Radiomic Phenotypes for Improving Early Prediction of Survival in Stage III Non-Small Cell Lung Cancer Adenocarcinoma after Chemoradiation
Abstract
:Simple Summary
Abstract
1. Introduction
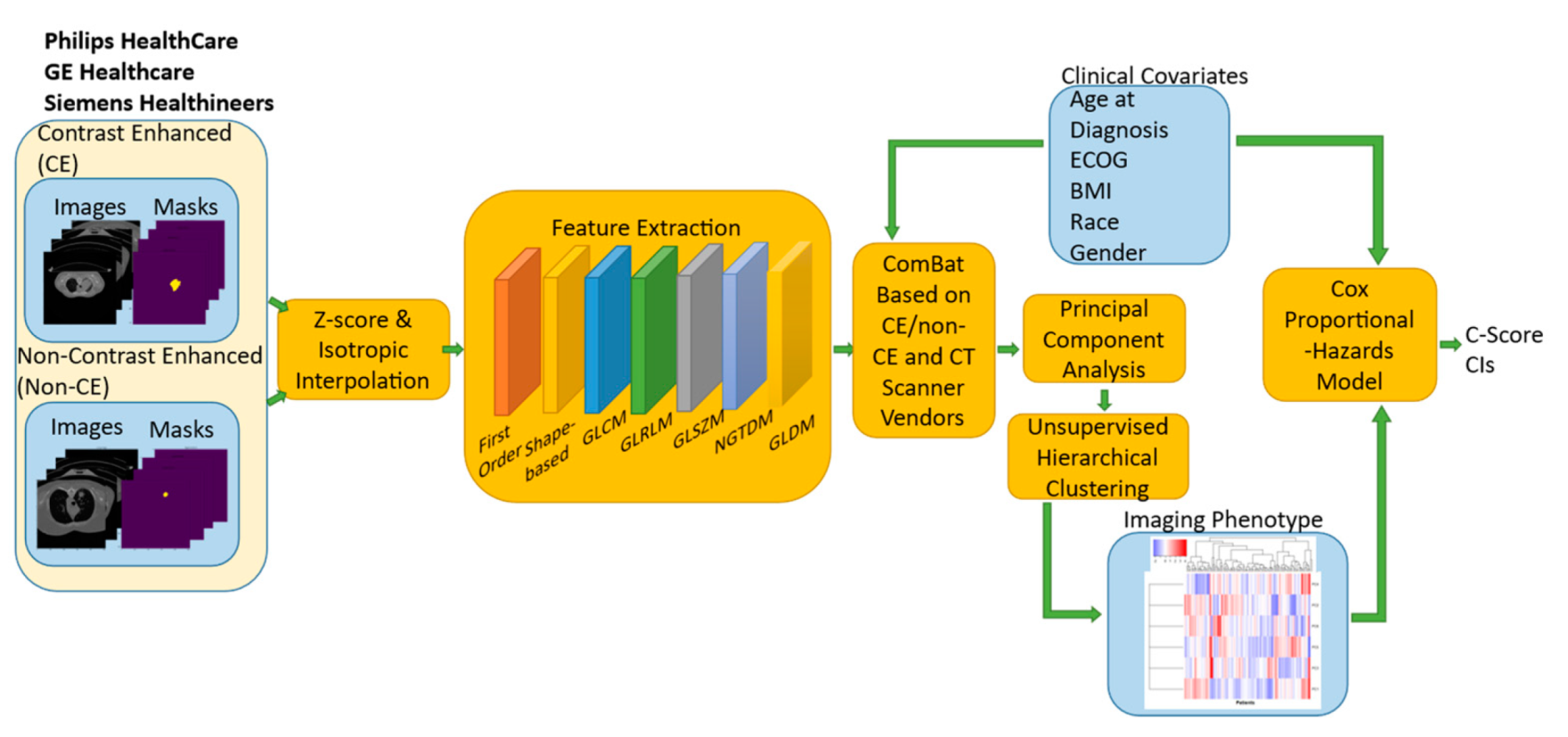
2. Materials and Methods
2.1. Patient Cohort
2.2. Radiomic Feature Extraction
2.3. Clinical Features
2.4. ComBat Harmonization
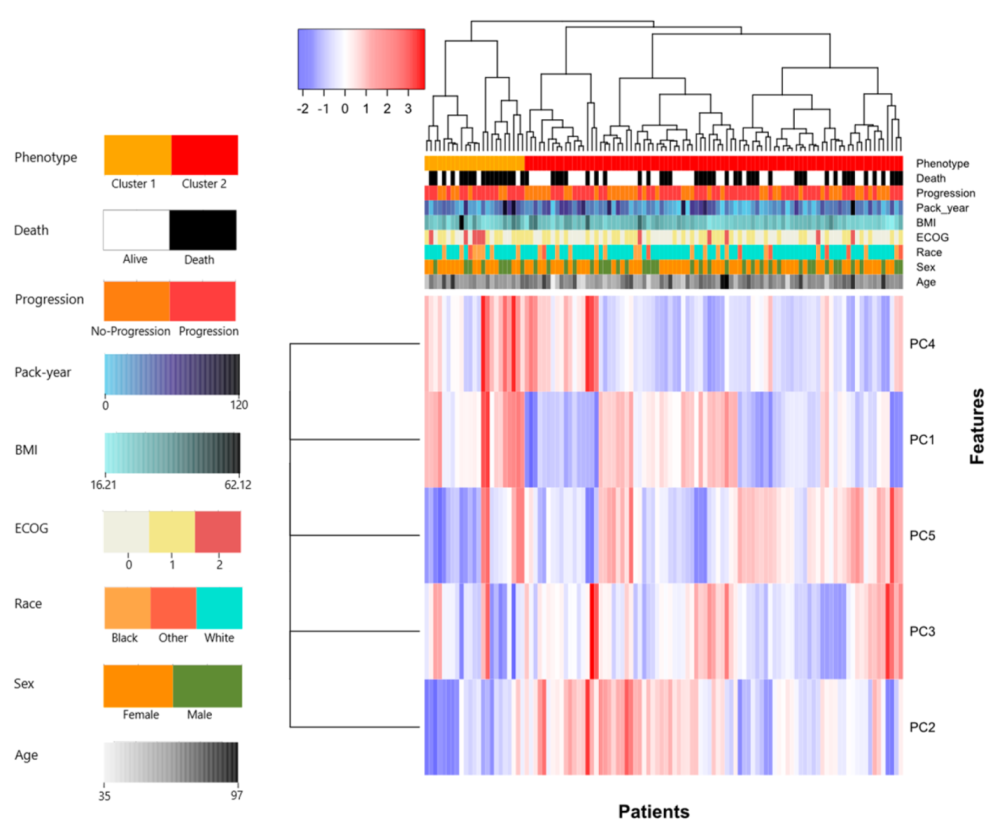
2.5. Unsupervised Hierarchical Clustering
2.6. Univariate Analysis
2.7. Multivariate Analysis
3. Results
3.1. Patient Characteristics
3.2. Univariate Analysis
3.3. Multivariate Analysis
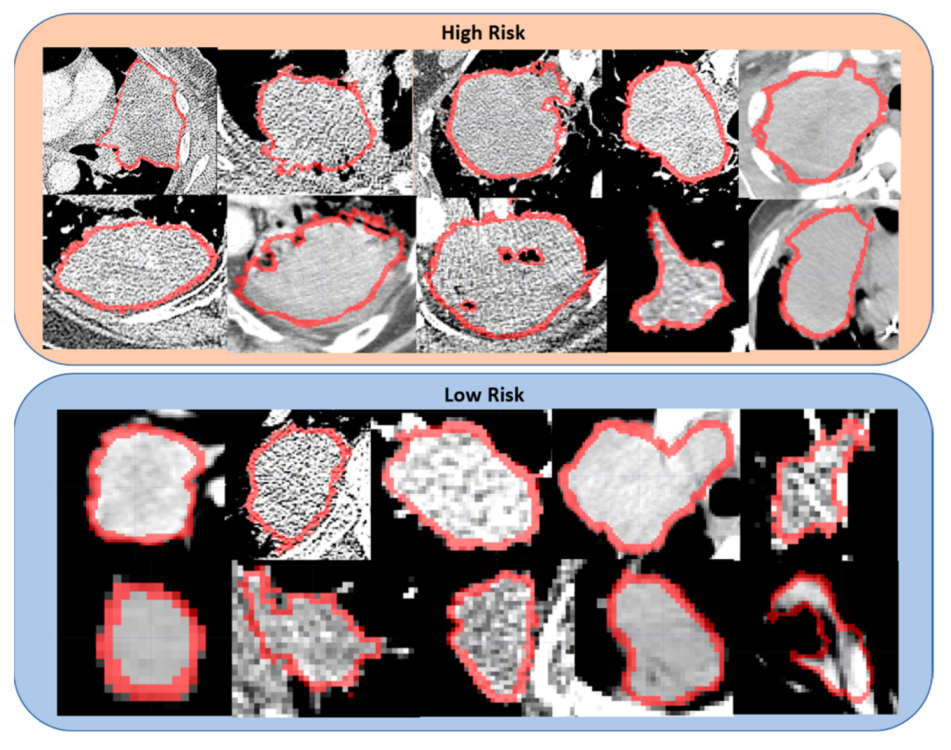
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung cancer statistics. Lung Cancer Pers. Med. 2016, 893, 1–19. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.; Krapcho, M.E.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D. SEER cancer statistics review, 1975–2016. Natl. Cancer Inst. 2019. Available online: https://seer.cancer.gov/archive/csr/1975_2016/ (accessed on 9 August 2021).
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.A.; Riely, G.J.; van Schil, P.E. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.; Wood, D.; Chair, V. Continue NCCN Guidelines Panel Disclosures NCCN Guidelines Version 3.2020 Non-Small Cell. Lung Cancer; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2020. [Google Scholar]
- Liang, J.; Bi, N.; Wu, S.; Chen, M.; Lv, C.; Zhao, L.; Shi, A.; Jiang, W.; Xu, Y.; Zhou, Z. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non-small cell lung cancer: A multicenter randomized phase III trial. Ann. Oncol. 2017, 28, 777–783. [Google Scholar] [CrossRef]
- Senan, S.; Brade, A.; Wang, L.H.; Vansteenkiste, J.; Dakhil, S.; Biesma, B.; Aguillo, M.M.; Aerts, J.; Govindan, R.; Rubio-Viqueira, B. Proclaim: Randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2016, 34, 953–962. [Google Scholar] [CrossRef]
- Albain, K.S.; Rusch, V.W.; Crowley, J.J.; Rice, T.W.; Turrisi, A., 3rd; Weick, J.K.; Lonchyna, V.A.; Presant, C.A.; McKenna, R.J.; Gandara, D.R. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: Mature results of southwest oncology group phase II study 8805. J. Clin. Oncol. 1995, 13, 1880–1892. [Google Scholar] [CrossRef]
