthesis Pathways Identified from Fecal Microbiome Associated with Prostate Cancer. Eur. Urol. 2018, 74, 575–582. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Markowski, M.C.; Peiffer, L.B.; Ernst, S.E.; White, J.R.; Pienta, K.J.; Antonarakis, E.S.; Ross, A.E. Compositional Differences in Gastrointestinal Microbiota in Prostate Cancer Patients Treated with Androgen Axis-Targeted Therapies. Prostate Cancer Prostatic Dis. 2018, 21, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Li, L.; Chen, J.; Wei, G.; Ji, Y.; Chen, X.; Liu, J.; Huo, J. Human Gut-Microbiome Interplay: Analysis of Clinical Studies for the Emerging Roles of Diagnostic Microbiology in Inflammation, Oncogenesis and Cancer Management. Infect. Genet. Evol. 2021, 93, 104946. [Google Scholar] [CrossRef]
- Suvorov, A. Gut Microbiota, Probiotics, and Human Health. Biosci. Microbiota Food Health 2013, 32, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Sha, S.; Ni, L.; Stefil, M.; Dixon, M.; Mouraviev, V. The Human Gastrointestinal Microbiota and Prostate Cancer Development and Treatment. Investig. Clin. Urol. 2020, 61, S43–S50. [Google Scholar] [CrossRef]
- Kassayová, M.; Bobrov, N.; Strojný, L.; Orendáš, P.; Demečková, V.; Jendželovský, R.; Kubatka, P.; Kisková, T.; Kružliak, P.; Adamkov, M.; et al. Anticancer and Immunomodulatory Effects of Lactobacillus Plantarum LS/07, Inulin and Melatonin in NMU-Induced Rat Model of Breast Cancer. Anticancer Res. 2016, 36, 2719–2728. [Google Scholar]
- Kassayová, M.; Bobrov, N.; Strojný, L.; Kisková, T.; Mikeš, J.; Demečková, V.; Orendáš, P.; Bojková, B.; Péč, M.; Kubatka, P.; et al. Preventive Effects of Probiotic Bacteria Lactobacillus Plantarum and Dietary Fiber in Chemically-Induced Mammary Carcinogenesis. Anticancer Res. 2014, 34, 4969–4975. [Google Scholar]
- Rosa, L.S.; Santos, M.L.; Abreu, J.P.; Balthazar, C.F.; Rocha, R.S.; Silva, H.L.A.; Esmerino, E.A.; Duarte, M.C.K.H.; Pimentel, T.C.; Freitas, M.Q.; et al. Antiproliferative and Apoptotic Effects of Probiotic Whey Dairy Beverages in Human Prostate Cell Lines. Food Res. Int. 2020, 137, 109450. [Google Scholar] [CrossRef]
- Frugé, A.D.; Ptacek, T.; Tsuruta, Y.; Morrow, C.D.; Azrad, M.; Desmond, R.A.; Hunter, G.R.; Rais-Bahrami, S.; Demark-Wahnefried, W. Dietary Changes Impact the Gut Microbe Composition in Overweight and Obese Men with Prostate Cancer Undergoing Radical Prostatectomy. J. Acad. Nutr. Diet 2018, 118, 714–723.e1. [Google Scholar] [CrossRef]
- Mandair, D.; Rossi, R.E.; Pericleous, M.; Whyand, T.; Caplin, M.E. Prostate Cancer and the Influence of Dietary Factors and Supplements: A Systematic Review. Nutr. Metab. 2014, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Golubnitschaja, O.; Baban, B.; Boniolo, G.; Wang, W.; Bubnov, R.; Kapalla, M.; Krapfenbauer, K.; Mozaffari, M.S.; Costigliola, V. Medicine in the Early Twenty-First Century: Paradigm and Anticipation—EPMA Position Paper 2016. EPMA J. 2016, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Ponti, G.; Maccaferri, M.; Manfredini, M.; Micali, S.; Torricelli, F.; Milandri, R.; Del Prete, C.; Ciarrocchi, A.; Ruini, C.; Benassi, L.; et al. Quick Assessment of Cell-Free DNA in Seminal Fluid and Fragment Size for Early Non-Invasive Prostate Cancer