Targeted Therapy for Older Patients with Non-Small Cell Lung Cancer: Systematic Review and Guidelines from the French Society of Geriatric Oncology (SoFOG) and the French-Language Society of Pulmonology (SPLF)/French-Language Oncology Group (GOLF)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Questions
- (1)
- For which older patients with non-small cell lung cancer (NSCLC) can we propose the following TKI: EGFR tyrosine kinase inhibitors, ALK tyrosine kinase inhibitors, ROS1 tyrosine kinase inhibitors, and inhibitors of other molecular alterations BRAF/MET?
- (2)
- For which older patients with NSCLC must we consider mono-therapy (TKI alone)?
- (3)
- For which older patients with NSCLC can we consider a combination of several TKI?
2.2. Inclusion and Exclusion Criteria
2.3. Data and Research Algorithm
2.4. Article Selection
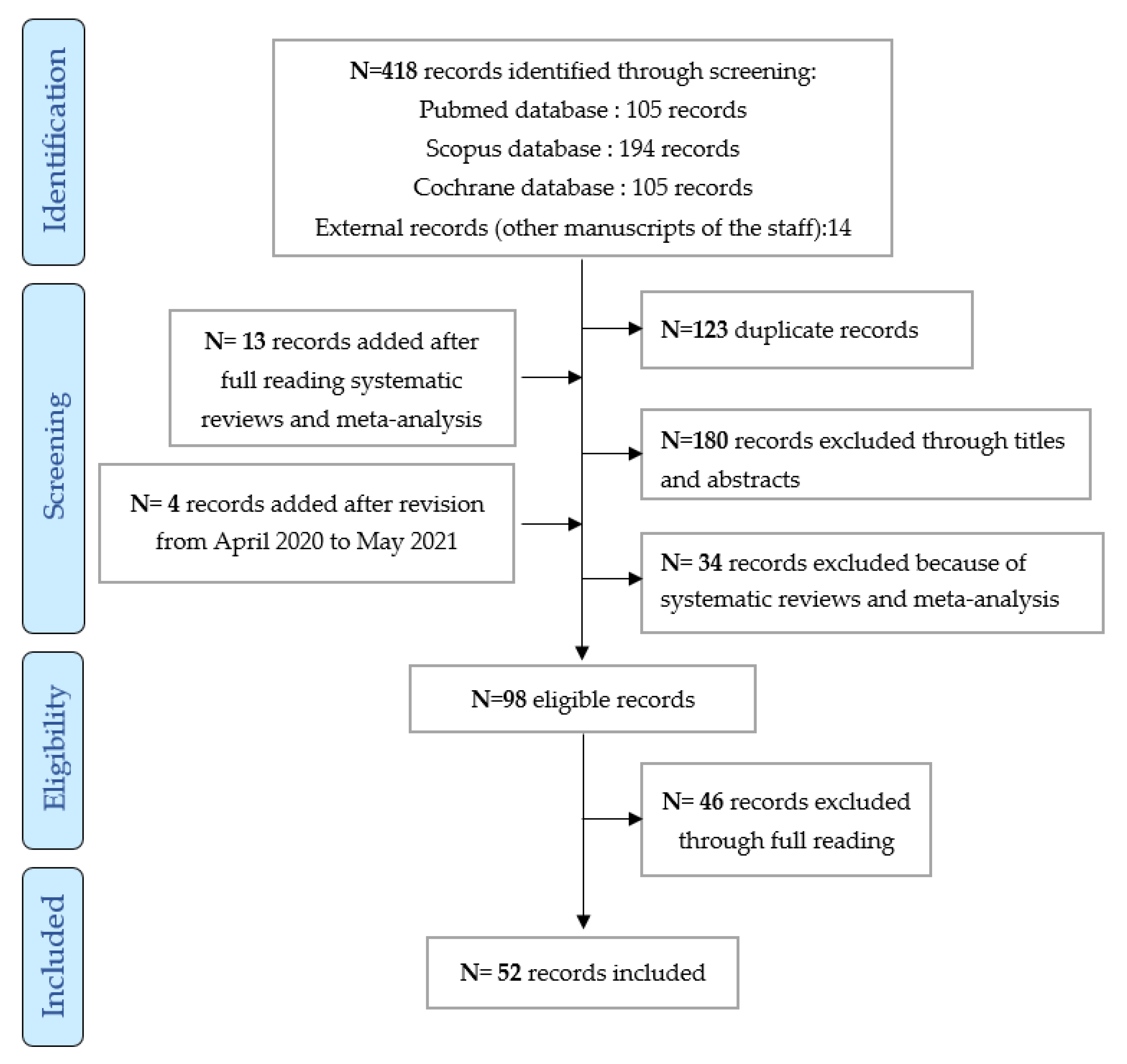
3. Results
- 28 articles on the efficacy of EGFR TKI (only articles mentioning the mutation were included: 17 prospective observational and retrospective non-randomized studies dedicated to older subjects [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42], and 11 prospective randomized or non-randomized studies not dedicated to older subjects [43,44,45,46,47,48,49,50,51,52,53]; 5 articles on the efficacy of ALK TKI were analyzed: 2 prospective non-randomized observational studies not dedicated to older subjects (subgroup of older subjects) and 3 randomized prospective studies not dedicated to older subjects (subgroup of older subjects) [16,17,54,55,56]; only one study on the efficacy of ROS1 TKI as crizotinib was included [57].
- 36 articles on toxicity: 26 retrospective or prospective studies specific to older subjects [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,42,58,59,60,61,62,63,64,65,66,67], 5 randomized studies dedicated to older subjects [7,68,69,70,71], 4 prospective studies with a subgroup of older subjects [55,72,73,74], and a randomized study including a subgroup of older subjects [44].
- 36 articles on feasibility: 26 prospective and retrospective studies, 4 prospective studies with a subgroup of older subjects, 5 randomized studies specific to older subjects, and a randomized study with a subgroup of older subjects.
