Primary Tumor Resection Decelerates Disease Progression in an Orthotopic Mouse Model of Metastatic Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
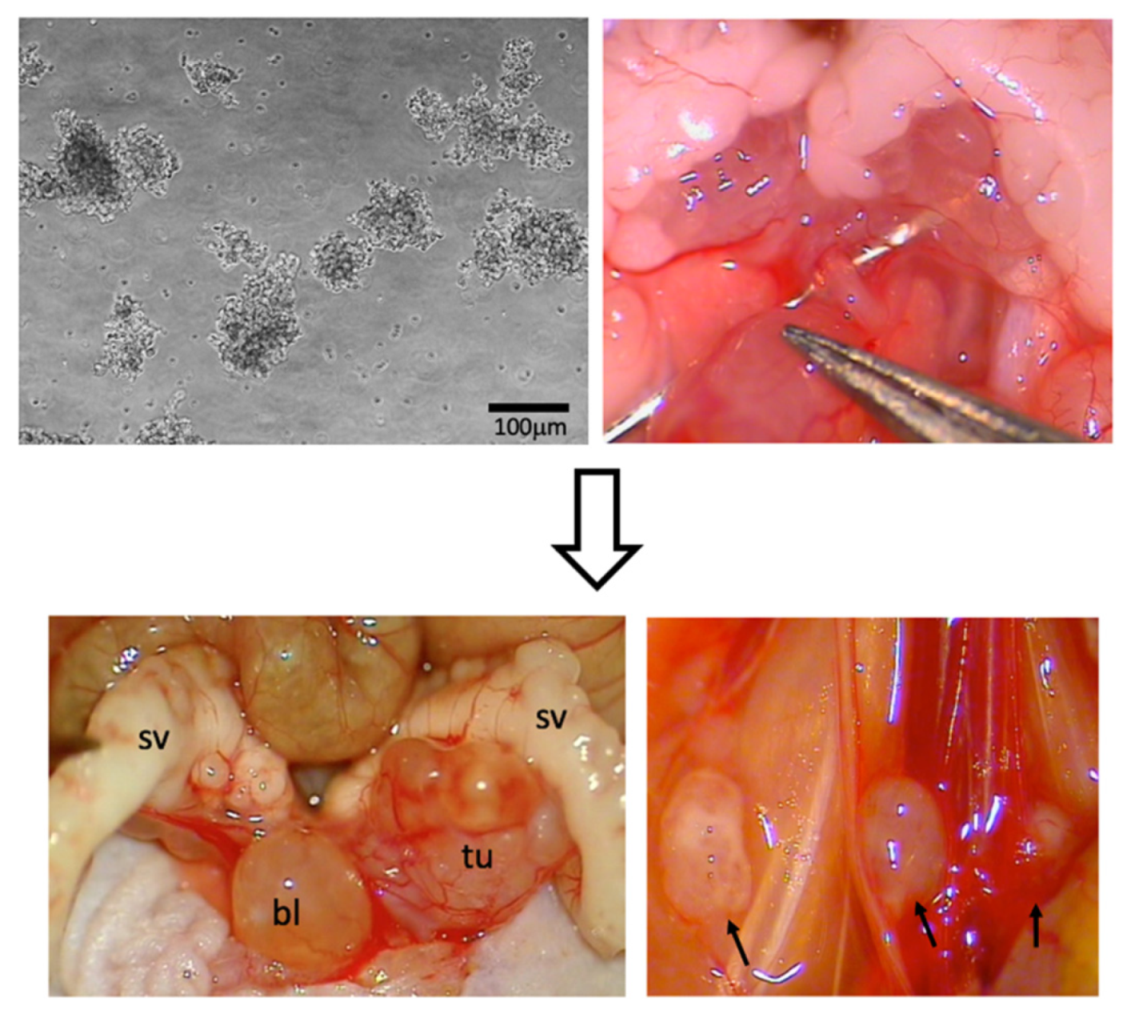
2.1. Cell Culture
2.2. Mice
2.3. Intraprostatic Tumor Cell Inoculation
2.4. Monitoring of Disease Burden
2.5. Lymph Node Dissection
2.6. Primary Tumor Resection/Sham Operation
2.7. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaloupka, M.; Herlemann, A.; Spek, A.; Gratzke, C.; Stief, C. Die zytoreduktive radikale Prostatektomie beim metastasierten Prostatakarzinom Cytoreductive, radical prostatectomy in metastatic prostate cancer. Der Urol. 2017, 56, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, R.; Korn, S.M.; Bensalah, K.; Kramer, G.; Shariat, S.F. Cytoreductive radical prostatectomy in metastatic prostate cancer: Does it really make sense? World J. Urol. 2017, 35, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.; Wells, J.C.; Rini, B.I.; Beuselinck, B.; Lee, J.-L.; Knox, J.J.; Bjarnason, G.A.; Pal, S.K.; Kollmannsberger, C.K.; Yuasa, T.; et al. Cytoreductive Nephrectomy in Patients with Synchronous Metastases from Renal Cell Carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur. Urol. 2014, 66, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Xie, W.; Kollmannsberger, C.; North, S.; Knox, J.J.; Lampard, J.G.; McDermott, D.F.; Rini, B.I.; Heng, D.Y. The Impact of Cytoreductive Nephrectomy on Survival of Patients With Metastatic Renal Cell Carcinoma Receiving Vascular Endothelial Growth Factor Targeted Therapy. J. Urol. 2011, 185, 60–66. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Gundem, G.; Van Loo, P.; Kremeyer, B.; Alexandrov, L.B.; Tubio, J.M.; Papaemmanuil, E.; Brewer, D.S.; Kallio, H.M.; Högnäs, G.; Annala, M. The evolutionary history of lethal metastatic prostate cancer. Nature 2015, 520, 353–357. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Rafii, S.; Lyden, D. Preparing the “soil”: The premetastatic niche. Cancer Res. 2006, 66, 11089–11093. [Google Scholar] [CrossRef] [Green Version]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; De Marzo, A.M.; Nelson, P.S.; Yegnasubramanian, S. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 2021, 18, 79–92. [Google Scholar] [CrossRef]
- Culp, S.H.; Schellhammer, P.F.; Williams, M.B. Might Men Diagnosed with Metastatic Prostate Cancer Benefit from Definitive Treatment of the Primary Tumor? A SEER-Based Study. Eur. Urol. 2014, 65, 1058–1066. [Google Scholar] [CrossRef]
- Swanson, G.; Thompson, I.; Basler, J.; Crawford, E.D. Metastatic Prostate Cancer—Does Treatment of the Primary Tumor Matter? J. Urol. 2006, 176, 1292–1298. [Google Scholar] [CrossRef]
- Heidenreich, A.; Pfister, D. Radical cytoreductive prostatectomy in men with prostate cancer and oligometastatic disease. Curr. Opin. Urol. 2020, 30, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef] [Green Version]
- Niklas, C.; Saar, M.; Nini, A.; Linxweiler, J.; Siemer, S.; Junker, K.; Stoeckle, M. Can local treatment prolong the sensitivity of metastatic prostate cancer to androgen deprivation or even prevent castration resistance? World J. Urol. 2021, 39, 3231–3237. [Google Scholar] [CrossRef]