Diagnosis. Clin. Chim. Acta 2019, 497, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Heidrich, I.; Ačkar, L.; Mossahebi Mohammadi, P.; Pantel, K. Liquid Biopsies: Potential and Challenges. Int. J. Cancer 2021, 148, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Haldrup, C.; Pedersen, A.L.; Øgaard, N.; Strand, S.H.; Høyer, S.; Borre, M.; Ørntoft, T.F.; Sørensen, K.D. Biomarker Potential of ST6GALNAC3 and ZNF660 Promoter Hypermethylation in Prostate Cancer Tissue and Liquid Biopsies. Mol. Oncol. 2018, 12, 545–560. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-P.; Lai, H.-M.; Guo, Z. Prostate Cancer Early Diagnosis: Circulating MicroRNA Pairs Potentially beyond Single MicroRNAs upon 1231 Serum Samples. Brief. Bioinform. 2021, 22, bbaa111. [Google Scholar] [CrossRef]
- Espinoza, A.R.; Lavi, J.; Otaño, N.; Arenilla, W.; León, R.; Espinoza, A.; Salvador, N.; León, A. Serum cellular inflammatory markers in the diagnosis of prostate cancer. Arch. Esp. Urol. 2019, 72, 641–646. [Google Scholar]
- Boerrigter, E.; Groen, L.N.; Van Erp, N.P.; Verhaegh, G.W.; Schalken, J.A. Clinical Utility of Emerging Biomarkers in Prostate Cancer Liquid Biopsies. Expert Rev. Mol. Diagn. 2020, 20, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Campos-Fernández, E.; Barcelos, L.S.; de Souza, A.G.; Goulart, L.R.; Alonso-Goulart, V. Research Landscape of Liquid Biopsies in Prostate Cancer. Am. J. Cancer Res. 2019, 9, 1309–1328. [Google Scholar]
- Farahani, H.; Alaee, M.; Amri, J.; Baghinia, M.-R.; Rafiee, M. Serum and Saliva Concentrations of Biochemical Parameters in Men with Prostate Cancer and Benign Prostate Hyperplasia. Lab. Med. 2020, 51, 243–251. [Google Scholar] [CrossRef]
- Abbasabad, G.D.; Khojasteh, S.M.B.; Naji, H.E.; Zamani, M.R.; Hajipour, H.; Serati-Nouri, H. An Interleukin-6 Single Nucleotide Polymorphism and Susceptibility to Prostate Adenocarcinoma and Bone Metastasis in an Iranian Population. Asian Pac. J. Cancer Prev. 2018, 19, 1717–1720. [Google Scholar] [CrossRef]
- Kelly, R.S.; Heiden, M.V.; Giovannucci, E.L.; Mucci, L.A. Metabolomic Biomarkers of Prostate Cancer: Prediction, Diagnosis, Progression, Prognosis and Recurrence. Cancer Epidemiol. Biomark. Prev. 2016, 25, 887–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constâncio, V.; Nunes, S.P.; Moreira-Barbosa, C.; Freitas, R.; Oliveira, J.; Pousa, I.; Oliveira, J.; Soares, M.; Dias, C.G.; Dias, T.; et al. Early Detection of the Major Male Cancer Types in Blood-Based Liquid Biopsies Using a DNA Methylation Panel. Clin. Epigenet. 2019, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Hongo, H.; Oya, M. Complete Response with Early Introduction of Cabazitaxel in a Patient with Multiple Lung Metastases of Castration-Resistant Prostate Cancer Following the Early Detection of Metastases Using Liquid Biopsy: A Case Report. BMC Cancer 2019, 19, 562. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.; Tuzova, A.V.; Walsh, A.L.; Russell, N.M.; O’Brien, O.; Kelly, S.; Dhomhnallain, O.N.; DeBarra, L.; Dale, C.M.; Brugman, R.; et al. EpiCaPture: A Urine DNA Methylation Test for Early Detection of Aggressive Prostate Cancer. JCO Precis. Oncol. 