- Belani, C.P.; Choy, H.; Bonomi, P.; Scott, C.; Travis, P.; Haluschak, J.; Curran, W.J., Jr. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non–small-cell lung cancer: A randomized phase II locally advanced multi-modality protocol. J. Clin. Oncol. 2005, 23, 5883–5891. [Google Scholar] [CrossRef]
- Hanna, N.; Neubauer, M.; Yiannoutsos, C.; McGarry, R.; Arseneau, J.; Ansari, R.; Reynolds, C.; Govindan, R.; Melnyk, A.; Fisher, W. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: The hoosier oncology group and US oncology. J. Clin. Oncol. 2008, 26, 5755–5760. [Google Scholar] [CrossRef]
- Gandara, D.R.; Chansky, K.; Albain, K.S.; Leigh, B.R.; Gaspar, L.E.; Lara, P.N., Jr.; Burris, H.; Gumerlock, P.; Kuebler, J.P.; Bearden, J.D., III. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non–small-cell lung cancer: Phase II southwest oncology group study S9504. J. Clin. Oncol. 2003, 21, 2004–2010. [Google Scholar] [CrossRef]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar]
- Higgins, K.A.; O’Connell, K.; Liu, Y.; Gillespie, T.W.; McDonald, M.W.; Pillai, R.N.; Patel, K.R.; Patel, P.R.; Robinson, C.G.; Simone II, C.B. National cancer database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.G.; Hu, C.; Choy, H.; Komaki, R.U.; Timmerman, R.D.; Schild, S.E.; Bogart, J.A.; Dobelbower, M.C.; Bosch, W.; Galvin, J.M. Impact of intensity-modulated radiation therapy technique for locally advanced non–small-cell lung cancer: A secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J. Clin. Oncol. 2017, 35, 56. [Google Scholar] [CrossRef] [PubMed]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.; Spigel, D.; Garassino, M.; Reck, M.; Senan, S. LBA49 Durvalumab after chemoradiotherapy in stage III NSCLC: 4-year survival update from the phase III pacific trial. Ann. Oncol. 2020, 31, S1178–S1179. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.; Özgüroğlu, M.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): A randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1670–1680. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Spira, A.; Raben, D.; Planchard, D.; Cho, B.; Özgüroğlu, M.; Daniel, D.; Villegas, A.; Vicente, D.; Hui, R. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann. Oncol. 