- Hajili, T.; Ohlmann, C.H.; Linxweiler, J.; Niklas, C.; Janssen, M.; Siemer, S.; Stoeckle, M.; Saar, M. Radical prostatectomy in T4 prostate cancer after inductive androgen deprivation: Results of a single-institution series with long-term follow-up. BJU Int. 2018, 123, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Rexer, H. Metastatic, hormone-naive prostate cancer interventional study: Multicenter, prospective, randomized study to evaluate the effect of standard drug therapy with or without radical prostatectomy in patients with limited bone metastasized prostate cancer (G-RAMPP—The AUO AP 75/13 study). Urol. A 2015, 54, 1613–1616. [Google Scholar]
- Steuber, T.; Berg, K.D.; Røder, M.A.; Brasso, K.; Iversen, P.; Huland, H.; Tiebel, A.; Schlomm, T.; Haese, A.; Salomon, G.; et al. Does Cytoreductive Prostatectomy Really Have an Impact on Prognosis in Prostate Cancer Patients with Low-volume Bone Metastasis? Results from a Prospective Case-Control Study. Eur. Urol. Focus 2017, 3, 646–649. [Google Scholar] [CrossRef]
- Poelaert, F.; Verbaeys, C.; Rappe, B.; Kimpe, B.; Billiet, I.; Plancke, H.; Decaestecker, K.; Fonteyne, V.; Buelens, S.; Lumen, N. Cytoreductive Prostatectomy for Metastatic Prostate Cancer: First Lessons Learned From the Multicentric Prospective Local Treatment of Metastatic Prostate Cancer (LoMP) Trial. Urology 2017, 106, 146–152. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Connor, M.J.; Dubash, S.; Bass, E.J.; Tam, H.; Barwick, T.; Khoo, V.; Winkler, M.; Ahmed, H.U. Clinical Translation of Positive Metastases Identified on Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Imaging in the Management of De Novo Synchronous Oligometastatic Prostate Cancer. Eur. Urol. Focus 2020, 7, 951–954. [Google Scholar] [CrossRef]
- Saar, M.; Körbel, C.; Linxweiler, J.; Jung, V.; Kamradt, J.; Hasenfus, A.; Stöckle, M.; Unteregger, G.; Menger, M.D. Orthotopic tumorgrafts in nude mice: A new method to study human prostate cancer. Prostate 2015, 75, 1526–1537. [Google Scholar] [CrossRef]
- Valta, M.P.; Zhao, H.; Saar, M.; Tuomela, J.; Nolley, R.; Linxweiler, J.; Sandholm, J.; Lehtimäki, J.; Härkönen, P.; Coleman, I.; et al. Spheroid culture of LuCaP 136 patient-derived xenograft enables versatile preclinical models of prostate cancer. Clin. Exp. Metastasis 2016, 33, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Linxweiler, J.; Körbel, C.; Müller, A.; Hammer, M.; Veith, C.; Bohle, R.M.; Stöckle, M.; Junker, K.; Menger, M.D.; Saar, M. A novel mouse model of human prostate cancer to study intraprostatic tumor growth and the development of lymph node metastases. Prostate 2018, 78, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Linxweiler, J.; Hajili, T.; Körbel, C.; Berchem, C.; Zeuschner, P.; Müller, A.; Stöckle, M.; Menger, M.D.; Junker, K.; Saar, M. Cancer-associated fibroblasts stimulate primary tumor growth and metastatic spread in an orthotopic prostate cancer xenograft model. Sci. Rep. 2020, 10, 12575. [Google Scholar] [CrossRef]