2019, 2019, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Brikun, I.; Nusskern, D.; Decatus, A.; Harvey, E.; Li, L.; Freije, D. A Panel of DNA Methylation Markers for the Detection of Prostate Cancer from FV and DRE Urine DNA. Clin. Epigenet. 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markou, A.; Lazaridou, M.; Paraskevopoulos, P.; Chen, S.; Świerczewska, M.; Budna, J.; Kuske, A.; Gorges, T.M.; Joosse, S.A.; Kroneis, T.; et al. Multiplex Gene Expression Profiling of In Vivo Isolated Circulating Tumor Cells in High-Risk Prostate Cancer Patients. Clin. Chem. 2018, 64, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Budna-Tukan, J.; Świerczewska, M.; Mazel, M.; Cieślikowski, W.A.; Ida, A.; Jankowiak, A.; Antczak, A.; Nowicki, M.; Pantel, K.; Azria, D.; et al. Analysis of Circulating Tumor Cells in Patients with Non-Metastatic High-Risk Prostate Cancer before and after Radiotherapy Using Three Different Enumeration Assays. Cancers 2019, 11, 802. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Cao, S.; Situ, B.; Zhong, J.; Hu, Y.; Li, S.; Huang, J.; Xu, J.; Wu, S.; Lin, J.; et al. Metabolic Reprogramming-Based Characterization of Circulating Tumor Cells in Prostate Cancer. J. Exp. Clin. Cancer Res. 2018, 37, 127. [Google Scholar] [CrossRef]
- Panigrahi, G.K.; Praharaj, P.P.; Kittaka, H.; Mridha, A.R.; Black, O.M.; Singh, R.; Mercer, R.; van Bokhoven, A.; Torkko, K.C.; Agarwal, C.; et al. Exosome Proteomic Analyses Identify Inflammatory Phenotype and Novel Biomarkers in African American Prostate Cancer Patients. Cancer Med. 2019, 8, 1110–1123. [Google Scholar] [CrossRef]
- Hofmann, L.; Sallinger, K.; Haudum, C.; Smolle, M.; Heitzer, E.; Moser, T.; Novy, M.; Gesson, K.; Kroneis, T.; Bauernhofer, T.; et al. A Multi-Analyte Approach for Improved Sensitivity of Liquid Biopsies in Prostate Cancer. Cancers 2020, 12, 2247. [Google Scholar] [CrossRef]
- Cheung, P.K.; Ma, M.H.; Tse, H.F.; Yeung, K.F.; Tsang, H.F.; Chu, M.K.M.; Kan, C.M.; Cho, W.C.S.; Ng, L.B.W.; Chan, L.W.C.; et al. The Applications of Metabolomics in the Molecular Diagnostics of Cancer. Expert Rev. Mol. Diagn. 2019, 19, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Crigna, A.T.; Samec, M.; Koklesova, L.; Liskova, A.; Giordano, F.A.; Kubatka, P.; Golubnitschaja, O. Cell-Free Nucleic Acid Patterns in Disease Prediction and Monitoring-Hype or Hope? EPMA J. 2020, 11, 603–627. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Nishimura, S.; Ito, T.; Oka, N.; Akagi, M. Limitations and usefulness of biopsy techniques for the diagnosis of metastatic bone and soft tissue tumors. Ann. Med. Surg. 2021, 68, 102581. [Google Scholar] [CrossRef] [PubMed]
- Gerner, C.; Costigliola, V.; Golubnitschaja, O. Multiomic Patterns in Body Fluids: Technological Challenge with a Great Potential to Implement the Advanced Paradigm of 3p Medicine. Mass Spectrom. Rev. 2020, 39, 442–451. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Liskova, A.; Koklesova, L.; Samec, M.; Biringer, K.; Büsselberg, D.; Podbielska, H.; Kunin, A.A.; Evsevyeva, M.E.; Shapira, N.; et al. Caution, “normal” BMI: Health risks associated with potentially masked individual underweight EPMA Position Paper 2021. EPMA J. 2021, 12, 243–264. [Google Scholar] [CrossRef]

| Type of Patient Stratification | Type of Biomarker | Characterisation | References |