3.1. Efficacy
3.1.1. EGFR Tyrosine Kinase Inhibitors
3.1.2. ALK Tyrosine Kinase Inhibitors in Older Subjects
3.1.3. ROS1 Tyrosine Kinase Inhibitors in Older Subjects, Case of the Crizotinib
3.1.4. Erlotinib Combined with Bevacizumab in Older Subjects
3.2. Toxicity
3.2.1. EGFR Tyrosine Kinase Inhibitors
- Combination of erlotinib (150 mg/day) + sorafenib (800 mg/day):
3.2.2. ALK Tyrosine Kinase Inhibitors
- Alectinib (600 mg twice/day) [55]:
- Ceritinib (450 mg/day) [55]:
- Crizotinib (250 mg twice/day) [55]:
3.3. Feasibility
3.3.1. EGFR Tyrosine Kinase Inhibitors
- Erlotinib or Gefitinib + Chemotherapy [66]:
- Combination of Targeted Therapy (Sorafenib + Erlotinib) [70]:
3.3.2. ALK Tyrosine Kinase Inhibitors
- Alectinib (600 mg twice/day) [55]:
- Ceritinib (450 mg/day) [55]:
- Crizotinib (250 mg twice/day) [55]:
3.4. Quality of Life
3.5. Geriatric Data
4. Discussions
4.1. For Which Older Patients with NSCLC Can We Propose the Following TKI?
4.1.1. EGFR Tyrosine Kinase Inhibitors
4.1.2. ALK Tyrosine Kinase Inhibitors
4.1.3. The ROS Tyrosine Kinase Inhibitor Found in this Systematic Review in Older Subjects: Crizotinib
4.2. For Which Older Patients with NSCLC Should We Consider Monotherapy (TKI Alone)?
4.3. For Which Older Patients with NSCLC Can We Consider a Combination of Several TKI?
4.4. General Recommendations for Prescribing TKI for the Treatment of NSCLC in Older Patients
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Owonikoko, T.K.; Ragin, C.C.; Belani, C.P.; Oton, A.B.; Gooding, W.E.; Taioli, E.; Ramalingam, S.S. Lung cancer in elderly patients: An analysis of the surveillance, epidemiology, and end results database. J. Clin. Oncol. 2007, 25, 5570–5577. [Google Scholar] [CrossRef] [PubMed]
- SEER Cancer Statistics Review, 1975–2010—Previous Version—SEER Cancer Statistics Review. Available online: https://seer.cancer.gov/archive/csr/1975_2010/index.html (accessed on 18 December 2021).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, R.; Burggraaf, J.; Rissmann, R. Under-representation of elderly in clinical trials: An analysis of the initial approval documents in the food and drug administration database. Br. J. Clin. Pharmacol. 2019, 85, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Jackson, S.H.D. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br. J. Clin. Pharmacol. 2004, 57, 6–14. [Google Scholar] [CrossRef]
- Giroux Leprieur, E.; Labrune, S.; Giraud, V.; Gendry, T.; Cobarzan, D.; Chinet, T. Delay between the initial symptoms, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin. Lung. Cancer 2012, 13, 363–368. [Google Scholar] [CrossRef]
- Schulkes, K.J.G.; Hamaker, M.E.; van den Bos, F.; van Elden, L.J.R. Relevance of a geriatric assessment for elderly patients with lung cancer-a systematic review. Clin. Lung. Cancer 2016, 17, 341–349.e3. [Google Scholar] [CrossRef]
- Driessen, E.J.M.; Schulkes, K.J.G.; Dingemans, A.-M.C.; van Loon, J.G.M.; Hamaker, M.E.; Aarts, M.J.; Janssen-Heijnen, M.L.G. Patterns of treatment and survival among older patients with stage III non-small cell lung cancer. Lung Cancer 2018, 116, 55–61. [Google Scholar] [CrossRef]
- Balducci, L. Frailty: A common pathway in aging and cancer. Interdiscip. Top. Gerontol. 2013, 38, 61–72. [Google Scholar] [CrossRef]
- Mislang, A.R.; Wildes, T.M.; Kanesvaran, R.; Baldini, C.; Holmes, H.M.; Nightingale, G.; Coolbrandt, A.; Biganzoli, L. Adherence to oral cancer therapy in older adults: The international society of geriatric oncology (SIOG) taskforce recommendations. Cancer Treat. Rev. 2017, 57, 58–66. [Google Scholar] [CrossRef]
- Tufman, A.; Kahnert, K.; Duell, T.; Kauffmann-Guerrero, D.; Milger, K.; Schneider, C.; Stump, J.; Syunyaeva, Z.; Huber, R.M.; Reu, S. Frequency and clinical relevance of EGFR mutations and EML4-ALK translocations in octogenarians with non-small cell lung cancer. Onco Targets Ther. 2017, 10, 5179–5186. [Google Scholar] [CrossRef]
- Enewold, L.; Thomas, A. Real-world patterns of EGFR testing and treatment with erlotinib for non-small cell lung cancer in the United States. PLoS ONE 2016, 11, e0156728. [Google Scholar] [CrossRef]
- Barlesi, F.; Scherpereel, A.; Rittmeyer, A.; Pazzola, A.; Ferrer Tur, N.; Kim, J.-H.; Ahn, M.-J.; Aerts, J.G.J.V.; Gorbunova, V.; Vikström, A.; et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J. Clin. Oncol. 2013, 31, 3004–3011. [Google Scholar] [CrossRef]
- Fumagalli, C.; Catania, C.; Ranghiero, A.; Bosi, C.; Viale, G.; de Marinis, F.; Barberis, M.; Guerini-Rocco, E. Molecular profile of advanced non-small cell lung cancers in octogenarians: The door to precision medicine in elderly patients. J. Clin. Med. 2019, 8, 112. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.-H.; Han, J.-Y.; Lee, J.-S.; Hochmair, M.J.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.-W.; Ou, S.-H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Soria, J.-C.; Tan, D.S.W.; Chiari, R.; Wu, Y.-L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.-J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced alk-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Solomon, B.J.; Mok, T.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef]
- Frampton, G.M.; Ali, S.M.; Rosenzweig, M.; Chmielecki, J.; Lu, X.; Bauer, T.M.; Akimov, M.; Bufill, J.A.; Lee, C.; Jentz, D.; et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015, 5, 850–859. [Google Scholar] [CrossRef]
- Awad, M.M.; Leonardi, G.C.; Kravets, S.; Dahlberg, S.E.; Drilon, A.; Noonan, S.A.; Camidge, D.R.; Ou, S.-H.I.; Costa, D.B.; Gadgeel, S.M.; et al. Impact of MET inhibitors on survival among patients with non-small cell lung cancer harboring MET Exon 14 mutations: A retrospective analysis. Lung Cancer 2019, 133, 96–102. [Google Scholar] [CrossRef]
- Drilon, A.E.; Camidge, D.R.; Ou, S.-H.I.; Clark, J.W.; Socinski, M.A.; Weiss, J.; Riely, G.J.; Winter, M.; Wang, S.C.; Monti, K.; et al. Efficacy and safety of crizotinib in patients (Pts) with advanced MET exon 14-altered non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2016, 34, 108. [Google Scholar] [CrossRef]
- Couraud, S.; Westeel, V.; Ranchon, F.; Toffart, A.-C.; Souquet, P.J. Comité de Rédaction des Référentiels Auvergne Rhône-Alpes en; Oncologie Thoracique; Comité de Rédaction de L’édition 2021 Référentiel sur le Cancer Bronchique non à Petites-Cellules: Actualisation 2021; ARISTOT: Paris, France, 2021. [Google Scholar]
- La Valeur de l’outil « Risque de Biais » de la Cochrane Collaboration Dans les Synthèses Méthodiques. Available online: http://www.minerva-ebm.be/FR/Article/2109 (accessed on 12 August 2021).