2020, 31, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Rodrigues, G.; Choy, H.; Bradley, J.; Rosenzweig, K.E.; Bogart, J.; Curran, W.J., Jr.; Gore, E.; Langer, C.; Louie, A.V.; Lutz, S. Definitive radiation therapy in locally advanced non-small cell lung cancer: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based clinical practice guideline. Pract. Radiat. Oncol. 2015, 5, 141–148. [Google Scholar] [CrossRef]
- Rodrigues, G.; Choy, H.; Bradley, J.; Rosenzweig, K.E.; Bogart, J.; Curran, W.J., Jr.; Gore, E.; Langer, C.; Louie, A.V.; Lutz, S. Adjuvant radiation therapy in locally advanced non-small cell lung cancer: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based clinical practice guideline. Pract. Radiat. Oncol. 2015, 5, 149–155. [Google Scholar] [CrossRef]
- Zhu, X.; Hou, R.; Li, X.; Jiang, C.; Xia, W.; Fu, X. Predictive model of the first failure pattern in patients receiving definitive chemoradiotherapy for inoperable locally advanced non-small cell lung cancer (LA-NSCLC). Radiat. Oncol. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Yoon, S.; Shaikh, T.; Ross, E.; Bauman, J.; Borghaei, H.; Mehra, R.; Meyer, J.; Hallman, M. Patterns and predictors of failure following definitive chemoradiation for locally advanced Non-Small Cell Lung Cancer (NSCLC). Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, E477–E478. [Google Scholar] [CrossRef]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bredholt, G.; Mannelqvist, M.; Stefansson, I.M.; Birkeland, E.; Bø, T.H.; Øyan, A.M.; Trovik, J.; Kalland, K.-H.; Jonassen, I.; Salvesen, H.B. Tumor necrosis is an important hallmark of aggressive endometrial cancer and associates with hypoxia, angiogenesis and inflammation responses. Oncotarget 2015, 6, 39676. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, H.-S.; Jang, H.-J.; Lee, G.K.; Chung, K.Y.; Zo, J.I. Tumor necrosis as a prognostic factor for stage IA non-small cell lung cancer. Ann. Thorac. Surg. 2011, 91, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Parisi, A.; Bonanno, A.; Paparo, D.; Quattrocchi, E.; Branca, G.; Scardigno, M.; Fedele, F. Histologic coagulative tumour necrosis as a prognostic indicator of aggressiveness in renal, lung, thyroid and colorectal carcinomas: A brief review. Oncol. Lett. 2012, 3, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Van Stiphout, R.G.; Starmans, M.H.; Rios-Velazquez, E.; Nalbantov, G.; Aerts, H.J.; Roelofs, E.; Van Elmpt, W.; Boutros, P.C.; Granone, P. Predicting outcomes in radiation oncology—multifactorial decision support systems. Nat. Rev. Clin. Oncol. 