|---|---|---|---|
| PCa versus benign adenomas | cfDNA | Cell free DNA in seminal fluid | [123] |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Seminal fluid | |||
| ctDNA | ct-DNA methylation panel: ST6GALNAC3, CCDC181, HAPLN3 | [124,125] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Serum | |||
| miRNA | 1231 high-throughput miRNA-profiled serum samples | [126] | |
| The pairwise model was composed of five circulating miRNAs coupled to miR-5100 and miR-1290. | |||
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Serum | |||
| Proteins | Circulating inflammatory markers (Cytokines)-neutrophil/lymphocyte (NLR), neutrophil/monocyte (NMR) and platelet/lymphocyte (PLR) | [127] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Blood | |||
| miRNA | mir-200-family (including miRNA-141-3p) and miR-375 | [128] | |
| Clinical relevance: Prognosis | |||
| Liquid biopsy: Urine | |||
| cfDNA | Long non-coding RNAs- MALAT1-Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) | [128] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Blood plasma | |||
| miRNA | let-7c, miR-30c, miR-141, miR-375 | [129] | |
| Clinical relevance: Diagnosis, treatment algorithms | |||
| Liquid biopsy: Blood plasma | |||
| miRNA | miR-572, miR-1290, miR-141 and miR-145 (in EV) | [129] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Urine | |||
| miRNA | miR-21, miR-141, miR-214, miR-375 and let-7c (in EV) | [129] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Urine | |||
| Proteins | ERG + PCA3 transcripts (ExoDx Prostate Intelliscore) (in EV) | [129] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Urine | |||
| Proteins | Sensitivity and Specificity Analysis for Salivary PSA, B2M, CK-BB, MT, Zinc, Creatinine and Urea | [130] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Saliva, blood serum | |||
| Proteins | Inflammatory biomarkers: Interleukin-6 Single Nucleotide Polymorphism | [131] | |
| Clinical relevance: Prediction, diagnosis | |||
| Liquid biopsy: Blood | |||
| cfDNA | ALU sequence quantification and integrity | [129] | |
| Clinical relevance: Prediction, prognosis | |||
| Liquid biopsy: Blood plasma | |||
| Metabolites | Acetoacetate, cystine, glutamate, lysine, tyrosine, lipids | [132] | |
| Clinical relevance: Prediction, diagnosis | |||
| Liquid biopsy: Blood serum | |||
| Metabolites | Metabolites-based disease modelling: dihyroxybutanoic acid and xylonic acid (upregulated), pyrimidine, xylopyranose and ribofuranoside (downregulated) | [132] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Urine | |||
| PCa with versus PCa without metastatic potential | cfDNA | DNA methylation | [133] |
| Promotor methylation levels of APCme, FOXA1me, GSTP1me, HOXD3me, RARβ2me, RASSF1Ame, SEPT9me and SOX17me | |||
| Clinical relevance: Prediction, prognosis | |||
| Liquid biopsy: Blood plasma | |||
| CTC enumeration/molecular biological characterisation | AR-signalling-dependent cancer cells | [134] | |
| Clinical relevance: Treatment algorithms | |||
| Liquid biopsy: Blood | |||
| ctDNA | DNA methylation panel (epigenetic regulation) | [135] | |
| Gene panel—GSTP1, SFRP2, IGFBP3, IGFBP7, APC, PTGS2 | |||
| Clinical relevance: Prognosis | |||
| Liquid biopsy: Urine | |||