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Available online: https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000097 (accessed on 12 August 2021).
- Haute Autorité de Santé. Etat des Lieux-Niveau de Preuve et Gradation des Recommandations de Bonne Pratique; Haute Autorité de Santé: Saint-Denis La Plaine Cedex, France, 2013; ISBN 978-2-11-138037-0. [Google Scholar]
- Tanaka, H.; Taima, K.; Tanaka, Y.; Itoga, M.; Ishioka, Y.; Nakagawa, H.; Baba, K.; Hasegawa, Y.; Takanashi, S.; Tasaka, S. A Phase I study of afatinib for patients aged 75 or older with advanced non-small cell lung cancer harboring EGFR mutations. Med. Oncol. 2018, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Kaira, K.; Suzuki, K.; Anzai, M.; Tsuda, T.; Ishizuka, T.; Kuwako, T.; Naruse, I.; Nemoto, K.; Uchino, J.; et al. A phase II study of afatinib treatment for elderly patients with previously untreated advanced non-small-cell lung cancer harboring EGFR mutations. Lung Cancer 2018, 126, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, Y.; Yamaguchi, O.; Sugawara, S.; Kuyama, S.; Watanabe, S.; Usui, K.; Mori, M.; Hataji, O.; Nukiwa, T.; Morita, S.; et al. A phase II study of first-line afatinib for patients aged ≥75 years with EGFR mutation-positive advanced non-small cell lung cancer: North east japan study group trial NEJ027. BMC Cancer 2021, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, K.; Ichiyama, T.; Hirai, K.; Agatsuma, T.; Koyama, S.; Hachiya, T.; Morozumi, N.; Shiina, T.; Koizumi, T. Clinical outcomes in elderly patients administered gefitinib as first-line treatment in epidermal growth factor receptor-mutated non-small-cell lung cancer: Retrospective analysis in a nagano lung cancer research group study. Med. Oncol. 2013, 30, 1–6. [Google Scholar] [CrossRef]
- Fujita, S.; Katakami, N.; Masago, K.; Yoshioka, H.; Tomii, K.; Kaneda, T.; Hirabayashi, M.; Kunimasa, K.; Morizane, T.; Mio, T. Customized chemotherapy based on epidermal growth factor receptormutation status for elderly patients with advanced non-small-cell lung cancer: A phase II trial. BMC Cancer 2012, 12, 185. [Google Scholar] [CrossRef]
- Morikawa, N.; Minegishi, Y.; Inoue, A.; Maemondo, M.; Kobayashi, K.; Sugawara, S.; Harada, M.; Hagiwara, K.; Okinaga, S.; Oizumi, S.; et al. First-line gefitinib for elderly patients with advanced NSCLC harboring EGFR mutations. A combined analysis of North-East Japan Study Group studies. Expert Opin. Pharmacother. 2015, 16, 465–472. [Google Scholar] [CrossRef]
- Maemondo, M.; Minegishi, Y.; Inoue, A.; Kobayashi, K.; Harada, M.; Okinaga, S.; Morikawa, N.; Oizumi, S.; Tanaka, T.; Isobe, H.; et al. First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J. Thorac. Oncol. 2012, 7, 1417–1422. [Google Scholar] [CrossRef]
- Takahashi, K.; Saito, H.; Hasegawa, Y.; Ando, M.; Yamamoto, M.; Kojima, E.; Sugino, Y.; Kimura, T.; Nomura, F.; Ogasawara, T.; et al. First-line gefitinib therapy for elderly patients with non-small cell lung cancer harboring EGFR mutation: Central Japan lung study group 0901. Cancer Chemother. Pharmacol. 2014, 74, 721–727. [Google Scholar] [CrossRef]
- Kuwako, T.; Imai, H.; Masuda, T.; Miura, Y.; Seki, K.; Yoshino, R.; Kaira, K.; Utsugi, M.; Shimizu, K.; Sunaga, N.; et al. First-line gefitinib treatment in elderly patients (Aged ≥75 Years) with non-small cell lung cancer harboring EGFR mutations. Cancer Chemother. Pharmacol. 2015, 76, 761–769. [Google Scholar] [CrossRef]
- Asami, K.; Koizumi, T.; Hirai, K.; Ameshima, S.; Tsukadaira, A.; Morozumi, N.; Morikawa, A.; Atagi, S.; Kawahara, M. Gefitinib as first-line treatment in elderly epidermal growth factor receptor-mutated patients with advanced lung adenocarcinoma: Results of a nagano lung cancer research group study. Clin. Lung Cancer 2011, 12, 387–392. [Google Scholar] [CrossRef]
- Corre, R.; Gervais, R.; Guisier, F.; Tassy, L.; Vinas, F.; Lamy, R.; Fraboulet, G.; Greillier, L.; Doubre, H.; Descourt, R.; et al. Octogenarians with EGFR-mutated non-small cell lung cancer treated by tyrosine-kinase inhibitor: A multicentric real-world study assessing tolerance and efficacy (OCTOMUT Study). Oncotarget 2018, 9, 8253–8262. [Google Scholar] [CrossRef]
- Furuta, H.; Uemura, T.; Yoshida, T.; Kobara, M.; Yamaguchi, T.; Watanabe, N.; Shimizu, J.; Horio, Y.; Kuroda, H.; Sakao, Y.; et al. Efficacy and safety data of osimertinib in elderly patients with NSCLC who harbor the EGFR T790M mutation after failure of initial EGFR-TKI treatment. Anticancer Res. 2018, 38, 5231–5237. [Google Scholar] [CrossRef]