2013, 10, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Scrivener, M.; de Jong, E.E.; van Timmeren, J.E.; Pieters, T.; Ghaye, B.; Geets, X. Radiomics applied to lung cancer: A review. Transl. Cancer Res 2016, 5, 398–409. [Google Scholar] [CrossRef]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“How-to” guide and critical reflection. Insights Imaging 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Deng, J.; She, Y.; Zhang, L.; Wang, B.; Ren, Y.; Wu, J.; Xie, D.; Sun, X.; Chen, C. Radiomics signature predicts the recurrence-free survival in stage I non-small cell lung cancer. Ann. Thorac. Surg. 2020, 109, 1741–1749. [Google Scholar] [CrossRef]
- Arshad, M.A.; Thornton, A.; Lu, H.; Tam, H.; Wallitt, K.; Rodgers, N.; Scarsbrook, A.; McDermott, G.; Cook, G.J.; Landau, D. Discovery of pre-therapy 2-deoxy-2-18 F-fluoro-D-glucose positron emission tomography-based radiomics classifiers of survival outcome in non-small-cell lung cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 455–466. [Google Scholar] [CrossRef]
- Lee, G.; Park, H.; Sohn, I.; Lee, S.H.; Song, S.H.; Kim, H.; Lee, K.S.; Shim, Y.M.; Lee, H.Y. Comprehensive computed tomography radiomics analysis of lung adenocarcinoma for prognostication. Oncology 2018, 23, 806. [Google Scholar] [CrossRef] [PubMed]
- Van Timmeren, J.E.; van Elmpt, W.; Leijenaar, R.T.; Reymen, B.; Monshouwer, R.; Bussink, J.; Paelinck, L.; Bogaert, E.; De Wagter, C.; Elhaseen, E. Longitudinal radiomics of cone-beam CT images from non-small cell lung cancer patients: Evaluation of the added prognostic value for overall survival and locoregional recurrence. Radiother. Oncol. 2019, 136, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Lee, H.; Kim, S.; Hyun, S. Pre-treatment 18F-FDG PET-based radiomics predict survival in resected non-small cell lung cancer. Clin. Radiol. 2019, 74, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Van Griethuysen, J.J.