Sociocognitive Functioning and Psychosocial Burden in Patients with Brain Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
3. Results
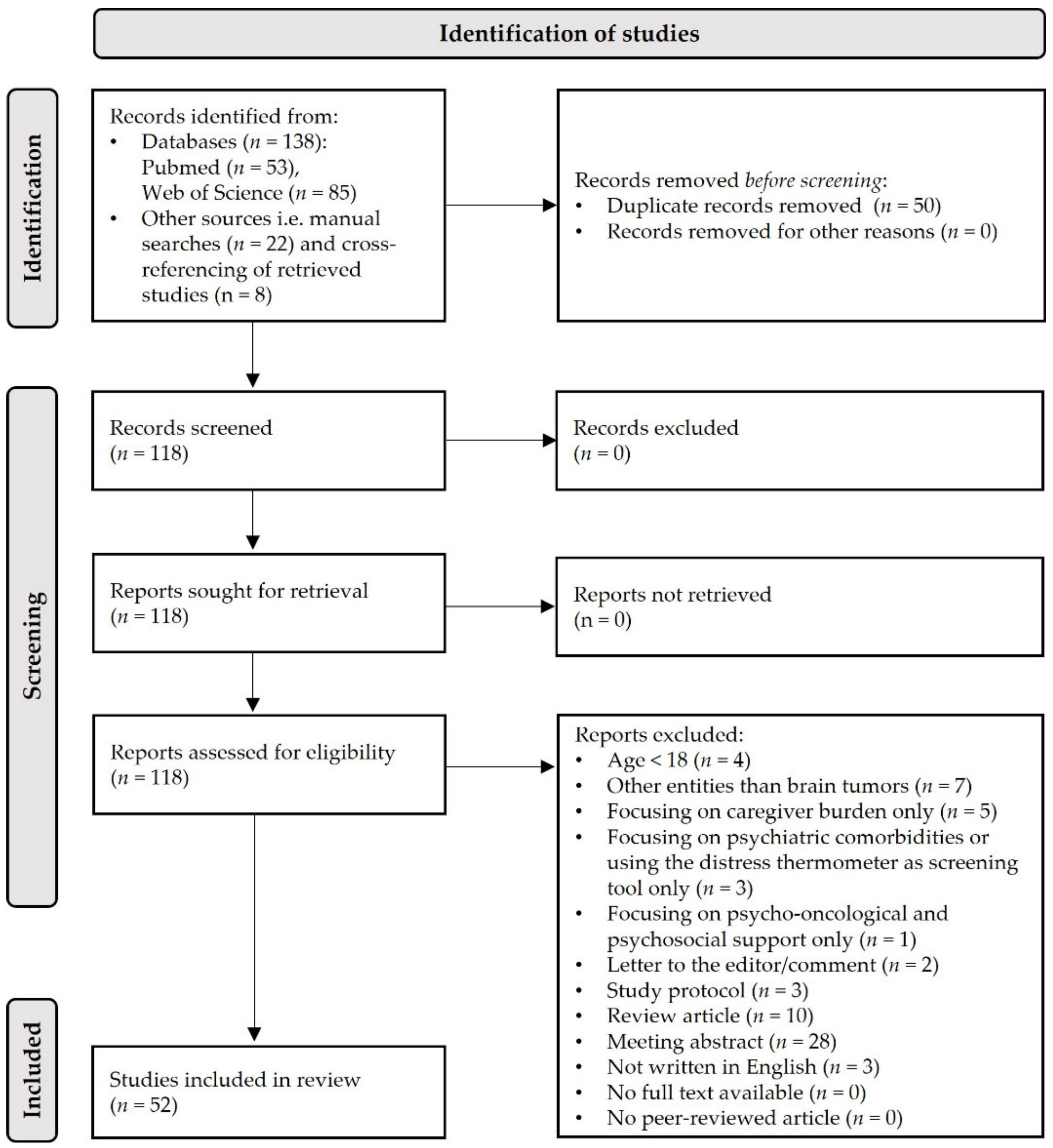
3.1. Genereral Search Results
3.2. Psychosocial Burden
3.3. Sociocognitive Functioning
4. Discussion
4.1. Summary of Main Findings
4.2. Clinical and Therapeutical Implications
4.3. Limitations of Current Research
4.4. Outlook and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lieberman, A.N.; Foo, S.H.; Ransohoff, J.; Wise, A.; George, A.; Gordon, W.; Walker, R. Long term survival among patients with malignant brain tumors. Neurosurgery 1982, 10, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Boele, F.W.; Douw, L.; Reijneveld, J.C.; Robben, R.; Klein, M. Health-Related Quality of Life in Stable, Long-Term Survivors of Low-Grade Glioma. J. Clin. Oncol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Beier, D.; Proescholdt, M.; Reinert, C.; Pietsch, T.; Jones, D.T.W.; Pfister, S.M.; Hattingen, E.; Seidel, C.; Dirven, L.; Luerding, R.; et al. Multicenter pilot study of radiochemotherapy as first-line treatment for adults with medulloblastoma (NOA-07). Neuro-Oncology 2018, 20, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Seidel, S.; Pels, H.; Schlömer, S.; Kowoll, A.; Fliessbach, K.; Engert, A.; Vogt-Schaden, M.; Egerer, G.; Reichmann, H.; Schackert, G.; et al. Twenty-year follow-up of a pilot/phase II trial on the Bonn protocol for primary CNS lymphoma. Neurology 2020, 95, e3138–e3144. [Google Scholar] [CrossRef]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.-D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA–09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar] [CrossRef]
- Olson, J.D.; Riedel, E.; DeAngelis, L.M. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology 2000, 54, 1442–1448. [Google Scholar] [CrossRef]
- Weller, J.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Hau, P.; Krex, D.; Grauer, O.; Goldbrunner, R.; Bähr, O.; et al. Health-related quality of life and neurocognitive functioning with lomustine–temozolomide versus temozolomide in patients with newly diagnosed, MGMT-methylated glioblastoma (CeTeG/NOA-09): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1444–1453. [Google Scholar] [CrossRef]
- Klein, M. Neurocognitive functioning in adult WHO grade II gliomas: Impact of old and new treatment modalities. Neuro-Oncology 2012, 14 (Suppl. 4), iv17–iv24. [Google Scholar] [CrossRef]
- Taphoorn, M.J.B.; Klein, M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004, 3, 159–168. [Google Scholar] [CrossRef]
- Fountain, D.M.; Allen, D.; Joannides, A.J.; Nandi, D.; Santarius, T.; Chari, A. Reporting of patient-reported health-related quality of life in adults with diffuse low-grade glioma: A systematic review. Neuro-Oncology 2016, 18, 1475–1486. [Google Scholar] [CrossRef] [Green Version]
- Butenschoen, V.M.; Kelm, A.; Meyer, B.; Krieg, S.M. Quality-adjusted life years in glioma patients: A systematic review on currently available data and the lack of evidence-based utilities. J. Neurooncol. 2019, 144, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Coomans, M.B.; Dirven, L.; Aaronson, N.K.; Baumert, B.G.; van den Bent, M.; Bottomley, A.; Brandes, A.A.; Chinot, O.; Coens, C.; Gorlia, T.; et al. Calculating the net clinical benefit in neuro-oncology clinical trials using two methods: Quality-adjusted survival effect sizes and joint modeling. Neurooncol. Adv. 2020, 2, vdaa147. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.C.; Shaw, E.G.; Pugh, S.L.; Chakravarti, A.; Gilbert, M.R.; Barger, G.R.; Coons, S.; Ricci, P.; Bullard, D.; Brown, P.D.; et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N. Engl. J. Med. 2016, 374, 1344–1355. [Google Scholar] [CrossRef]
- Juergens, A.; Pels, H.; Rogowski, S.; Fliessbach, K.; Glasmacher, A.; Engert, A.; Reiser, M.; Diehl, V.; Vogt-Schaden, M.; Egerer, G.; et al. Long-term survival with favorable cognitive outcome after chemotherapy in primary central nervous system lymphoma. Ann. Neurol. 2010, 67, 182–189. [Google Scholar] [CrossRef]
- Lawrie, T.A.; Gillespie, D.; Dowswell, T.; Evans, J.; Erridge, S.; Vale, L.; Kernohan, A.; Grant, R. Long-term neurocognitive and other side effects of radiotherapy, with or without chemotherapy, for glioma. Cochrane Database Syst. Rev. 2019, 8, CD013047. [Google Scholar] [CrossRef]
- Brown, P.D.; Buckner, J.C.; O’Fallon, J.R.; Iturria, N.L.; Brown, C.A.; O’Neill, B.P.; Scheithauer, B.W.; Dinapoli, R.P.; Arusell, R.M.; Curran, W.J.; et al. Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the folstein mini-mental state examination. J. Clin. Oncol. 2003, 21, 2519–2524. [Google Scholar] [CrossRef] [PubMed]
- Jalali, R.; Gupta, T.; Goda, J.S.; Goswami, S.; Shah, N.; Dutta, D.; Krishna, U.; Deodhar, J.; Menon, P.; Kannan, S.; et al. Efficacy of Stereotactic Conformal Radiotherapy vs Conventional Radiotherapy on Benign and Low-Grade Brain Tumors: A Randomized Clinical Trial. JAMA Oncol. 2017, 3, 1368–1376. [Google Scholar] [CrossRef] [Green Version]
- Kiebert, G.M.; Curran, D.; Aaronson, N.K.; Bolla, M.; Menten, J.; Rutten, E.H.; Nordman, E.; Silvestre, M.E.; Pierart, M.; Karim, A.B. Quality of life after radiation therapy of cerebral low-grade gliomas of the adult: Results of a randomised phase III trial on dose response (EORTC trial 22844). EORTC Radiotherapy Co-operative Group. Eur. J. Cancer 1998, 34, 1902–1909. [Google Scholar] [CrossRef]
- Klein, M.; Heimans, J.J.; Aaronson, N.K.; van der Ploeg, H.M.; Grit, J.; Muller, M.; Postma, T.J.; Mooij, J.J.; Boerman, R.H.; Beute, G.N.; et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: A comparative study. Lancet 2002, 360, 1361–1368. [Google Scholar] [CrossRef] [Green Version]
- Douw, L.; Klein, M.; Fagel, S.S.; van den Heuvel, J.; Taphoorn, M.J.B.; Aaronson, N.K.; Postma, T.J.; Vandertop, W.P.; Mooij, J.J.; Boerman, R.H.; et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: Long-term follow-up. Lancet Neurol. 2009, 8, 810–818. [Google Scholar] [CrossRef]
- Prabhu, R.S.; Won, M.; Shaw, E.G.; Hu, C.; Brachman, D.G.; Buckner, J.C.; Stelzer, K.J.; Barger, G.R.; Brown, P.D.; Gilbert, M.R.; et al. Effect of the addition of chemotherapy to radiotherapy on cognitive function in patients with low-grade glioma: Secondary analysis of RTOG 98-02. J. Clin. Oncol. 2014, 32, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Reijneveld, J.C.; Taphoorn, M.J.B.; Coens, C.; Bromberg, J.E.C.; Mason, W.P.; Hoang-Xuan, K.; Ryan, G.; Hassel, M.B.; Enting, R.H.; Brandes, A.A.; et al. Health-related quality of life in patients with high-risk low-grade glioma (EORTC 22033-26033): A randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016, 17, 1533–1542. [Google Scholar] [CrossRef]
- Vigliani, M.-C.; Sichez, N.; Poisson, M.; Delattre, J.-Y. A prospective study of cognitive functions following conventional radiotherapy for supratentorial gliomas in young adults: 4-year results. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 527–533. [Google Scholar] [CrossRef]