- Nakao, A.; Hiranuma, O.; Uchino, J.; Sakaguchi, C.; Araya, T.; Hiraoka, N.; Ishizuka, T.; Takeda, T.; Kawasaki, M.; Goto, Y.; et al. Final results from a phase II trial of osimertinib for elderly patients with epidermal growth factor receptor T790m-positive non-small cell lung cancer that progressed during previous treatment. J. Clin. Med. 2020, 9, 1762. [Google Scholar] [CrossRef]
- Auliac, J.B.; Saboundji, K.; Andre, M.; Madelaine, J.; Quere, G.; Masson, P.; Vergnenegre, A.; Lamy, R.; Raymond, S.; Chiappa, A.M.; et al. Real-life efficacy of osimertinib in pretreated octogenarian patients with T790M-mutated advanced non-small cell lung cancer. Target. Oncol. 2019, 14, 307–314. [Google Scholar] [CrossRef]
- Kato, Y.; Hosomi, Y.; Watanabe, K.; Yomota, M.; Kawai, S.; Okuma, Y.; Kubota, K.; Seike, M.; Gemma, A.; Okamura, T. Impact of clinical features on the efficacy of osimertinib therapy in patients with T790M-positive non-small cell lung cancer and acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors. J. Thorac. Dis. 2019, 11, 2350–2360. [Google Scholar] [CrossRef]
- Miyamoto, S.; Azuma, K.; Ishii, H.; Bessho, A.; Hosokawa, S.; Fukamatsu, N.; Kunitoh, H.; Ishii, M.; Tanaka, H.; Aono, H.; et al. Low-dose erlotinib treatment in elderly or frail patients with EGFR mutation-positive non-small cell lung cancer: A multicenter phase 2 trial. JAMA Oncol. 2020, 6, e201250. [Google Scholar] [CrossRef]
- Inoue, Y.; Inui, N.; Asada, K.; Karayama, M.; Matsuda, H.; Yokomura, K.; Koshimizu, N.; Imokawa, S.; Yamada, T.; Shirai, T.; et al. Phase II study of erlotinib in elderly patients with non-small cell lung cancer harboring epidermal growth factor receptor mutations. Cancer Chemother. Pharmacol. 2015, 76, 155–161. [Google Scholar] [CrossRef]
- Park, K.; Tan, E.-H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.-H.; Lee, K.H.; Lu, S.; et al. Afatinib versus Gefitinib as First-Line Treatment of Patients with EGFR Mutation-Positive Non-Small-Cell Lung Cancer (LUX-Lung 7): A Phase 2B, Open-Label, Randomised Controlled Trial. Lancet Oncol 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Wu, Y.L.; Sequist, L.V.; Tan, E.H.; Geater, S.L.; Orlov, S.; Zhang, L.; Lee, K.H.; Tsai, C.M.; Kato, T.; Barrios, C.H.; et al. Afatinib as first-line treatment of older patients with EGFR mutation-positive non-small-cell lung cancer: Subgroup analyses of the LUX-lung 3, LUX-lung 6, and LUX-lung 7 lrials. Clin. Lung Cancer 2018, 19, e465–e479. [Google Scholar] [CrossRef]
- Sequist, L.V.; Yang, J.C.-H.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.-M.; Boyer, M.; et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR Mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Tsuji, F.; Linke, R.; Rosell, R.; Corral, J.; et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 1454–1466. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.-L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in untreatedEGFR-mutated advanced non–small-cell lung cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.M.E.; et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Ostoros, G.; Cobo, M.; Ciuleanu, T.; McCormack, R.; Webster, A.; Milenkova, T. First-line gefitinib in caucasian EGFR mutation-positive NSCLC patients: A phase-IV, open-label, single-arm study. Br. J. Cancer. 2014, 110, 55–62. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Tan, E.-H.; O’Byrne, K.; Zhang, L.; Hirsh, V.; Boyer, M.; Yang, J.C.-H.; Mok, T.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: Overall survival data from the phase IIb LUX-lung 7 trial. Ann. Oncol. 2017, 28, 270–277. [Google Scholar] [CrossRef]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Bedas, A.; Peled, N.; Maimon Rabinovich, N.; Mishaeli, M.; Shochat, T.; Zer, A.; Rotem, O.; Allen, A.M.; Bar, J.; Dudnik, E. Efficacy and safety of ALK tyrosine kinase inhibitors in elderly patients with advanced ALK-positive non-small cell lung cancer: Findings from the real-life cohort. Oncol. Res. Treat. 2019, 42, 275–282. [Google Scholar] [CrossRef]
- Camidge, D.R.; Bang, Y.-J.; Kwak, E.L.; Iafrate, A.J.; Varella-Garcia, M.; Fox, S.B.; Riely, G.J.; Solomon, B.; Ou, S.-H.I.; Kim, D.-W.; et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: Updated results from a phase 1 study. Lancet Oncol. 2012, 13, 1011–1019. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Yang, J.C.-H.; Kim, D.-W.; Lu, S.; Zhou, J.; Seto, T.; Yang, J.-J.; Yamamoto, N.; Ahn, M.-J.; Takahashi, T.; et al. Phase II study of crizotinib in east asian patients with ROS1-positive advanced non-small-cell lung cancer. J. Clin. Oncol. 2018, 36, 1405–1411. [Google Scholar] [CrossRef]