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Dercle, L.; Fronheiser, M.; Lu, L.; Du, S.; Hayes, W.; Leung, D.K.; Roy, A.; Wilkerson, J.; Guo, P.; Fojo, A.T. Identification of non–small cell lung cancer sensitive to systemic cancer therapies using radiomics. Clin. Cancer Res. 2020, 26, 2151–2162. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, J.; Sun, T.; Zheng, X.; Li, J.; Sun, H.; Zhou, X.; Zhou, C.; Zhang, H.; Cheng, Z. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep. 2017, 7, 1339. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zheng, W.; Johansson, M.; Lan, Q.; Park, Y.; White, E.; Matthews, C.E.; Sawada, N.; Gao, Y.-T.; Robien, K. Overall and central obesity and risk of lung cancer: A pooled analysis. JNCI J. Natl. Cancer Inst. 2018, 110, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Matsuoka, R.; Sakurada, T.; Goda, M.; Chuma, M.; Yagi, K.; Zamami, Y.; Nishioka, Y.; Ishizawa, K. Risk factors of immune checkpoint inhibitor-related interstitial lung disease in patients with lung cancer: A single-institution retrospective study. Sci. Rep. 2020, 10, 13773. [Google Scholar] [CrossRef]
- Richards, T.B.; Henley, S.J.; Puckett, M.C.; Weir, H.K.; Huang, B.; Tucker, T.C.; Allemani, C. Lung cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer 2017, 123, 5079–5099. [Google Scholar] [CrossRef]
- Botticelli, A.; Salati, M.; Di Pietro, F.R.; Strigari, L.; Cerbelli, B.; Zizzari, I.G.; Giusti, R.; Mazzotta, M.; Mazzuca, F.; Roberto, M. A nomogram to predict survival in non-small cell lung cancer patients treated with nivolumab. J. Transl. Med. 2019, 17, 99. [Google Scholar] [CrossRef]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Whitney, H.M.; Li, H.; Ji, Y.; Liu, P.; Giger, M.L. Harmonization of radiomic features of breast lesions across international DCE-MRI datasets. J. Med. Imaging 2020, 7, 012707. [Google Scholar] [CrossRef]
- Lucia, F.; Visvikis, D.; Vallières, M.; Desseroit, M.-C.; Miranda, O.; Robin, P.; Bonaffini, P.A.; Alfieri, J.; Masson, I.; Mervoyer, A. External validation of a combined PET and MRI radiomics model for prediction of recurrence in cervical cancer patients treated with chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Masson, I.; Da-ano, R.; Lucia, F.; Doré, M.; Castelli, J.; Goislard de Monsabert, C.; Ramée, J.F.; Sellami, S.; Visvikis, D.; Hatt, M. Statistical harmonization can improve the development of a multicenter CT based radiomic model predictive of non-response to induction chemotherapy in laryngeal cancers. Med. Phys. 2021, 48, 4099–4109. [Google Scholar] [CrossRef] [PubMed]