- Habets, E.J.J.; Taphoorn, M.J.B.; Nederend, S.; Klein, M.; Delgadillo, D.; Hoang-Xuan, K.; Bottomley, A.; Allgeier, A.; Seute, T.; Gijtenbeek, A.M.M.; et al. Health-related quality of life and cognitive functioning in long-term anaplastic oligodendroglioma and oligoastrocytoma survivors. J. Neurooncol. 2014, 116, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taphoorn, M.J.B.; van den Bent, M.J.; Mauer, M.E.L.; Coens, C.; Delattre, J.-Y.; Brandes, A.A.; Sillevis Smitt, P.A.E.; Bernsen, H.J.J.A.; Frénay, M.; Tijssen, C.C.; et al. Health-related quality of life in patients treated for anaplastic oligodendroglioma with adjuvant chemotherapy: Results of a European Organisation for Research and Treatment of Cancer randomized clinical trial. J. Clin. Oncol. 2007, 25, 5723–5730. [Google Scholar] [CrossRef]
- Wang, M.; Cairncross, G.; Shaw, E.; Jenkins, R.; Scheithauer, B.; Brachman, D.; Buckner, J.; Fink, K.; Souhami, L.; Laperriere, N.; et al. Cognition and quality of life after chemotherapy plus radiotherapy (RT) vs. RT for pure and mixed anaplastic oligodendrogliomas: Radiation therapy oncology group trial 9402. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 662–669. [Google Scholar] [CrossRef] [Green Version]
- Correa, D.D.; Shi, W.; Thaler, H.T.; Cheung, A.M.; DeAngelis, L.M.; Abrey, L.E. Longitudinal cognitive follow-up in low grade gliomas. J. Neurooncol. 2008, 86, 321–327. [Google Scholar] [CrossRef]
- Surma-aho, O.; Niemelä, M.; Vilkki, J.; Kouri, M.; Brander, A.; Salonen, O.; Paetau, A.; Kallio, M.; Pyykkönen, J.; Jääskeläinen, J. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology 2001, 56, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Hermann, B.P.; Mehta, M.P.; Tomé, W.A. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e487–e493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wefel, J.S.; Cloughesy, T.; Zazzali, J.L.; Zheng, M.; Prados, M.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.K.A.; et al. Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab. Neuro-Oncology 2011, 13, 660–668. [Google Scholar] [CrossRef]
- Armstrong, T.S.; Wefel, J.S.; Wang, M.; Gilbert, M.R.; Won, M.; Bottomley, A.; Mendoza, T.R.; Coens, C.; Werner-Wasik, M.; Brachman, D.G.; et al. Net clinical benefit analysis of radiation therapy oncology group 0525: A phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2013, 31, 4076–4084. [Google Scholar] [CrossRef] [Green Version]
- Wefel, J.S.; Kayl, A.E.; Meyers, C.A. Neuropsychological dysfunction associated with cancer and cancer therapies: A conceptual review of an emerging target. Br. J. Cancer 2004, 90, 1691–1696. [Google Scholar] [CrossRef] [Green Version]
- Correa, D.D.; DeAngelis, L.M.; Shi, W.; Thaler, H.; Glass, A.; Abrey, L.E. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology 2004, 62, 548–555. [Google Scholar] [CrossRef]
- Fliessbach, K.; Helmstaedter, C.; Urbach, H.; Althaus, A.; Pels, H.; Linnebank, M.; Juergens, A.; Glasmacher, A.; Schmidt-Wolf, I.G.; Klockgether, T.; et al. Neuropsychological outcome after chemotherapy for primary CNS lymphoma: A prospective study. Neurology 2005, 64, 1184–1188. [Google Scholar] [CrossRef]
- Fliessbach, K.; Urbach, H.; Helmstaedter, C.; Pels, H.; Glasmacher, A.; Kraus, J.A.; Klockgether, T.; Schmidt-Wolf, I.; Schlegel, U. Cognitive performance and magnetic resonance imaging findings after high-dose systemic and intraventricular chemotherapy for primary central nervous system lymphoma. Arch. Neurol. 2003, 60, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrey, L.E. The impact of chemotherapy on cognitive outcomes in adults with primary brain tumors. J. Neurooncol. 2012, 108, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Froklage, F.E.; Oosterbaan, L.J.; Sizoo, E.M.; de Groot, M.; Bosma, I.; Sanchez, E.; Douw, L.; Heimans, J.J.; Reijneveld, J.C.; Lagerwaard, F.J.; et al. Central neurotoxicity of standard treatment in patients with newly-diagnosed high-grade glioma: A prospective longitudinal study. J. Neurooncol. 2014, 116, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.J.; Mundt, A.J.; Sweeney, P.J.; Llanes-Macy, S.; Dunaway, L.; Castillo, M.; Macdonald, R.L. A longitudinal neuropsychological study of partial brain radiation in adults with brain tumors. Neurology 2003, 60, 1113–1118. [Google Scholar] [CrossRef]
- Laack, N.N.; Brown, P.D.; Ivnik, R.J.; Furth, A.F.; Ballman, K.V.; Hammack, J.E.; Arusell, R.M.; Shaw, E.G.; Buckner, J.C. Cognitive function after radiotherapy for supratentorial low-grade glioma: A North Central Cancer Treatment Group prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Talacchi, A.; Santini, B.; Savazzi, S.; Gerosa, M. Cognitive effects of tumour and surgical treatment in glioma patients. J. Neurooncol. 2011, 103, 541–549. [Google Scholar] [CrossRef]
- Tucha, O.; Smely, C.; Preier, M.; Lange, K.W. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery 2000, 47, 324–333, discussion 333-4. [Google Scholar] [CrossRef]
- Lageman, S.K.; Cerhan, J.H.; Locke, D.E.C.; Anderson, S.K.; Wu, W.; Brown, P.D. Comparing neuropsychological tasks to optimize brief cognitive batteries for brain tumor clinical trials. J. Neurooncol. 2010, 96, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.A.; Dunn, R.H.; Logue, P.E.; King, J.H.; Edwards, C.L.; Halperin, E.C. Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 992–999. [Google Scholar] [CrossRef]
- Bosma, I.; Douw, L.; Bartolomei, F.; Heimans, J.J.; van Dijk, B.W.; Postma, T.J.; Stam, C.J.; Reijneveld, J.C.; Klein, M. Synchronized brain activity and neurocognitive function in patients with low-grade glioma: A magnetoencephalography study. Neuro-Oncology 2008, 10, 734–744. [Google Scholar] [CrossRef]
- Ek, L.; Almkvist, O.; Wiberg, M.K.; Stragliotto, G.; Smits, A. Early cognitive impairment in a subset of patients with presumed low-grade glioma. Neurocase 2010, 16, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Krupp, W.; Klein, C.; Koschny, R.; Holland, H.; Seifert, V.; Meixensberger, J. Assessment of neuropsychological parameters and quality of life to evaluate outcome in patients with surgically treated supratentorial meningiomas. Neurosurgery 2009, 64. [Google Scholar] [CrossRef]
- Ownsworth, T. Coping with the Unthinkable: Psychosocial Advances in the Management of Primary Brain Tumour. Brain Impair. 2016, 17, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Loughan, A.R.; Lanoye, A.; Aslanzadeh, F.J.; Villanueva, A.A.L.; Boutte, R.; Husain, M.; Braun, S. Fear of Cancer Recurrence and Death Anxiety: Unaddressed Concerns for Adult Neuro-oncology Patients. J. Clin. Psychol. Med. Settings 2021, 28, 16–30. [Google Scholar] [CrossRef]
- Carlson, L.E.; Angen, M.; Cullum, J.; Goodey, E.; Koopmans, J.; Lamont, L.; MacRae, J.H.; Martin, M.; Pelletier, G.; Robinson, J.; et al. High levels of untreated distress and fatigue in cancer patients. Br. J. Cancer 2004, 90, 2297–2304. [Google Scholar] [CrossRef]
- Keir, S.T.; Calhoun-Eagan, R.D.; Swartz, J.J.; Saleh, O.A.; Friedman, H.S. Screening for distress in patients with brain cancer using the NCCN’s rapid screening measure. Psychooncology 2008, 17, 621–625. [Google Scholar] [CrossRef]