- Inomata, M.; Shimokawa, K.; Tokui, K.; Taka, C.; Okazawa, S.; Kambara, K.; Yamada, T.; Miwa, T.; Hayashi, R.; Kashii, T.; et al. Appetite loss as an adverse effect during treatment with EGFR-Tkis in elderly patients with non-small cell lung cancer. Anticancer Res. 2016, 36, 4951–4954. [Google Scholar] [CrossRef]
- Wu, C.-H.; Fan, W.-C.; Chen, Y.-M.; Chou, K.-T.; Shih, J.-F.; Tsai, C.-M.; Lee, Y.-C.; Perng, R.-P. Second-line therapy for elderly patients with non-small cell lung cancer who failed previous chemotherapy is as effective as for younger patients. J. Thorac. Oncol. 2010, 5, 376–379. [Google Scholar] [CrossRef]
- Nakao, A.; Hiranuma, O.; Uchino, J.; Sakaguchi, C.; Kita, T.; Hiraoka, N.; Ishizuka, T.; Kubota, Y.; Kawasaki, M.; Goto, Y.; et al. Osimertinib in elderly patients with epidermal growth factor receptor T790M-positive non-small-cell lung cancer who progressed during prior treatment: A phase II trial. Oncologist 2019, 24, 593–e170. [Google Scholar] [CrossRef]
- Minemura, H.; Yokouchi, H.; Azuma, K.; Hirai, K.-I.; Sekine, S.; Oshima, K.; Kanazawa, K.; Tanino, Y.; Inokoshi, Y.; Ishii, T.; et al. A phase II trial of erlotinib monotherapy for pretreated elderly patients with advanced EGFR wild-type non-small cell lung cancer cancer. BMC Res. Notes 2015, 8, 1–6. [Google Scholar] [CrossRef][Green Version]
- Rossi, D.; Dennetta, D.; Ugolini, M.; Catalano, V.; Alessandroni, P.; Giordani, P.; Baldelli, A.M.; Casadei, V.; Graziano, F.; Fedeli, S.L. Activity and safety of erlotinib as second- and third-line treatment in elderly patients with advanced non-small cell lung cancer: A phase II trial. Target. Oncol. 2010, 5, 231–235. [Google Scholar] [CrossRef]
- Yoshioka, H.; Komuta, K.; Imamura, F.; Kudoh, S.; Seki, A.; Fukuoka, M. Efficacy and safety of erlotinib in elderly patients in the phase IV POLARSTAR surveillance study of japanese patients with non-small-cell lung cancer. Lung Cancer 2014, 86, 201–206. [Google Scholar] [CrossRef]
- Brueckl, W.M.; Achenbach, H.J.; Ficker, J.H.; Schuette, W. Erlotinib treatment after platinum-based therapy in elderly patients with non-small-cell lung cancer in routine clinical practice—Results from the ElderTac study. BMC Cancer 2018, 18, 333. [Google Scholar] [CrossRef]
- Yamada, K.; Azuma, K.; Takeshita, M.; Uchino, J.; Nishida, C.; Suetsugu, T.; Kondo, A.; Harada, T.; Eida, H.; Kishimoto, J.; et al. Phase II trial of erlotinib in elderly patients with previously treated non-small cell lung cancer: Results of the lung oncology group in kyushu (LOGiK-0802). Anticancer Res. 2016, 36, 2881–2887. [Google Scholar]
- Aoshima, Y.; Karayama, M.; Inui, N.; Yasui, H.; Hozumi, H.; Suzuki, Y.; Furuhashi, K.; Fujisawa, T.; Enomoto, N.; Nakamura, Y.; et al. Erlotinib and bevacizumab in elderly patients ≥75 years old with non-small cell lung cancer harboring epidermal growth factor receptor mutations. Invest. New Drugs 2021, 39, 210–216. [Google Scholar] [CrossRef]
- Kobayashi, M.; Matsui, K.; Katakami, N.; Takeda, K.; Moriyama, A.; Iwamoto, Y.; Takada, M.; Yoshioka, H.; Sueoka-Aragane, N.; Nakagawa, K. Phase II study of gefitinib as a first-line therapy in elderly patients with pulmonary adenocarcinoma: West japan thoracic oncology group study 0402. Jpn. J. Clin. Oncol. 2011, 41, 948–952. [Google Scholar] [CrossRef]
- Heigener, D.; Deppermann, K.; Pawel, J.; Fischer, J.; Kortsik, C.; Bohnet, S.; Eiff, M.; Koester, W.; Thomas, M.; Schnabel, P.; et al. Open, randomized, multi-center phase II study comparing efficacy and tolerability of Erlotinib vs. Carboplatin/Vinorelbin in elderly patients (>70 years of age) with untreated non-small cell lung cancer. Lung Cancer 2014, 84, 62–66. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Tsai, C.-M.; Fan, W.-C.; Shih, J.-F.; Liu, S.-H.; Wu, C.-H.; Chou, T.-Y.; Lee, Y.-C.; Perng, R.-P.; Whang-Peng, J. Phase II Randomized Trial of Erlotinib or Vinorelbine in Chemonaive, Advanced, Non-small Cell Lung Cancer Patients Aged 70 Years or Older. J. Thorac. Oncol. 2012, 7, 412–418. [Google Scholar] [CrossRef]
- Gridelli, C.; Morgillo, F.; Favaretto, A.; de Marinis, F.; Chella, A.; Cerea, G.; Mattioli, R.; Tortora, G.; Rossi, A.; Fasano, M.; et al. Sorafenib in combination with erlotinib or with gemcitabine in elderly patients with advanced non-small-cell lung cancer: A randomized phase II study. Ann. Oncol. 2011, 22, 1528–1534. [Google Scholar] [CrossRef]
- Quoix, E.; Westeel, V.; Moreau, L.; Pichon, E.; Lavolé, A.; Dauba, J.; Debieuvre, D.; Souquet, P.J.; Bigay-Game, L.; Dansin, E.; et al. Second-line therapy in elderly patients with advanced nonsmall cell lung cancer. Eur. Respir. J. 2014, 43, 240–249. [Google Scholar] [CrossRef]