- Dissaux, G.; Visvikis, D.; Da-Ano, R.; Pradier, O.; Chajon, E.; Barillot, I.; Duverge, L.; Masson, I.; Abgral, R.; Ribeiro, M.-J.S. Pretreatment 18F-FDG PET/CT radiomics predict local recurrence in patients treated with stereotactic body radiotherapy for early-stage non–small cell lung cancer: A multicentric study. J. Nucl. Med. 2020, 61, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Garau, N.; Paganelli, C.; Summers, P.; Choi, W.; Alam, S.; Lu, W.; Fanciullo, C.; Bellomi, M.; Baroni, G.; Rampinelli, C. External validation of radiomics-based predictive models in low-dose CT screening for early lung cancer diagnosis. Med. Phys. 2020, 47, 4125–4136. [Google Scholar] [CrossRef] [PubMed]
- Shayesteh, S.; Nazari, M.; Salahshour, A.; Sandoughdaran, S.; Hajianfar, G.; Khateri, M.; Yaghobi Joybari, A.; Jozian, F.; Fatehi Feyzabad, S.H.; Arabi, H. Treatment response prediction using MRI-based pre-, post-, and delta-radiomic features and machine learning algorithms in colorectal cancer. Med. Phys. 2021, 48, 3691–3701. [Google Scholar] [CrossRef]
- Hotta, M.; Minamimoto, R.; Gohda, Y.; Miwa, K.; Otani, K.; Kiyomatsu, T.; Yano, H. Prognostic value of 18 F-FDG PET/CT with texture analysis in patients with rectal cancer treated by surgery. Ann. Nucl. Med. 2021, 35, 843–852. [Google Scholar] [CrossRef]
- Nakajo, M.; Jinguji, M.; Tani, A.; Kikuno, H.; Hirahara, D.; Togami, S.; Kobayashi, H.; Yoshiura, T. Application of a machine learning approach for the analysis of clinical and radiomic features of pretreatment [18F]-FDG PET/CT to predict prognosis of patients with endometrial cancer. Mol. Imaging Biol. 2021, 23, 756–765. [Google Scholar] [CrossRef]
- Crombé, A.; Kind, M.; Fadli, D.; Le Loarer, F.; Italiano, A.; Buy, X.; Saut, O. Intensity harmonization techniques influence radiomics features and radiomics-based predictions in sarcoma patients. Sci. Rep. 2020, 10, 15496. [Google Scholar] [CrossRef]
- Horng, H. Generalized-Combat. Available online: https://github.com/hannah-horng/generalized-combat (accessed on 9 August 2021).