- Riba, M.B.; Donovan, K.A.; Andersen, B.; Braun, I.; Breitbart, W.S.; Brewer, B.W.; Buchmann, L.O.; Clark, M.M.; Collins, M.; Corbett, C.; et al. Distress Management, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 1229–1249. [Google Scholar] [CrossRef]
- Randazzo, D.M.; McSherry, F.; Herndon, J.E.; Affronti, M.L.; Lipp, E.S.; Flahiff, C.; Miller, E.; Woodring, S.; Freeman, M.; Healy, P.; et al. A cross sectional analysis from a single institution’s experience of psychosocial distress and health-related quality of life in the primary brain tumor population. J. Neurooncol. 2017, 134, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Ownsworth, T.; Henderson, L.; Chambers, S.K. Social support buffers the impact of functional impairments on caregiver psychological well-being in the context of brain tumor and other cancers. Psychooncology 2010, 19, 1116–1122. [Google Scholar] [CrossRef] [Green Version]
- Ozbay, F.; Johnson, D.C.; Dimoulas, E.; Morgan, C.A.; Charney, D.; Southwick, S. Social Support and Resilience to Stress: From Neurobiology to Clinical Practice. Psychiatry 2007, 4, 35–40. [Google Scholar]
- Payne, S.; Jarrett, N.; Jeffs, D.; Brown, L. Implications of social isolation during cancer treatment. The implications of residence away from home during cancer treatment on patients’ experiences: A comparative study. Health Place 2001, 7, 273–282. [Google Scholar] [CrossRef]
- Cobb, S. Social support as a moderator of life stress. Psychosom. Med. 1976, 38, 300–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- House, J.S.; Landis, K.R.; Umberson, D. Social relationships and health. Science 1988, 241. [Google Scholar] [CrossRef]
- Berkman, L.F.; Syme, S.L. Social networks, host resistance, and mortality: A nine-year follow-up study of Alameda County residents. Am. J. Epidemiol. 1979, 109. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, S.; Genova, H. The Effect of Severe Traumatic Brain Injury on Social Cognition, Emotion Regulation, and Mood; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Kendall, E. Psychosocial Adjustment Following Closed Head Injury: A Model for Understanding Individual Differences and Predicting Outcome. Neuropsychol. Rehabil. 1996, 6, 101–132. [Google Scholar] [CrossRef]
- Cassel, A.; Mcdonald, S.; Kelly, M.; Togher, L. Learning from the minds of others: A review of social cognition treatments and their relevance to traumatic brain injury. Neuropsychol. Rehabil. 2016, 29, 22–55. [Google Scholar] [CrossRef]
- Keyes, C.L.M. Social Well-Being. Soc. Psychol. Q. 1998, 61, 121. [Google Scholar] [CrossRef]
- Adolphs, R. The neurobiology of social cognition. Curr. Opin. Neurobiol. 2001, 11. [Google Scholar] [CrossRef]
- Frith, C.D. Social cognition. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2008, 363, 2033–2039. [Google Scholar] [CrossRef] [Green Version]
- Adolphs, R. Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 2002, 12, 169–177. [Google Scholar] [CrossRef]
- Decety, J.; Lamm, C. Human empathy through the lens of social neuroscience. Sci. World J. 2006, 6, 1146–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamay-Tsoory, S.G.; Aharon-Peretz, J.; Perry, D. Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 2009, 132, 617–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvash, J.; Shamay-Tsoory, S.G. Theory of Mind and Empathy as Multidimensional Constructs. Top. Lang. Disord. 2014, 34, 282–295. [Google Scholar] [CrossRef] [Green Version]
- Shamay-Tsoory, S.G.; Aharon-Peretz, J. Dissociable prefrontal networks for cognitive and affective theory of mind: A lesion study. Neuropsychologia 2007, 45, 3054–3067. [Google Scholar] [CrossRef]
- Chang, E.C.; D’Zurilla, T.J.; Sanna, L.J. Social Problem Solving: Theory, Research, and Training; American Psychological Association: Washington, DC, USA, 2004; ISBN 1-59147-147-8. [Google Scholar]
- D’Zurilla, T.J.; Nezu, A.M. Development and preliminary evaluation of the Social Problem-Solving Inventory. Psychol. Assess. 1990, 2, 156–163. [Google Scholar] [CrossRef]
- Park, H.K.; Chun, S.Y.; Choi, Y.; Lee, S.Y.; Kim, S.J.; Park, E.-C. Effects of social activity on health-related quality of life according to age and gender: An observational study. Health Qual. Life Outcomes 2015, 13, 181. [Google Scholar] [CrossRef] [Green Version]
- Hawkley, L.C.; Cacioppo, J.T. Loneliness Matters: A Theoretical and Empirical Review of Consequences and Mechanisms. Ann. Behav. Med. 2010, 40, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertz, M.; Okoniewski, A.; Schlegel, U.; Thoma, P. Impairment of sociocognitive functions in patients with brain tumours. Neurosci. Biobehav. Rev. 2020, 108, 370–392. [Google Scholar] [CrossRef]
- Pertz, M.; Popkirov, S.; Schlegel, U.; Thoma, P. Research on cognitive and sociocognitive functions in patients with brain tumours: A bibliometric analysis and visualization of the scientific landscape. Neurol. Sci. 2020, 41, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Kinoshita, M.; Nakada, M.; Herbet, G. Social Cognition; Springer International Publishing: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Randazzo, D.; Peters, K.B. Psychosocial distress and its effects on the health-related quality of life of primary brain tumor patients. CNS Oncol. 2016, 5, 241–249. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Andrewes, D.G.; Kaye, A.; Murphy, M.; Harris, B.; Aitken, S.; Parr, C.; Bates, L. Emotional and social dysfunction in patients following surgical treatment for brain tumour. J. Clin. Neurosci. 2003, 10, 428–433. [Google Scholar] [CrossRef]
- Baird, A.; Dewar, B.-K.; Critchley, H.; Dolan, R.; Shallice, T.; Cipolotti, L. Social and emotional functions in three patients with medial frontal lobe damage including the anterior cingulate cortex. Cogn. Neuropsychiatry 2006, 11, 369–388. [Google Scholar] [CrossRef] [Green Version]
- Baird, A.D.; Walker, D.G.; Biggs, V.; Robinson, G.A. Selective preservation of the beat in apperceptive music agnosia: A case study. Cortex 2014, 53, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Bowers, D.; Heilman, K.M. Dissociation between the processing of affective and nonaffective faces: A case study. J. Clin. Neuropsychol. 1984, 6, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Bunston, T.; Mings, D.; Laperriere, N.; Malcolm, J.; Williams, D. The impact of psychosocial need and needs resolution on quality of life in patients with brain tumors. FOC 1998, 4, E9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campanella, F.; Shallice, T.; Ius, T.; Fabbro, F.; Skrap, M. Impact of brain tumour location on emotion and personality: A voxel-based lesion–symptom mapping study on mentalization processes. Brain 2014, 137, 2532–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campanella, F.; Fabbro, F.; Ius, T.; Shallice, T.; Skrap, M. Acute effects of surgery on emotion and personality of brain tumor patients: Surgery impact, histological aspects, and recovery. Neuro-Oncology 2015, 17, 1121–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavers, D.; Hacking, B.; Erridge, S.E.; Kendall, M.; Morris, P.G.; Murray, S.A. Social, psychological and existential well-being in patients with glioma and their caregivers: A qualitative study. CMAJ 2012, 184, E373–E382. [Google Scholar] [CrossRef] [Green Version]