- Young, S.R.; Saar, M.; Santos, J.; Nguyen, H.M.; Vessella, R.L.; Peehl, D.M. Establishment and serial passage of cell cultures derived from LuCaP xenografts. Prostate 2013, 73, 1251–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.M.; Vessella, R.L.; Morrissey, C.; Brown, L.G.; Coleman, I.M.; Higano, C.S.; Mostaghel, E.A.; Zhang, X.; True, L.D.; Lam, H.-M.; et al. LuCaP Prostate Cancer Patient-Derived Xenografts Reflect the Molecular Heterogeneity of Advanced Disease and Serve as Models for Evaluating Cancer Therapeutics. Prostate 2017, 77, 654–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Broeck, W.; Derore, A.; Simoens, P. Anatomy and nomenclature of murine lymph nodes: Descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J. Immunol. Methods 2006, 312, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, F.F.; Valenzuela, R.H.; Contreras, H.R.; Castellón, E.A. Surgical cytoreduction of the primary tumor reduces metastatic progression in a mouse model of prostate cancer. Oncol. Rep. 2015, 34, 2837–2844. [Google Scholar] [CrossRef] [Green Version]
- Di Trapani, E.; Nini, A.; Locatelli, I.; Buono, R.; Russo, A.; Dell’Oglio, P.; Castiglione, F.; La Croce, G.; Benigni, F.; Montorsi, F.; et al. Development of the First Model of Radical Prostatectomy in the Mouse: A Feasibility Study. Eur. Urol. 2018, 73, 482–484. [Google Scholar] [CrossRef]
- Greenberg, N.M.; DeMayo, F.; Finegold, M.J.; Medina, D.; Tilley, W.D.; Aspinall, J.O.; Cunha, G.R.; Donjacour, A.A.; Matusik, R.J.; Rosen, J.M. Prostate cancer in a transgenic mouse. Proc. Natl. Acad. Sci. USA 1995, 92, 3439–3443. [Google Scholar] [CrossRef] [Green Version]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.-Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef] [Green Version]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Linxweiler, J.; Junker, K. Extracellular vesicles in urological malignancies: An update. Nat. Rev. Urol. 2020, 17, 11–27. [Google Scholar] [CrossRef] [PubMed]




| Time after Tumor Cell Implantation | 6 Weeks | 8 Weeks | 10 Weeks |
|---|---|---|---|
| primary tumor resectable | yes | yes | No |
| presence of lymph node metastases | 2/3 mice | 3/3 mice | 3/3 mice |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linxweiler, J.; Hajili, T.; Zeuschner, P.; Menger, M.D.; Stöckle, M.; Junker, K.; Saar, M. Primary Tumor Resection Decelerates Disease Progression in an Orthotopic Mouse Model of Metastatic Prostate Cancer. Cancers 2022, 14, 737. https://doi.org/10.3390/cancers14030737
Linxweiler J, Hajili T, Zeuschner P, Menger MD, Stöckle M, Junker K, Saar M. Primary Tumor Resection Decelerates Disease Progression in an Orthotopic Mouse Model of Metastatic Prostate Cancer. Cancers. 2022; 14(3):737. https://doi.org/10.3390/cancers14030737
Chicago/Turabian StyleLinxweiler, Johannes, Turkan Hajili, Philip Zeuschner, Michael D. Menger, Michael Stöckle, Kerstin Junker, and Matthias Saar. 2022. "Primary Tumor Resection Decelerates Disease Progression in an Orthotopic Mouse Model of Metastatic Prostate Cancer" Cancers 14, no. 3: 737. https://doi.org/10.3390/cancers14030737
APA StyleLinxweiler, J., Hajili, T., Zeuschner, P., Menger, M. D., Stöckle, M., Junker, K., & Saar, M. (2022). Primary Tumor Resection Decelerates Disease Progression in an Orthotopic Mouse Model of Metastatic Prostate Cancer. Cancers, 14(3), 737. https://doi.org/10.3390/cancers14030737