| ctDNA | Methylation Gene panel—ADCY4, AOX1, APC, CXCL14, EPHX3, GFRA2, GSTP1, HEMK1, HOXA7, HOXB5, HOXD3a, HOXD3b, HOXD9 HOXD10, KIFC2, MOXD1, NEUROG3, NODAL, RASSF5, NSD1 | [124,136] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Urine | |||
| CTC enumeration/molecular biological characterisation | Gene panel-(KRT19, EpCAM, CDH1, HMBS, PSCA, ALDH1A1, PROM1, HPRT1, TWIST1, VIM, CDH2, B2M, PLS3 and PSA) | [124,137] | |
| Clinical relevance: Diagnosis, prognosis | |||
| Liquid biopsy: Blood | |||
| CTC enumeration/molecular biological characterisation | Circulating tumour cells (CTC) using the CellSearch system, dual fluoro-EPISPOT assay and CellCollector | [124,138] | |
| Clinical relevance: Prediction, prognosis | |||
| Liquid biopsy: Blood | |||
| CTC enumeration/molecular biological characterisation | Metabolic characterisation of CTCs in the peripheral blood of PCa patients PGK1 and G6PD (GM markers) | [124,139] | |
| Clinical relevance: Diagnosis, prognosis | |||
| Liquid biopsy: Blood | |||
| Proteins | African American men (AAM) with PCa compared to healthy AAM; Disease specific protein patterns: Isoform 2 of Coiled-coil and C2 domain-containing protein 1A, Keratin, type I cytoskeletal 10, UPF0728 protein C10orf53, DnaJ homolog subfamily C member 13, Prothrombin, Apolipoprotein (a), Coiled-coil domain-containing protein 172. | [140] | |
| Clinical relevance: Diagnosis, prognosis | |||
| Liquid biopsy: Blood serum | |||
| CTC enumeration/molecular biological characterisation | CTC enumeration and characterisation-AR-V7, AR-FL, KLK3 mRNA expression AR-V7, AR-FL, KLK3 mRNA expression AR amplification in ct-DNA | [141] | |
| Clinical relevance: Diagnosis, prognosis | |||
| Liquid biopsy: CTCs, RNA from whole blood lysates and plasma DNA | |||
| CTC enumeration/molecular biological characterisation | (KLK3, FOLH1, NPY)-tumour derived biomarkers | [128] | |
| Clinical relevance: Prognosis | |||
| Liquid biopsy: Blood | |||
| cfDNA | KLK3 and TMPRSS2-ERG | [128] | |
| Clinical relevance: Prognosis | |||
| Liquid biopsy: Urine and blood | |||
| cfDNA | SCHLAP1 SWI/SNF COMPLEX Antagonist Associated with Prostate Cancer 1 (SCHLAP1) lncRNA | [128] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Urine | |||
| cfDNA | MALAT1 | [128] | |
| Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) | |||
| Clinical relevance: Prediction, prognosis | |||
| Liquid biopsy: Blood plasma | |||
| CTC enumeration/molecular biological characterisation | Androgen receptor variant 7 protein (AR-V7) | [129] | |
| Clinical relevance: Prognosis | |||
| Liquid biopsy: Blood | |||
| CTC enumeration/molecular biological characterisation | mRNA of PSA, PSMA and EGFR in CTCs | [129] | |
| Clinical relevance: Treatment algorithms, prognosis | |||
| Liquid biopsy: Blood | |||
| CTC enumeration/molecular biological characterisation | Nuclear localisation of AR-V7 in CTCs | [129] | |
| Clinical relevance: Treatment algorithms, prognosis | |||
| Liquid biopsy: Blood | |||
| CTC enumeration/molecular biological characterisation | Albumin, LDH, PSA, haemoglobin and ALK (ALPHA) in serum and CTC enumeration | [129] | |
| Clinical relevance: Prediction, prognosis | |||
| Liquid biopsy: Blood | |||