- Channon, S.; Rule, A.; Maudgil, D.; Martinos, M.; Pellijeff, A.; Frankl, J.; Drury, H.; Shieff, C. Interpretation of mentalistic actions and sarcastic remarks: Effects of frontal and posterior lesions on mentalising. Neuropsychologia 2007, 45, 1725–1734. [Google Scholar] [CrossRef]
- Chen, P.; Wang, G.; Ma, R.; Jing, F.; Zhang, Y.; Wang, Y.; Zhang, P.; Niu, C.; Zhang, X. Multidimensional assessment of empathic abilities in patients with insular glioma. Cogn. Affect. Behav. Neurosci. 2016, 16, 962–975. [Google Scholar] [CrossRef] [Green Version]
- Cornwell, P.; Dicks, B.; Fleming, J.; Haines, T.P.; Olson, S. Care and support needs of patients and carers early post-discharge following treatment for non-malignant brain tumour: Establishing a new reality. Support. Care Cancer 2012, 20, 2595–2610. [Google Scholar] [CrossRef] [PubMed]
- Cubis, L.; Ownsworth, T.; Pinkham, M.B.; Foote, M.; Legg, M.; Chambers, S. The importance of staying connected: Mediating and moderating effects of social group memberships on psychological well-being after brain tumor. Psychooncology 2019, 28, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Giussani, C.; Pirillo, D.; Roux, F.-E. Mirror of the soul: A cortical stimulation study on recognition of facial emotions. J. Neurosurg. 2010, 112, 520–527. [Google Scholar] [CrossRef]
- Goebel, S.; von Harscher, M.; Mehdorn, H.M. Comorbid mental disorders and psychosocial distress in patients with brain tumours and their spouses in the early treatment phase. Support. Care Cancer 2011, 19, 1797–1805. [Google Scholar] [CrossRef]
- Goebel, S.; Stark, A.M.; Kaup, L.; von Harscher, M.; Mehdorn, H.M. Distress in patients with newly diagnosed brain tumours. Psychooncology 2011, 20, 623–630. [Google Scholar] [CrossRef]
- Goebel, S.; Mehdorn, H.M.; Wiesner, C.D. Social cognition in patients with intracranial tumors: Do we forget something in the routine neuropsychological examination? J. Neurooncol. 2018, 140, 687–696. [Google Scholar] [CrossRef]
- Gu, X.; Gao, Z.; Wang, X.; Liu, X.; Knight, R.T.; Hof, P.R.; Fan, J. Anterior insular cortex is necessary for empathetic pain perception. Brain 2012, 135, 2726–2735. [Google Scholar] [CrossRef] [Green Version]
- Guha-Thakurta, N.; Damek, D.; Pollack, C.; Hochberg, F.H. Intravenous methotrexate as initial treatment for primary central nervous system lymphoma: Response to therapy and quality of life of patients. J. Neurooncol. 1999, 43, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Herbet, G.; Lafargue, G.; Bonnetblanc, F.; Moritz-Gasser, S.; Duffau, H. Is the right frontal cortex really crucial in the mentalizing network? A longitudinal study in patients with a slow-growing lesion. Cortex 2013, 49, 2711–2727. [Google Scholar] [CrossRef] [PubMed]
- Herbet, G.; Lafargue, G.; Bonnetblanc, F.; Moritz-Gasser, S.; Menjot de Champfleur, N.; Duffau, H. Inferring a dual-stream model of mentalizing from associative white matter fibres disconnection. Brain 2014, 137, 944–959. [Google Scholar] [CrossRef] [PubMed]
- Herbet, G.; Lafargue, G.; Moritz-Gasser, S.; Menjot de Champfleur, N.; Costi, E.; Bonnetblanc, F.; Duffau, H. A disconnection account of subjective empathy impairments in diffuse low-grade glioma patients. Neuropsychologia 2015, 70, 165–176. [Google Scholar] [CrossRef]
- Herbet, G.; Lafargue, G.; Moritz-Gasser, S.; Bonnetblanc, F.; Duffau, H. Interfering with the neural activity of mirror-related frontal areas impairs mentalistic inferences. Brain Struct. Funct. 2015, 220, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, L.M.; Andrewes, D.G.; Nicholas, C.L.; Drummond, K.J.; Moffat, B.A.; Phal, P.; Desmond, P.; Kessels, R.P.C. Social cognition in patients following surgery to the prefrontal cortex. Psychiatry Res. Neuroimaging 2014, 224, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Kangas, M.; Williams, J.R.; Smee, R.I. Benefit Finding in Adults Treated for Benign Meningioma Brain Tumours: Relations with Psychosocial Wellbeing. Brain Impair. 2011, 12, 105–116. [Google Scholar] [CrossRef]
- Kangas, M.; Tate, R.L.; Williams, J.R.; Smee, R.I. The effects of radiotherapy on psychosocial and cognitive functioning in adults with a primary brain tumor: A prospective evaluation. Neuro-Oncology 2012, 14, 1485–1502. [Google Scholar] [CrossRef] [PubMed]
- Kanter, C.; D’Agostino, N.M.; Daniels, M.; Stone, A.; Edelstein, K. Together and apart: Providing psychosocial support for patients and families living with brain tumors. Support. Care Cancer 2014, 22, 43–52. [Google Scholar] [CrossRef]
- Langbecker, D.; Yates, P. Primary brain tumor patients’ supportive care needs and multidisciplinary rehabilitation, community and psychosocial support services: Awareness, referral and utilization. J. Neurooncol. 2016, 127, 91–102. [Google Scholar] [CrossRef]
- Lucas, M.R. Psychosocial Implications for the Patient With a High-Grade Glioma. J. Neurosci. Nurs. 2010, 42, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Luherne-du Boullay, V.; Plaza, M.; Perrault, A.; Capelle, L.; Chaby, L. Atypical crossmodal emotional integration in patients with gliomas. Brain Cogn. 2014, 92, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Mattavelli, G.; Pisoni, A.; Casarotti, A.; Comi, A.; Sera, G.; Riva, M.; Bizzi, A.; Rossi, M.; Bello, L.; Papagno, C. Consequences of brain tumour resection on emotion recognition. J. Neuropsychol. 2017, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.-G.; Huang, L.-J.; Li, S.-Y.; Ke, C.; Chen, Y.; Jin, Y.; Chen, Z.-P. Working memory and the identification of facial expression in patients with left frontal glioma. Neuro-Oncology 2012, 14, iv81–iv89. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, R.; Kinoshita, M.; Okita, H.; Yahata, T.; Matsui, M.; Nakada, M. Neural Networks Mediating High-Level Mentalizing in Patients with Right Cerebral Hemispheric Gliomas. Front. Behav. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, R.; Yordanova, Y.N.; Duffau, H.; Herbet, G. Neuropsychological evidence for the crucial role of the right arcuate fasciculus in the face-based mentalizing network: A disconnection analysis. Neuropsychologia 2018, 115, 179–187. [Google Scholar] [CrossRef]
- Nakajima, R.; Kinoshita, M.; Okita, H.; Liu, Z.; Nakada, M. Preserving Right Pre-motor and Posterior Prefrontal Cortices Contribute to Maintaining Overall Basic Emotion. Front. Hum. Neurosci. 2021, 15, 97. [Google Scholar] [CrossRef]
- Ownsworth, T.; Chambers, S.; Hawkes, A.; Walker, D.G.; Shum, D. Making sense of brain tumour: A qualitative investigation of personal and social processes of adjustment. Neuropsychol. Rehabil. 2011, 21, 117–137. [Google Scholar] [CrossRef]
- Ownsworth, T.; Chambers, S.; Damborg, E.; Casey, L.; Walker, D.G.; Shum, D.H.K. Evaluation of the making sense of brain tumor program: A randomized controlled trial of a home-based psychosocial intervention. Psycho-Oncology 2015, 24, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Papagno, C.; Pisoni, A.; Mattavelli, G.; Casarotti, A.; Comi, A.; Fumagalli, F.; Vernice, M.; Fava, E.; Riva, M.; Bello, L. Specific disgust processing in the left insula: New evidence from direct electrical stimulation. Neuropsychologia 2016, 84, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Peper, M.; Irle, E. Categorical and Dimensional Decoding of Emotional Intonations in Patients with Focal Brain Lesions. Brain Lang. 1997, 58, 233–264. [Google Scholar] [CrossRef] [PubMed]