- Fortin, J.P.; Robert Fitzgerald, T. Neurocombat. Available online: https://github.com/Jfortin1/neuroCombat (accessed on 15 July 2021).
- Team, R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2017. Available online: https://www.r-project.org/ (accessed on 15 October 2021).
- Beare, R.; Lowekamp, B.; Yaniv, Z. Image segmentation, registration and characterization in R with SimpleITK. J. Stat. Softw. 2018, 86, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Davidson-Pilon, C. Lifelines: Survival analysis in Python. J. Open Source Softw. 2019, 4, 1317. [Google Scholar] [CrossRef]
- Van Rossum, G.; Drake Jr, F.L. Python Reference Manual; Centrum voor Wiskunde en Informatica: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Alfons, A. Robust model selection for high-dimensional data with the R package robustHD. In Proceedings of the The R User Conference, useR!, University of Castilla-La Mancha, Albacete, Spain, 10–12 July 2013; p. 86. [Google Scholar]
- Warnes, M.G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W. Package “gplots”. Various R programming tools for plotting data 2020. Available online: https://cran.r-project.org/web/packages/gplots/gplots.pdf (accessed on 15 May 2021).
- Huan, G.H.; Liu, Y.; Marron, J.; Huang, M.H. Package “Sigclust”. Statistical Significance of Clustering. 2015. Available online: https://cran.r-project.org/web/packages/sigclust/sigclust.pdf (accessed on 17 May 2021).
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. J. Br. Surg. 2015, 102, 148–158. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Spigel, D.R.; Garassino, M.C.; Reck, M.; Senan, S. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC—An update from the Pacific trial. J. Thorac. Oncol. 2021, 16, 860–867. [Google Scholar] [CrossRef]

| Categorical Features | Classes | No. of Patients | (%) |
|---|---|---|---|
| Contrast Enhancement | Non-Contrast Enhanced | 82 | 74.5 |
| Contrast Enhanced | 28 | 25.5 | |
| CT Scanner Manufacturer | Philips Healthcare | 67 | 60.9 |
| Siemens Healthineers | 36 | 32.7 | |
| GE Healthcare | 7 | 6.4 | |
| Sex | Female | 68 | 61.8 |
| Male | 42 | 38.2 | |
| Race | White | 80 | 72.7 |
| African American | 22 | 20.0 | |
| Asian | 3 | 2.7 | |
| Native American | 1 | 0.9 | |
| Other | 4 | 3.6 | |
| Marital Status | Married | 66 | 60.0 |
| Single | 24 | 21.8 | |
| Divorced | 8 | 7.3 | |
| Widowed | 8 | 7.3 | |
| Separated | 4 | 3.6 | |
| Radiation Modality | Proton | 61 | 55.5 |
| Linac | 49 | 44.5 | |
| ECOG Status | 0 | 50 | 45.5 |
| 1 | 48 | 43.6 | |
| 2 | 10 | 9.1 | |
| Unknown | 2 | 1.8 | |
| Tobacco Use | Former Smoker | 91 | 82.7 |
| Current Smoker | 13 | 11.8 | |
| Never Smoker | 6 | 5.5 | |
| Histology | Adenocarcinoma | 110 | 100.0 |