| CTC enumeration/molecular biological characterisation | CTC enumeration, stem cell-related genes (ABCG2, PROM1 and PSCA) and EMT-related genes (TWIST1 and vimentin) in PBMCs | [129] | |
| Clinical relevance: Prediction, prognosis | |||
| Liquid biopsy: Blood | |||
| CTC enumeration/molecular biological characterisation | CD117/c-kit, CD133/prominin-1, CD34, CD184/CXCR4 and EpCAM/CD326 in lymphocytes | [129] | |
| Clinical relevance: Treatment algorithms, prognosis | |||
| Liquid biopsy: Blood | |||
| CTC enumeration/molecular biological characterisation | CTC enumeration and AR-V7 mRNA in CTCs | [129] | |
| Clinical relevance: Treatment algorithms, prognosis | |||
| Liquid biopsy: Blood | |||
| CTC enumeration/molecular biological characterisation | CTC enumeration and Ki67 and vimentin in CTCs | [129] | |
| Clinical relevance: Treatment algorithms, prognosis | |||
| Liquid biopsy: Blood | |||
| CTC enumeration/molecular biological characterisation | Telomerase activity in CTCs and CTC enumeration | [129] | |
| Clinical relevance: Diagnosis, treatment algorithms, | |||
| prognosis | |||
| Liquid biopsy: Blood | |||
| CTC enumeration/molecular biological characterisation | mRNA of KLK3, KLK2, HOXB13, GRHL2 and FOXA1 in whole blood and CTC enumeration | [129] | |
| Clinical relevance: Treatment algorithms | |||
| Liquid biopsy: Blood | |||
| CTC enumeration/molecular biological characterisation | mRNA of antioxidant genes (GPX1 and SOD2) and prostate genes (AR, cyclin B and bFGF) in CTCs | [129] | |
| Clinical relevance: Prediction, prognosis | |||
| Liquid biopsy: Blood | |||
| CTC enumeration/molecular biological characterisation | mRNA of anti-oxidant genes (GPX1, SOD2 and TXNRD1), epithelial gene (CK20) and organ-specific genes (AR, PSA, PSMA) in CTCs | [129] | |
| Clinical relevance: Prediction, prognosis | |||
| Liquid biopsy: Blood | |||
| cfDNA | Copy number of cancer-related genes, AR and CTC numeration | [129] | |
| Clinical relevance: Prediction, prognosis | |||
| Liquid biopsy: Blood plasma | |||
| cfDNA | cBMP6 mRNA, cf-DNA, apoptotic nucleosomes and H3K27me3 | [129] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Blood plasma | |||
| cfDNA | cfDNA quantification | [129] | |
| Clinical relevance: Prediction, prognosis | |||
| Liquid biopsy: Blood plasma | |||
| cfDNA | cfDNA quantification | [129] | |
| Clinical relevance: Prediction, prognosis | |||
| Liquid biopsy: Blood serum | |||
| cfDNA | AR copy number and 19 cancer associated genes | [129] | |
| Clinical relevance: Prognosis | |||
| Liquid biopsy: Blood plasma | |||
| Proteins | Prostate-specific transcripts such as KLK3, PCA3 and ERG; kidney- and bladder-specific transcripts in EVs | [129] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Urine | |||
| Proteins | ADSV-TGM4 and CD63-GLPK5-SPHM-PSA-PAPP | [129] | |
| Clinical relevance: Diagnosis, prognosis | |||
| Liquid biopsy: Urine | |||
| CTC enumeration/molecular biological characterisation | PSMA in prostate microparticles and CTC enumeration | [129] | |
| Clinical relevance: Prediction, prognosis | |||
| Liquid biopsy: Blood plasma | |||
| miRNA | RNA copy numbers of ERG and PCA3 (EXO106 score) | [129] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Urine | |||