- Peper, M.; Irle, E. The Decoding of Emotional Concepts in Patients with Focal Cerebral Lesions. Brain Cogn. 1997, 34, 360–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertz, M.; Kowalski, T.; Thoma, P.; Schlegel, U. What Is on Your Mind?: Impaired Social Cognition in Primary Central Nervous System Lymphoma Patients Despite Ongoing Complete Remission. Cancers 2021, 13, 943. [Google Scholar] [CrossRef]
- Prat-Acín, R.; Galeano-Senabre, I.; López-Ruiz, P.; Ayuso-Sacido, A.; Espert-Tortajada, R. Intraoperative brain mapping of language, cognitive functions, and social cognition in awake surgery of low-grade gliomas located in the right non-dominant hemisphere. Clin. Neurol. Neurosurg. 2021, 200, 106363. [Google Scholar] [CrossRef]
- Saver, J.L.; Damasio, A.R. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia 1991, 29, 1241–1249. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kizilbash, S.H.; Robinson, S.I.; Uhm, J.H.; Hammack, J.E.; Lachance, D.H.; Buckner, J.C.; Jatoi, A. Seizures in patients with primary brain tumors: What is their psychosocial impact? J. Neurooncol. 2016, 128, 285–291. [Google Scholar] [CrossRef]
- Sinha, R.; Dijkshoorn, A.B.C.; Li, C.; Manly, T.; Price, S.J. Glioblastoma surgery related emotion recognition deficits are associated with right cerebral hemisphere tract changes. Brain Commun. 2020. [Google Scholar] [CrossRef]
- Szelag, E.; Fersten, E. Recognition of faces expressing emotions in patients with unilateral brain damage. Acta Neurobiol. Exp. 1991, 51, 115–123. [Google Scholar]
- Trejnowska, A.; Goodall, K.; Rush, R.; Ellison, M.; McVittie, C. The relationship between adult attachment and coping with brain tumour: The mediating role of social support. Psycho-Oncology 2020, 29, 729–736. [Google Scholar] [CrossRef]
- Troschel, F.M.; Ahndorf, F.; Wille, L.-M.; Brandt, R.; Jost, J.; Rekowski, S.; Eich, H.T.; Stummer, W.; Wiewrodt, R.; Jetschke, K.; et al. Quality of Life in Brain Tumor Patients and Their Relatives Heavily Depends on Social Support Factors during the COVID-19 Pandemic. Cancers 2021, 13, 1276. [Google Scholar] [CrossRef]
- Voß, H.; Scholz-Kreisel, P.; Richter, C.; Ringel, F.; Singer, S.; Renovanz, M. Development of screening questions for doctor–patient consultation assessing the quality of life and psychosocial burden of glioma patients: An explorative study. Qual. Life Res. 2021, 30, 1513–1522. [Google Scholar] [CrossRef]
- Wang, X.; Gu, X.; Fan, J.; Wang, S.; Zhao, F.; Hof, P.R.; Liu, P.; Gao, Z. Recovery of empathetic function following resection of insular gliomas. J. Neurooncol. 2014, 117, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Weitzner, M.A.; Meyers, C.A.; Byrne, K. Psychosocial functioning and quality of life in patients with primary brain tumors. J. Neurosurg. 1996, 84, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, Y.N.; Duffau, H.; Herbet, G. Neural pathways subserving face-based mentalizing. Brain Struct. Funct. 2017, 222, 3087–3105. [Google Scholar] [CrossRef]
- Yuksek, E.; Eroz, S.; Yassa, A.; Akturk, D.; Zakirov, F.; Akcam, F.E.; Emul, M. The Influences of Whole Brain Radiotherapy on Social Cognition and Association with Hippocampal and Frontal Dosimetry. Psychiatr. Q. 2015, 86, 533–543. [Google Scholar] [CrossRef]
- The cooperative human. Nat. Hum. Behav. 2018, 2, 427–428. [CrossRef] [PubMed]
- Arnold, S.D.; Forman, L.M.; Brigidi, B.D.; Carter, K.E.; Schweitzer, H.A.; Quinn, H.E.; Guill, A.B.; Herndon, J.E.; Raynor, R.H. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro-Oncology 2008, 10, 171–181. [Google Scholar] [CrossRef]
- Manne, S.; Badr, H. Intimacy and relationship processes in couples’ psychosocial adaptation to cancer. Cancer 2008, 112, 2541–2555. [Google Scholar] [CrossRef] [Green Version]
- Doolittle, N.D.; Korfel, A.; Lubow, M.A.; Schorb, E.; Schlegel, U.; Rogowski, S.; Fu, R.; Dósa, E.; Illerhaus, G.; Kraemer, D.F.; et al. Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology 2013, 81, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Day, J.; Gillespie, D.C.; Rooney, A.G.; Bulbeck, H.J.; Zienius, K.; Boele, F.; Grant, R. Neurocognitive Deficits and Neurocognitive Rehabilitation in Adult Brain Tumors. Curr. Treat. Options Neurol. 2016, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Noll, K.R.; Bradshaw, M.E.; Weinberg, J.S.; Wefel, J.S. Relationships between neurocognitive functioning, mood, and quality of life in patients with temporal lobe glioma. Psychooncology 2017, 26, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litofsky, N.S.; Farace, E.; Anderson, F.; Meyers, C.A.; Huang, W.; Laws, E.R. Depression in patients with high-grade glioma: Results of the Glioma Outcomes Project. Neurosurgery 2004, 54, 358–366, discussion 366–367. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.D.; von Hippel, W.; Molenberghs, P.; Lee, T.; Sachdev, P.S. Clinical assessment of social cognitive function in neurological disorders. Nat. Rev. Neurol. 2016, 12, 28–39. [Google Scholar] [CrossRef]
- Burgess, P.; Alderman, N.; Forbes, C.; Costello, A.; Coates, L.M.; Dawson, D.; Anderson, N.; Gilbert, S.; Dumontheil, I.; Channon, S. The case for the development and use of “ecologically valid” measures of executive function in experimental and clinical neuropsychology. J. Int. Neuropsychol. Soc. 2006, 12, 194–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njomboro, P. Social Cognition Deficits: Current Position and Future Directions for Neuropsychological Interventions in Cerebrovascular Disease. Behav. Neurol. 2017, 1–11. [Google Scholar] [CrossRef]
- Giovagnoli, A.R. Investigation of cognitive impairments in people with brain tumors. J. Neurooncol. 2012, 108, 277–283. [Google Scholar] [CrossRef]
- Wallis, K.; Kelly, M.; McRae, S.E.; Mcdonald, S.; Campbell, L.E. Domains and measures of social cognition in acquired brain injury: A scoping review. Neuropsychol. Rehabil. 2021, 34, 1–35. [Google Scholar] [CrossRef]
- Shamay-Tsoory, S.G. The Neural Bases for Empathy. Neuroscientist 2011, 17, 18–24. [Google Scholar] [CrossRef]
- Channon, S. Frontal lobe dysfunction and everyday problem-solving: Social and non-social contributions. Acta Psychol. 2004, 115, 235–254. [Google Scholar] [CrossRef]
- Rowe, A.D.; Bullock, P.R.; Polkey, C.E.; Morris, R.G. ‘Theory of mind’ impairments and their relationship to executive functioning following frontal lobe excisions. Brain 2001, 124, 600–616. [Google Scholar] [CrossRef] [PubMed]