- Merimsky, O.; Cheng, C.-K.; Au, J.S.-K.; Von Pawel, J.; Reck, M. Efficacy and safety of first-line erlotinib in elderly patients with advanced non-small cell lung cancer. Oncol. Rep. 2012, 28, 721–727. [Google Scholar] [CrossRef]
- Kurishima, K.; Satoh, H.; Kaburagi, T.; Nishimura, Y.; Shinohara, Y.; Inagaki, M.; Endo, T.; Saito, T.; Hayashihara, K.; Hizawa, N.; et al. Erlotinib for elderly patients with non-small-cell lung cancer: Subset analysis from a population-based observational study by the ibaraki thoracic integrative (POSITIVE) research group. Mol. Clin. Oncol. 2013, 1, 828–832. [Google Scholar] [CrossRef][Green Version]
- Tam, T.C.-C.; Ho, J.C.-M.; Wong, M.K.-Y.; Wong, W.-M.; Wang, J.K.-L.; Lam, J.C.-M.; Lui, M.M.-S.; Lam, W.-K.; Ip, M.S.-M.; Lam, D.C.-L. Treatment outcomes in elderly with advanced-stage non-small cell lung cancer. Lung 2013, 191, 645–654. [Google Scholar] [CrossRef]
- Stinchcombe, T.E.; Roder, J.; Peterman, A.H.; Grigorieva, J.; Lee, C.B.; Moore, D.T.; Socinski, M.A. A retrospective analysis of veristrat status on outcome of a randomized phase II trial of first-line therapy with gemcitabine, erlotinib, or the combination in elderly patients (age 70 years or older) with stage IIIB/IV non–small-cell lung cancer. J. Thorac. Oncol. 2013, 8, 443–451. [Google Scholar] [CrossRef]
- Stinchcombe, T.E.; Peterman, A.H.; Lee, C.B.; Moore, D.T.; Beaumont, J.L.; Bradford, D.S.; Bakri, K.; Taylor, M.; Crane, J.M.; Schwartz, G.; et al. A randomized phase ii trial of first-line treatment with gemcitabine, erlotinib, or gemcitabine and erlotinib in elderly patients (age ≥ 70 years) with stage IIIB/IV non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Shariati, M.; Meric-Bernstam, F. Targeting AKT for cancer therapy. Expert Opin. Investig. Drugs 2019, 28, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, C.; Wang, L. Molecular-targeted agents combination therapy for cancer: Developments and potentials. Int. J. Cancer 2014, 134, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Soubeyran, P.; Bellera, C.; Goyard, J.; Heitz, D.; Curé, H.; Rousselot, H.; Albrand, G.; Servent, V.; Jean, O.S.; van Praagh, I.; et al. Screening for vulnerability in older cancer patients: The ONCODAGE prospective multicenter cohort study. PLoS ONE 2014, 9, e115060. [Google Scholar] [CrossRef]
- Guigoz, Y.; Vellas, B. The Mini Assessment (MNA) for grading the nutritional state of elderly patients: Presentation of the MNA, history and validation. Nestle Nutr. Workshop Ser. Clin. Perform. Programme 1999, 1, 3–11, discussion 11–12. [Google Scholar] [CrossRef]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.S.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O.; et al. practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J. Clin. Oncol. 2018, 36, 2326–2347. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. J. Am. Med. Assoc. 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Miller, M.D.; Paradis, C.F.; Houck, P.R.; Mazumdar, S.; Stack, J.A.; Rifai, A.H.; Mulsant, B.; Reynolds, C.F. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the cumulative illness rating scale. Psychiatry Res. 1992, 41, 237–248. [Google Scholar] [CrossRef]
| Publication/Country | Targeted Therapy | Patient Number | ECOG-PS ≥ 2 (%) | Median Age, Years | Objective Tumor Response (95% CI) | Median PFS, Months (95% CI) | Median OS, Months (95% CI) |
|---|---|---|---|---|---|---|---|
| Tanaka 2018 [26]/Japan | Afatinib 40 mg/day 30 mg/day 20 mg/day | 15 | 13.3 | 79 | 73.3 (NR) | 22 (13.1-NR) | |
| Imai 2018 [27]/Japan | Afatinib 30 mg/day | 40 | 2.5 | 77 | 72.5 (58.6–86.3) | 12.9 (8.8–19.3) | NR At 1 year: 87.4%, 2 years: 60.6% |
| Minegishi 2021 [28]/Japan | Afatinib 40 mg/day | 37 | 0 | 77.5 | 75.7 (58.8–88.2) | 14.2 (9.5–19.0) | 35.2 (35.2-NR) At 1 year: 83.8%, 2 years: 78.3% |
| Tateishi 2013 [29]/Japan | Gefitinib 250 mg/day | 55 | 16.4 | 81.1 | 72.7 (59.5–82.9) | 13.8 (9.9–18.8) | 29.1 (22.4-NR) At 2 years: 59.5% |
| Fujita 2012 [30]/Japan | Gefitinib 250 mg/day | 54 | 0 | 81 | 45.5 (24.4–67.8) | 27.9 At 1 year: 90% | |
| Morikawa 2015 [31]/Japan | Gefitinib 250 mg/day versuscaboplatine/ paclitaxel | 71 | 8 | 75 | 73.2 (61.3–83.0) | 14.3 | 30.8 |
| Maemondo 2012 [32]/Japan | Gefitinib 250 mg/day | 31 | 6 | 80.3 | 74.2 (57.9–90.5) | 12.1 | 33.8 At 1 year: 83.9%, at 2 years: 58.1 |
| Takahashi 2014 [33]/Japan | Gefitinib 250 mg/day | 20 | 10 | 79.5 | 70 (45.7–88.1) | 10.0 | 26.4 |
| Kuwako 2015 [34]/Japan | Gefitinib 250 mg/day | 62 | 29 | 80 | 61.3 | 13.2 | 19 |
| Asami 2011 [35]/Japan | Gefitinib 250 mg/day | 17 | 17 | 81 | 59 (33–81) | 12.9 (2.2–23.6) | OS (NR) At 1 year: 88% |