| miRNA | Serum miR-141 and miR-37; and urine miR-107 and miR-574-3p | [129] | |
| Clinical relevance: Diagnosis, prognosis | |||
| Liquid biopsy: Serum, plasma and urine | |||
| Proteins | Interleukin-6 Single Nucleotide Polymorphism | [129] | |
| Clinical relevance: Prediction | |||
| Liquid biopsy: Blood | |||
| miRNA | lncRNA-p21 | [129] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Urine | |||
| Metabolites | sarcosine, choline, phosphocholine | [142] | |
| Liquid biopsy: Diagnosis | |||
| Liquid biopsy: Blood, urine | |||
| Metabolites | Biomarker profile: Lysophosphatidylcholines (LPC) with saturated fatty acid chains, serotonin, monoamine, aspartic acid (Asp) and ornithine | [132] | |
| Clinical relevance: Diagnosis, prognosis | |||
| Liquid biopsy: Blood serum | |||
| Metabolites | Sarcosine (sediment) | [132] | |
| Sarcosine (supernatant) | |||
| Clinical relevance: Diagnosis, prognosis | |||
| Liquid biopsy: Urine | |||
| Metabolites | Acylcarnitine and arachidonoyl amine | [132] | |
| Clinical relevance: Diagnosis | |||
| Liquid biopsy: Blood plasma | |||
| Metabolites | Ala, Ile, Orn, Lys (downregulated), Gln, Val, Trp and Arg (upregulated) | [132] | |
| Clinical relevance: Treatment monitoring, prognosis | |||
| Liquid biopsy: Blood plasma | |||
| Metabolites | Metabolites-based disease modelling: Pyrimidine, Creatinine, Purine, Glucopyranoside, Xylopyranose and Ribofuranoside (downregulated), Propenoic acid, Dihyroxybutanoic acid and Xylonic acid | [132] | |
| Clinical relevance: Diagnosis, prognosis | |||
| Liquid biopsy: Urine | |||
| Metabolites | Ureido isobutyric acid, indolylacryloyglycine, acetylvanilalinine 2-oxoglutarate | [132] | |
| Clinical relevance: Prognosis | |||
| Liquid biopsy: Urine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubnitschaja, O.; Kubatka, P.; Mazurakova, A.; Samec, M.; Alajati, A.; Giordano, F.A.; Costigliola, V.; Ellinger, J.; Ritter, M. Systemic Effects Reflected in Specific Biomarker Patterns Are Instrumental for the Paradigm Change in Prostate Cancer Management: A Strategic Paper. Cancers 2022, 14, 675. https://doi.org/10.3390/cancers14030675
Golubnitschaja O, Kubatka P, Mazurakova A, Samec M, Alajati A, Giordano FA, Costigliola V, Ellinger J, Ritter M. Systemic Effects Reflected in Specific Biomarker Patterns Are Instrumental for the Paradigm Change in Prostate Cancer Management: A Strategic Paper. Cancers. 2022; 14(3):675. https://doi.org/10.3390/cancers14030675
Chicago/Turabian StyleGolubnitschaja, Olga, Peter Kubatka, Alena Mazurakova, Marek Samec, Abdullah Alajati, Frank A. Giordano, Vincenzo Costigliola, Jörg Ellinger, and Manuel Ritter. 2022. "Systemic Effects Reflected in Specific Biomarker Patterns Are Instrumental for the Paradigm Change in Prostate Cancer Management: A Strategic Paper" Cancers 14, no. 3: 675. https://doi.org/10.3390/cancers14030675
APA StyleGolubnitschaja, O., Kubatka, P., Mazurakova, A., Samec, M., Alajati, A., Giordano, F. A., Costigliola, V., Ellinger, J., & Ritter, M. (2022). Systemic Effects Reflected in Specific Biomarker Patterns Are Instrumental for the Paradigm Change in Prostate Cancer Management: A Strategic Paper. Cancers, 14(3), 675. https://doi.org/10.3390/cancers14030675