- Fanning, J.R.; Bell, M.D.; Fiszdon, J.M. Is it possible to have impaired neurocognition but good social cognition in schizophrenia? Schizophr. Res. 2012, 135, 68–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagravinese, G.; Avanzino, L.; Raffo De Ferrari, A.; Marchese, R.; Serrati, C.; Mandich, P.; Abbruzzese, G.; Pelosin, E. Theory of Mind Is Impaired in Mild to Moderate Huntington’s Disease Independently from Global Cognitive Functioning. Front. Psychol. 2017, 8, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyers, C.A.; Wefel, J.S. The Use of the Mini-Mental State Examination to Assess Cognitive Functioning in Cancer Trials: No Ifs, Ands, Buts, or Sensitivity. J. Clin. Oncol. 2003, 21, 3557–3558. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, C.H.; Kubzansky, L.D.; Schernhammer, E.S.; Holmes, M.D.; Kawachi, I. Social networks, social support, and survival after breast cancer diagnosis. J. Clin. Oncol. 2006, 24. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; de Geest, K.; Bender, D.; Ahmed, A.; Goodheart, M.J.; Dahmoush, L.; Zimmerman, M.B.; Penedo, F.J.; Lucci, J.A.; Ganjei-Azar, P.; et al. Social influences on clinical outcomes of patients with ovarian cancer. J. Clin. Oncol. 2012, 30, 2885–2890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, S.; Lantz, C. The Brain Tumor Experience and Quality of Life: A Qualitative Study. J. Neurosci. Nurs. 1998, 30, 245–252. [Google Scholar] [CrossRef]
- Janda, M.; Eakin, E.G.; Bailey, L.; Walker, D.; Troy, K. Supportive care needs of people with brain tumours and their carers. Support. Care Cancer 2006, 14, 1094–1103. [Google Scholar] [CrossRef]
- Courtens, A.M.; Stevens, F.C.J.; Crebolder, H.F.J.M.; Philipsen, H. Longitudinal study on quality of life and social support in cancer patients. Cancer Nurs. 1996, 19, 162–169. [Google Scholar] [CrossRef]
- McConigley, R.; Halkett, G.; Lobb, E.; Nowak, A. Caring for someone with high-grade glioma: A time of rapid change for caregivers. Palliat. Med. 2010, 24, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Schmer, C.; Ward-Smith, P.; Latham, S.; Salacz, M. When a family member has a malignant brain tumor: The caregiver perspective. J. Neurosci. Nurs. 2008, 40, 78–84. [Google Scholar] [CrossRef]
- Schubart, J.R.; Kinzie, M.B.; Farace, E. Caring for the brain tumor patient: Family caregiver burden and unmet needs. Neuro-Oncology 2008, 10, 61–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiszdon, J.M.; Reddy, L.F. Review of social cognitive treatments for psychosis. Clin. Psychol. Rev. 2012, 32, 724–740. [Google Scholar] [CrossRef] [PubMed]
- Vallat-Azouvi, C.; Azouvi, P.; Le-Bornec, G.; Brunet-Gouet, E. Treatment of social cognition impairments in patients with traumatic brain injury: A critical review. Brain Inj. 2018, 33, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Cassel, A.; McDonald, S.; Kelly, M. Establishing ‘proof of concept’ for a social cognition group treatment program (SIFT IT) after traumatic brain injury: Two case studies. Brain Inj. 2020, 34, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, M.M.; Richardson, C.L. Social Cognitive Training for Schizophrenia: A Meta-Analytic Investigation of Controlled Research. Schizophr. Bull. 2012, 38, 1092–1104. [Google Scholar] [CrossRef] [Green Version]
- Combs, D.R.; Adams, S.D.; Penn, D.L.; Roberts, D.; Tiegreen, J.; Stem, P. Social Cognition and Interaction Training (SCIT) for inpatients with schizophrenia spectrum disorders: Preliminary findings. Schizophr. Res. 2007, 91, 112–116. [Google Scholar] [CrossRef]
- Bornhofen, C.; Mcdonald, S. Treating deficits in emotion perception following traumatic brain injury. Neuropsychol. Rehabil. 2008, 18, 22–44. [Google Scholar] [CrossRef]
- Bucher, J.A.; Loscalzo, M.; Zabora, J.; Houts, P.S.; BrintzenhofeSzoc, K. Problem-solving cancer care education for patients and caregivers. Cancer Pract. 2001, 9, 66–70. [Google Scholar] [CrossRef]
- Dinapoli, L.; Chiesa, S.; Dinapoli, N.; Gatta, R.; Beghella Bartoli, F.; Bracci, S.; Mazzarella, C.; Sanfilippo, M.Z.; Sabatino, G.; Gaudino, S.; et al. Personalised support of brain tumour patients during radiotherapy based on psychological profile and quality of life. Support. Care Cancer 2021, 29, 4555–4563. [Google Scholar] [CrossRef] [PubMed]
- Penton-Voak, I.S.; Munafò, M.R.; Looi, C.Y. Biased Facial-Emotion Perception in Mental Health Disorders: A Possible Target for Psychological Intervention? Curr. Dir. Psychol. Sci. 2017, 26, 294–301. [Google Scholar] [CrossRef] [Green Version]

| Authors | Topic | Design | Main Instrument of Sociocognitive Functioning or Psychosocial Burden |
|---|---|---|---|
| Andrewes et al. (2003) [79] | psychosocial burden | crosssectional | emotional and social dysfunction questionnaire |
| Baird et al. (2006) [80] | social cognition | crosssectional | facial emotional expression multimorph task: recognition of a neutral face gradually morphed through twenty 5% increment stages into 1 of 6 prototypical expressions (happiness, sadness, anger, disgust, fear and surprise); social situations task: judge the appropriateness of behaviors in short stories of social situations (normative versus violation); joke interpretation: state whether the scenario was amusing and why (correct answers referred directly to the thoughts, feelings and dispositions of the characters); advanced ToM: interpret and justify the behavior of the main protagonist in stories of naturalistic social situations |
| Baird et al. (2014) [81] | social cognition | case study | emotions portrayed by music excerpts (happy, peaceful, sad and scary); the Awareness of Social Inferences Test (happy, surprised, neutral, sad, angry, anxious) |
| Bowers & Heilman (1984) [82] | social cognition | case study | Neutral Facial Discrimination Task: state whether 2 faces (unfamiliar with neutral facial expression) were the same or a different person; Name the Facial Emotion Task: name the facial emotion depicted in a photograph with 1 of 4 facial emotions (happiness, sadness, anger or indifference); Choose the Facial Emotion Task: point to the face that depicted a target emotion named by the examiner (i.e., point to the sad face); Same-Different Facial Emotion Task: indicated whether the emotion portrayed by 2 same faces was the same or different; Affective Prosody Task: identify the affective intonation of a sentence (semantically neutral sentences recorded in 4 different affective intonations: happy, sad, angry, indifferent) |
| Bunston et al. (1998) [83] | psychosocial burden | crosssectional | Coping in stressful Situations Scale to measure 3 major coping styles: task-oriented, emotion-oriented and avoidance coping; FACT-Brain (subscales: physical, functional, social/family, emotional well-being, relationship with doctor, total score); Fatigue Severity Scale; The Life Event Survey to measure life event stress by assessing both the extent of desirability and personal impact; The Princess Margaret Hospital Needs Assessment Inventory to identify 58 specific needs grouped into 12 domains of need |
| Campanella et al. (2014) [84] | social cognition | prospective | Emotion recognition (Ekman Faces) word-to-picture matching task: 6 faces of the same person expressing 6 basic emotions (happiness, sadness, anger, surprise, fear and disgust); RMET; Toronto Alexithymia Scale; Temperament and Character Inventory |
| Campanella et al. (2015) [85] | social cognition | prospective | Emotion recognition (Ekman Faces) word-to-picture matching task: 6 faces of the same person expressing 6 basic emotions (happiness, sadness, anger, surprise, fear and disgust); RMET; Toronto Alexithymia Scale; Temperament and Character Inventory |