| Corre 2018 [36]/France | Gefitinib or erlotinib or afatinib | 114 | 28.4 | 83.9 | 63.3 | 11.9 (8.6–14.7) | 20.9 (14.3–27.1) |
| Furuta 2018 [37]/Japan | Osimertinib | 18 | 0 | 80 | 61 | 17.7 (8.4-NR) | 38.6 (14.3–52.8) |
| Nakao 2020 [38]/Japan | Osimertinib 80 mg/day | 36 | 0 | 80 | 58.3 (42.2–72.9) | 11.9 (7.9–17.5) | 22.0 (16.0-NR) at 1 year: 77.8%, at 2 years: 49.5% |
| Auliac 2019 [39]/France | Osimertinib 80 mg/day | 43 | 42.4 | 84.6 | 17.5 (12.2–19.0) | 22.8 (15.7-NR) | |
| Kato 2019 [40]/Japan | Osimertinib | 31 | 10 | 32.3 | 5.6 (3.6–14.8) | 19.4 (9.1-NR) | |
| 8 | 3 | 54 | 3.5 (1.6–14.8) | 5.3 | |||
| 23 | 7 | 75 | 6.4 (5-NR) HR 2.41; p = 0.041 | 19.4 HR 2.58; p = 0.067 | |||
| Miyamoto 2020 [41]/Japan | Erlotinib 50 mg/day | 80 | 32 | 80 | 60.0 (50.2–69.2) | 9.3 (7.2–11.4) | 26.2 (21.9–30.4) |
| Inoue 2015 [42]/Japan | Erlotinib 150 mg/day | 32 | 3.1 | 80 | 56.3 (39.4–72.0) | 15.5 (11.2-NR) | Median OS (NR) At 1 year: 83.9% (65.5–93.0) |
| Publication/ Country | Targeted Therapy | Patients Number | Age Group | ECOG-PS ≥ 2 (%) | PFS (Months) (95% CI) | OS (Months) (95% CI) |
|---|---|---|---|---|---|---|
| Park 2016 [43]/ International | Afatinib 40 mg/day or gefitinib 250 mg/day | 319 | 0 | |||
| 177 | <65 | afatinib 11.0 (9.2–17.0) gefitinib 9.2 (7.3–11.0) HR 0.68 (0.48–0.97) | ||||
| 142 | ≥65 | afatinib 11.0 (9.2–12.9) gefitinib 11.4 (10.8–12.9) HR 0.85 (0.59–1.22); p = 0.309 | ||||
| Wu 2018 [44]/ International | Afatinib 40 mg/day or gefitinib 250 mg/day | 319 | 0 | |||
| 177 | <75 | Afatinib 11.0 Gefitinib 10.9 HR 0.76 (0.58–1.00) | Afatinib 28.9 Gefitinib 25.2 HR 0.85 (0.64–1.12) | |||
| 142 | ≥75 | Afatinib 14.7 Gefitinib 10.8 HR 0.69 (0.33–1.44) | Afatinib 27.9 Gefitinib 19.7 HR 1.05 (0.5–2.21) | |||
| Paz-Ares 2017 [53]/International | Afatinib 40 mg/day or gefitinib 250 mg/day | 319 | 0 | |||
| 177 | <65 | 0.66 (0.46–0.94) HR 1.22 (0.82–1.81); p = 0.0228 | ||||
| 142 | ≥65 | |||||
| Sequist 2013 [45]/ International | Afatinib 40 mg/day or cisplatine/pemetrexed | 345 | ||||
| 211 | <65 | HR 0.53 (0.36–0.76) | ||||
| 134 | ≥65 | HR 0.64 (0.39–1.03); p = 0.58 | ||||
| Wu 2017 [46]/International | Dacomitinib 45 mg/day or gefitinib 250 mg/day | 452 | 0 | |||
| dacomitinib/ gefitinib | <65 | HR 0.51 (0.39–0.69) | ||||
| dacomitinib/ gefitinib | ≥65 | HR 0.69 (0.48–0.99) | ||||
| Zhou 2011 [47]/China | Erlotinib 150 mg/dayor gemcitabine/cisplatin | 165 | 9 | |||
| 64 | <65 | HR 0.19 (0.11–0.31) | ||||
| 19 | ≥65 | HR 0.17 (0.07–0.43) | ||||
| Rosell 2012 [48]/International | Erlotinib 150 mg/day or cisplatin/docetaxel or gemcitabin | 173 | 14 | |||
| 85 | <65 | HR 0.44 (0.25–0.75) | ||||
| 88 | ≥65 | HR 0.28 (0.16–0.51) p = 0.4962 | ||||
| Soria 2018 [49]/International | Osimertinib 80 mg/day or gefitinib 250 mg/day or erlotinib 150 mg/day | 556 | 0 | |||
| 298 | <65 | HR 0.44 (0.33–0.58) | ||||
| 258 | ≥65 | HR 0.49 (0.35–0.67) | ||||
| Ramalingam 2020 [50]/International | Osimertinib 80 mg/day or gefitinib 250 mg/day or erlotinib 150 mg/day | 556 | 0 | |||
| 298 | <65 | HR 0.72 (0.54–0.97) | ||||
| 258 | ≥65 | HR 0.87 (0.63–1.22) | ||||
| Mok 2017 [51]/ International | Osimertinib 80 mg/day | 279 | ||||
| 242 | <65 | HR 0.38 (0.28–0.54) | ||||
| 177 | ≥65 | HR 0.34 (0.23–0.50) | ||||
| Douillard 2014 [52]/International | Gefitinib 250 mg/day | 106 | 6.6 | |||
| 55 | ≤65 | 65.5 (52.3–76.6) | ||||
| 51 | > 65 | 74.5 (61.1–84.5) |
| Publication/ Country | Targeted Therapy | Patient Number | Age Group | ECOG-PS ≥ 2 (%) | Objective Response Rate (95% CI) | PFS (Months) (95% CI) | OS (Months) (95% CI) |
|---|---|---|---|---|---|---|---|
| Hida 2017 [54]/ Japan | Alectinib 300 mg twice a day or crizotinib 250 mg twice a day | 207 | 2 | ||||
| 185 | <75 | HR 0.34 (0.21–0.56) | |||||
| 22 | ≥75 | HR 0.28 (0.06–1.19) | |||||
| Peters 2017 [16]/ International | Alectinib 600 mg twice a day or crizotinib 250 mg twice a day | 303 | 7 | ||||
| 233 | <65 | HR 0.48 (0.34–0.70) | |||||
| 70 | ≥65 | HR 0.45 (0.24–0.87) | |||||
| Camidge 2012 [56]/International | Crizotinib 250 mg twice a day | 149 | 12 | ||||
| 123 | <65 | 60.2 (50.9–68.9) | |||||
| 20 | ≥65 | 65.0 (40.8–84.6) | |||||
| Soria 2017 [17]/ International | Ceritinib 750 mg/day or cisplatin/pemetrexed | 376 | 0 | ||||
| 295 | <65 | 17.1 (12.5–27.7) HR 0.58 (0.42–0.80) | |||||
| 81 | ≥65 | 14.0 (8.3-NR) HR 0.45 (0.24–0.86) | |||||
| Bedas 2019 [55]/ Israel | Crizotinib or alectinib or ceritinib | 53 | 11 | crizotinib 5.6 (2.5–14.7) | 25.1 (10.8–53.6) | ||
| 34 | <65 | ceritinib 23 (0.8–27.7) | |||||
| 19 | ≥65 | alectinib 5.6 (0.5-NR) |