| Chemotherapy Agents | Carboplatin-based Doublet | 61 | 55.5 |
| Cisplatin-based Doublet | 40 | 36.4 | |
| Platinum-based Triplet | 2 | 1.8 | |
| Unknown | 7 | 6.4 | |
| Chemotherapy | Concurrent | 89 | 80.9 |
| Sequential | 14 | 12.7 | |
| Unknown | 7 | 6.4 |
| Continuous Features | Median | Range * |
|---|---|---|
| Age (yr.) | 66 | (60–71) |
| Radiation Dose Delivered (Gy) | 66.6 | (60.0–66.7) |
| Dose per Fraction (Gy) | 1.8 | (1.8–1.8) |
| BMI (kg/m2) | 26.5 | (23.8–29.9) |
| Pack per year (smokers only) | 35.0 | (20.0–50.0) |
| Predictor | C-Score | 95% CI |
|---|---|---|
| ECOG Status | 0.62 | (0.55, 0.69) |
| Phenotype (ComBat) | 0.61 | (0.54, 0.67) |
| Age at Diagnosis | 0.58 | (0.50, 0.66) |
| Sex | 0.56 | (0.49, 0.63) |
| BMI | 0.55 | (0.44, 0.64) |
| Pack per year | 0.55 | (0.49, 0.62) |
| Phenotype (non-ComBat) | 0.48 | (0.47, 0.59) |
| Predictor | C-Score | 95% CI | p-Value (LRT) * |
|---|---|---|---|
| Phenotype (ComBat) + ECOG + Age | 0.69 | (0.62, 0.77) | 0.003 |
| Phenotype (non-ComBat) + ECOG + Age | 0.66 | (0.58, 0.74) | 0.15 |
| ECOG + Age (Baseline) | 0.65 | (0.57, 0.73) | -- |
| 2 PCs + ECOG + Age | 0.65 | (0.60, 0.75) | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna, J.M.; Barsky, A.R.; Shinohara, R.T.; Roshkovan, L.; Hershman, M.; Dreyfuss, A.D.; Horng, H.; Lou, C.; Noël, P.B.; Cengel, K.A.; et al. Radiomic Phenotypes for Improving Early Prediction of Survival in Stage III Non-Small Cell Lung Cancer Adenocarcinoma after Chemoradiation. Cancers 2022, 14, 700. https://doi.org/10.3390/cancers14030700
Luna JM, Barsky AR, Shinohara RT, Roshkovan L, Hershman M, Dreyfuss AD, Horng H, Lou C, Noël PB, Cengel KA, et al. Radiomic Phenotypes for Improving Early Prediction of Survival in Stage III Non-Small Cell Lung Cancer Adenocarcinoma after Chemoradiation. Cancers. 2022; 14(3):700. https://doi.org/10.3390/cancers14030700
Chicago/Turabian StyleLuna, José Marcio, Andrew R. Barsky, Russell T. Shinohara, Leonid Roshkovan, Michelle Hershman, Alexandra D. Dreyfuss, Hannah Horng, Carolyn Lou, Peter B. Noël, Keith A. Cengel, and et al. 2022. "Radiomic Phenotypes for Improving Early Prediction of Survival in Stage III Non-Small Cell Lung Cancer Adenocarcinoma after Chemoradiation" Cancers 14, no. 3: 700. https://doi.org/10.3390/cancers14030700
APA StyleLuna, J. M., Barsky, A. R., Shinohara, R. T., Roshkovan, L., Hershman, M., Dreyfuss, A. D., Horng, H., Lou, C., Noël, P. B., Cengel, K. A., Katz, S., Diffenderfer, E. S., & Kontos, D. (2022). Radiomic Phenotypes for Improving Early Prediction of Survival in Stage III Non-Small Cell Lung Cancer Adenocarcinoma after Chemoradiation. Cancers, 14(3), 700. https://doi.org/10.3390/cancers14030700