| Cavers et al. (2012) [86] | psychosocial burden | prospective | qualitative longitudinal multiperspective technique; interviews conducted over a period of 2 years to explore the experiences of patients and caregivers |
| Channon et al. (2007) [87] | social cognition | crosssectional | pragmatic comprehension task: social context with 4 different types of endings (control physical event, human action, direct sarcastic remark, indirect sarcastic remark), generation of appropriate interpretations of the final remark; selection of best interpretation among alternatives |
| Chen et al. (2016) [88] | social cognition | crosssectional | IRI; forced-choice facial Emotion Recognition Task with 5 basic emotions and neutral; perception of others’ pain task; emotional perspective taking; Toronto Alexithymia Scale |
| Cornwell et al. (2012) [89] | psychosocial burden | prospective | semi-structured interview with open questions; perspectives on issues related to patients’ health; in-depth interview asked about experiences and feelings of life at home since discharge, ongoing therapy and support services, perceived needs and barriers and facilitators to goal achievement |
| Cubis et al. (2019) [90] | psychosocial burden | crosssectional | FACT-cognitive function and general; The Exeter Identity Transition Scale (pre-existing social groups, the maintenance of social groups and new social groups); Social Subscale from the Traumatic Brain Injury Self-Efficacy Scale (confidence in support from social group membership); Satisfaction with Life Scale; the seven item depression Scale of Depression Anxiety Stress Scales; The Generalized Anxiety Disorder Scale |
| Giussani et al. (2010) [91] | social cognition | prospective | identify and name facial emotion expression (anger, happiness, fear, surprise, disgust and sadness); intraoperative facial emotion recognition task (anger, happiness, fear, surprise, disgust and sadness) |
| Goebel et al. (2011) [92] | psychosocial burden | crosssectional | clinical interview for diagnostic and statistical manual of mental disorders fourth edition; distress thermometer; HADS; Impact of Event Scale-revised; questionnaire to mark distressing events during illness |
| Goebel et al. (2011) [93] | psychosocial burden | crosssectional | distress thermometer and associated problem list of the distress thermometer (practical, family, emotional, spiritual-religious or physical problems); HADS, Questionnaire for the Assessment of social support |
| Goebel et al. (2018) [94] | social cognition and psychosocial burden | crosssectional | Karolinska directed emotional faces (emotion recognition, facial differentiation, emotional differentiation); ToM with the RMET; complex ToM reasoning with the Faux-Pas Test; nonverbal cognitive and affective ToM with Picture Stories; Empathy quotient; HADS; Marburg Competence Scale; Social Adjustment Scale; Social and occupational functional assessment scale (examiners rating) |
| Gu et al. (2012) [95] | social cognition | crosssectional | empathy for pain paradigm with explicit pain condition: judge whether the person in the photograph was suffering from pain or not and implicit pain condition: judge the laterality of the hand/foot |
| Guha-Thakurta et al. (1999) [96] | social cognition | crosssectional | modified FACT-Brain; Symptom Questionnaire; Social Adjustment Scale Self-Report; Problem Solving Inventory |
| Herbet et al. (2013) [97] | social cognition | prospective | RMET; Comic Strip Task |
| Herbet et al. (2014) [98] | social cognition | crosssectional | RMET; Comic Strip Task |
| Herbet et al. (2015) [99] | social cognition | crosssectional | Empathy quotient |
| Herbet et al. (2015) [100] | social cognition | crosssectional | RMET (preoperative: 4 response options, intraoperative: 2 response options) |
| Jenkins et al. (2014) [101] | social cognition | crosssectional | Emotion recognition Task: facial morphing with neutral faces changing into emotional (anger, disgust, fear, happiness, sadness and surprise) faces of differing intensities (20–100%); Perspective Taking Task: ToM scale (inferences on thoughts of the character), empathy scale (inferences on feelings of the character), physical scale (inferences on physical events) |
| Kangas et al. (2011) [102] | psychosocial burden | crosssectional | The Profile of Mood States; The Intrusion and Avoidance Subscale from the Impact of Event Scale-Revised; The Multidimensional Scale of Perceived Social Support |
| Kangas et al. (2012) [103] | psychosocial burden | prospective | Post-Traumatic Stress Disorder Checklist-Stressor Specific Version (group categorization in high and low symptoms); Impact of Event Scale-Revised; FACT-General and Brain; Profile of Mood States; Partner Response to Cancer Inventory (perceived positive support); Social Constraints Scale |
| Kanter et al. (2014) [104] | psychosocial burden | crosssectional | quantitative analyses of themes discussed in support groups |
| Langbecker & Yates (2016) [105] | psychosocial burden | prospective | Katz Index of Independence in Activities of daily living; Lwanton-Brody Instrumental Activities of daily living; Supportive Care Needs Survey short form and brain tumor-specific items; Distress thermometer; FACT-Brain |
| Lucas (2010) [106] | psychosocial burden | qualitative study | hundreds of unstructured interviews conducted between 2001–2008 in individual settings and in the group context |
| Luherne-du Boullay et al. (2014) [107] | social cognition | crosssectional | visual emotional recognition task from the Karolinska Directed Emotional Faces (happiness, sadness, disgust, anger, fear and neutral face); auditory emotional recognition task with 60 affect vocalizations (happiness, sadness, disgust, anger, fear and neutral) from the Montréal Affective Voices; crossmodal stimuli: emotional faces and voices congruently and simultaneously presented |
| Mattavelli et al. (2017) [108] | social cognition | prospective | Ekman 60 Faces test: recognition of emotional facial expressions (matching to sample procedure; surprise, happiness, fear, disgust, anger and sadness); recognition of emotion from prosody: new experimental paradigm (sentences consisting of pseudo-words with a prosody corresponding to 1 of 6 emotions) |
| Mu et al. (2012) [109] | social cognition | case-control study | Facial Expression Identification: photos from the Chinese static facial expression gallery with 6 types of basic emotions and neutral expressions |
| Nakajima et al. (2018) [110] | social cognition | prospective | intraoperative mentalizing test: False Belief Task; Cartoon format of the picture arrangement Task of the WAIS third edition |
| Nakajima et al. (2018) [111] | social cognition | crosssectional | RMET |
| Nakajima et al. (2021) [112] | social cognition | prospective | Intraoperative Basic Emotional Test with photos of modified Japanese facial expression of basic emotional series (eye region): selection of most suitable emotional state from 2 choices within 2 seconds; Expression Recognition Test for adults with 32 photographs of basic emotions (happiness, sadness, anger and surprise): selection of most reasonable mental state from 5 choices |
| Ownsworth et al. (2011) [113] | psychosocial burden | crosssectional | in depth semi-structured interviews |
| Ownsworth et al. (2015) [114] | psychosocial burden | randomized wait-list control study | McGill QoL Questionnaire: physical, psychological, existential and social well-being; Montgomery-Asberg Depression Rating Scale; Depression Anxiety Stress Scales-21; FACT-Brain |
| Papagno et al. (2016) [115] | social cognition | prospective | Forced-choice Emotion recognition Task (stimuli selected from FEEST to create a modified version of the Ekman test): selection of correct emotion among 5 alternatives written below the picture (orally or pointing), emotions of anger, fear, happiness, disgust (excluding sadness and surprise) and a mildly neutral expression (happiness at 25% of its intensity) |
| Peper & Irle (1997) [116] | social cognition | crosssectional | selection of category labels: name and select the correct label on a multiple-choice card of presented emotional vocalizations joy, anxiety, sadness and anger (unimodal multiple choice-task); crossmodal vocal-visual recognition of emotion categories with matching emotion categories between auditory and visual stimuli (matching to sample procedure): vocal probe stimulus followed by 2 Ekman & Friesen photographs displaying the same emotion category or a new category to choose from; recognition of affiliative emotion dimensions (valence and arousal) with matching emotion dimensions between auditory and visual stimuli: vocal probe stimulus displaying one emotion category followed by 2 photographs with 2 different emotion categories with corresponding emotion dimensions or not |
| Peper & Irle (1997) [117] | social cognition | crosssectional | selection of category labels: name and select the correct category label on a multiple-choice card of pictures (Ekman and Friesen’s Pictures of Facial Affect) displaying emotional categories (happiness, surprise, anger, anxiety, grief and disgust); selection of named emotion category: select the facial expression (6 emotional expressions of different faces) named by the examiner; matching emotion categories: matching to sample paradigm with 6 categories and an additional neutral stimulus (presentation of probe stimulus immediately followed by 2 choice photographs with the same emotion category and a new category to choose from); matching emotion dimension: probe face displaying one emotion category and 2 response photographs displaying 2 different emotion categories, with either a corresponding emotion dimension or not |
| Pertz et al. (2021) [118] | social cognition | crosssectional | IRI; Multifaceted Empathy Test; Social Problem Solving Fluency Task: ability to detect and interpret awkwardness in hypothetical real-life social situations; discomfort experienced in problematic social situations; capacity to freely generate and merely recognize appropriate solutions for social problems |
| Prat-Acin et al. (2021) [119] | social cognition | prospective | modified version of RMET |
| Saver & Damasio (1991) [120] | social cognition | case study | The Optional Thinking Test (ability to generate alternative solutions to hypothetical social dilemmas); The Awareness of Consequences Test (spontaneous inclination to consider the consequences of social actions); The Means-End Problem-solving procedure (ability to conceptualize efficacious step-by-step means to achieve social goals); The Carton Prediction Test (ability to predict the social consequences of events) |
| Shin et al. (2016) [121] | psychosocial burden | crosssectional | qualitative interview with semi-structured questions; questions included “How have your seizures affected your relationships?” |
| Sinha et al. (2020) [122] | social cognition | prospective | Affective Facial Expression Test: selection of correct emotional expression in faces (happiness, sadness, anger, surprise, fear, disgust); patient health questionnaire |
| Szelag & Fersten (1991) [123] | social cognition | crosssectional | emotion recognition with faces expressing positive, negative (happy and sad) and neutral emotions in a visual half field paradigm (left or right from a fixation point), effectiveness of perception in the left and right visual fields measured by number of errors |
| Trejnowska et al. (2020) [124] | psychosocial burden | crosssectional | Mini-Mental Adjustment to Cancer Scale; Experiences in Close Relationships-Revised questionnaire; Modified Medical Outcomes Study-Social Support Scale; FACT-Brain-physical well-being |
| Troschel et al. (2021) [125] | psychosocial burden | prospective | personal behavior (i.e., number of weekly contacts to friends, acquaintances, or family outside the home environment independent of contact in person, via telephone or via video tools); Isolation Questionnaire; HADS; Distress Thermometer; WHO5 well-being score |
| Voß et al. (2021) [126] | psychosocial burden | crosssectional | patient interviews covering 6 main areas: psyche, cognition, body, role functioning, social support, unmet needs; rating whether the issues affected them and the importance of these areas |
| Wang et al. (2014) [127] | social cognition | prospective | Empathy For Others Pain Task with pain condition and laterality condition; IRI; Toronto Alexithymia Scale |
| Weitzner et al. (1996) [128] | psychosocial burden | crosssectional | Ferrans and Powers QoL Index for Cancer (health and functioning, socioeconomic aspects, psychological/spiritual aspects, family); Psychosocial Adjustment to Illness Scale-Self Report (healthcare orientation, vocational environment, domestic environment, sexual relationships, extended family relationships, social environment, psychological distress) |
| Yordanova et al. (2017) [129] | social cognition | prospective | RMET; modified version of the RMET (only 2 mental state options, for each patient items with a wrong answer during preoperative assessment were excluded) |
| Yuksek et al. (2015) [130] | social cognition | prospective | Facial Emotion Recognition Test with Ekman and Friesen’s Faces (happy, surprised, fearful, sad, angry, disgusted and neutral facial expression) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pertz, M.; Schlegel, U.; Thoma, P. Sociocognitive Functioning and Psychosocial Burden in Patients with Brain Tumors. Cancers 2022, 14, 767. https://doi.org/10.3390/cancers14030767
Pertz M, Schlegel U, Thoma P. Sociocognitive Functioning and Psychosocial Burden in Patients with Brain Tumors. Cancers. 2022; 14(3):767. https://doi.org/10.3390/cancers14030767
Chicago/Turabian StylePertz, Milena, Uwe Schlegel, and Patrizia Thoma. 2022. "Sociocognitive Functioning and Psychosocial Burden in Patients with Brain Tumors" Cancers 14, no. 3: 767. https://doi.org/10.3390/cancers14030767
APA StylePertz, M., Schlegel, U., & Thoma, P. (2022). Sociocognitive Functioning and Psychosocial Burden in Patients with Brain Tumors. Cancers, 14(3), 767. https://doi.org/10.3390/cancers14030767