| Molecules | Afatinib | Gefitinib | Osimertinib | Crizotinib | Ceritinib | Alectinib | Erlotinib | Combination of TKI | Combination of TKI and Chemotherapy |
|---|---|---|---|---|---|---|---|---|---|
| Publications | Tanaka 2018 [26], Imai 2018 [27], Wu 2018 [44], Corre 2018 [36], Minegishi 2021 [28] | Wu 2018 [44], Inomata 2016 [58], Tateishi 2013 [29], Fujita 2012 [30], Morikawa 2015 [31], Maemondo 2012 [32], Takahashi 2014 [33], Kuwako 2015 [34], Asami 2011 [35], Corre 2018 [36], Wu 2015 [59], Kobayashi 2011 [67] | Furuta 2018 [37], Nakao 2020 [38], Nakao 2019 [60], Auliac 2019 [39], Kato 2019 [40] | Bedas 2019 [55] | Bedas 2019 [55] | Bedas 2019 [55] | Minemura 2015 [61], Stinchcombe 2011 [76], Rossi 2010 [62], Inomata 2016 [58], Yoshioka 2014 [63], Merimsky 2012 [72], Kurishima 2013 [73], Brueckl 2018 [64], Corre 2018 [36], Heigener 2014 [68], Chen 2012 [69], Inoue 2015 [42], Yamada 2016 [65], Quoix 2014 [71] | Gridelli 2011 [70] | Gridelli 2011 [70], Stinchcombe 2011 [76], Aoshima 2020 [66], Tam 2013 [74] |
| Anemia (%) | |||||||||
| Grade 1–2 | 4–60 | 6–50 | 28–75 | 6–80 | 3 | 3–12 | |||
| Grade 3–4 | 2 | 3–13 | 6–43 | 8 | |||||
| Leucopenia (%) | |||||||||
| Grade 1–2 | 2–3 | 4–10 | 17–36 | 3–20 | 16 | ||||
| Grade 3–4 | 1–2 | 3–17 | |||||||
| Neutropenia (%) | |||||||||
| Grade 1–2 | 3–17 | 1–3 | 39 | 3–10 | 19 | ||||
| Grade 3–4 | 1–2 | 3–6 | 1–2 | 2–3 | |||||
| Thrombocytopenia (%) | |||||||||
| Grade 1–2 | 21 | 1–10 | 56–58 | 17.5 | 3 | 3 | |||
| Grade 3–4 | 2 | 3 | 2 | 4–10 | |||||
| AST/ALT elevation (%) | |||||||||
| Grade 1–2 | 5–33 | 10–60 | 22–36 | 16 | 20 | 22 | 8–37.5 | 6 | 6–20 |
| Grade 3–4 | 5 | 7–50 | 6 | 1–6 | 2 | ||||
| Bilirubin elevation (%) | |||||||||
| Grade 1–2 | 3 | 10–13 | 8 | 40 | 6 | ||||
| Grade 3–4 | 3 | ||||||||
| AP elevation (%) | |||||||||
| Grade 1–2 | 25–34 | ||||||||
| Grade 3–4 | 27 | 6 | |||||||
| Creatinine elevation (%) | |||||||||
| Grade 1–2 | 17 | 13–16 | 25–31 | 16 | 40 | 6–40 | 9–12 | ||
| Grade 3–4 | |||||||||
| Grade 5 | 1 | 2 | |||||||
| Hypoalbuminemia (%) | |||||||||
| Grade 1–2 | 41 | 69–75 | |||||||
| Grade 3–4 | 3 | ||||||||
| Amylase-lipase elevation (%) | |||||||||
| Grade 1–2 | |||||||||
| Grade 3–4 | 3 | 3 | |||||||
| Hyperkalemia | |||||||||
| Grade 1–2 (%) | 23 | ||||||||
| Molecules | Afatinib | Gefitinib | Osimertinib | Crizotinib | Ceritinib | Alectinib | Erlotinib | Combination of TKI | Combination of TKI and Chemotherapy |
|---|---|---|---|---|---|---|---|---|---|
| Publications | Tanaka 2018 [26], Imai 2018 [27], Wu 2018 [44], Corre 2018 [36], Minegishi 2021 [28] | Wu 2018 [44], Inomata 2016 [58], Tateishi 2013 [29], Fujita 2012 [30], Morikawa 2015 [31], Maemondo 2012 [32], Takahashi 2014 [33], Kuwako 2015 [34], Asami 2011 [35], Corre 2018 [36], Wu 2015 [59], Kobayashi 2011 [67] | Furuta 2018 [37], Nakao 2020 [38], Nakao 2019 [60], Auliac 2019 [39], Kato 2019 [40] | Bedas 2019 [55] | Bedas 2019 [55] | Bedas 2019 [55] | Minemura 2015 [61], Stinchcombe 2011 [76], Rossi 2010 [62], Inomata 2016 [58], Yoshioka 2014 [63], Merimsky 2012 [72], Kurishima 2013 [73], Brueckl 2018 [64], Corre 2018 [36], Heigener 2014 [68], Chen 2012 [69], Inoue 2015 [42], Yamada 2016 [65], Quoix 2014 [71] | Gridelli 2011 [70] | Gridelli 2011 [70], Stinchcombe 2011 [76], Aoshima 2020 [66], Tam 2013 [74] |
| Nausea (%) | |||||||||
| Grade 1–2 | 8–50 | 2–19 | 42 | 60 | 22 | 2 | 6 | 16–24 | |
| Grade 3–4 | 3–17 | 2–3 | 5 | 20 | 1 | 16 | |||
| Vomiting (%) | |||||||||
| Grade 1–2 | 5–50 | 1–23 | 1–7.5 | 6 | |||||
| Grade 3–4 | 2–3 | 5 | 1 | ||||||
| Anorexia (%) | |||||||||
| Grade 1–2 | 17–33 | 13–50 | 28–31 | 12.5–50 | 14 | 3 | |||
| Grade 3–4 | 3–17 | 5–20 | 11 | 6 | |||||
| Dysgeusia (%) | |||||||||
| Grade 1–2 | 6 | 15 | |||||||
| Grade 3–4 | |||||||||
| Asthenia/fatigue (%) | |||||||||
| All grades | 17 | ||||||||
| Grade 1–2 | 13–67 | 6–40 | 28–31 | 32 | 40 | 44 | 2–42.5 | 28 | 19–12 |
| Grade 3–4 | 1–33 | 3 | 8–9 | 5 | 2–5 | 14 | 13–10 | ||
| Diarrhea (%) | |||||||||
| All grades | 30 | ||||||||
| Grade 1–2 | 67–100 | 6–52 | 22–39 | 32 | 60 | 12.5–80 | 38 | 9–32 | |
| Grade 3–4 | 8–33 | 1–17 | 2.8 | 10 | 3–17 | 17 | 3–6 | ||
| Grade 5 | 17 | ||||||||
| Skin rash (%) | |||||||||
| All grades | 69 | ||||||||
| Grade 1–2 | 33–74 | 31–90 | 22–36 | 3–95 | 35 | 26–30-60 | |||
| Grade 3–4 | 5–33 | 2–16 | 4–14 | 13 | 4–6–16 | ||||
| Acne (%) | |||||||||
| All grades | 47 | ||||||||
| Grade 1–2 | 45 | ||||||||
| Grade 3–4 | 31 | ||||||||
| Paronychia (%) | |||||||||
| Grade 1–2 | 26–50 | 19–30 | 33 | 6–37.5 | 3 | 3–36 | |||
| Grade 3–4 | 5–28 | 4–5 | 17–42 | ||||||
| Mucositis-stomatitis (%) | |||||||||
| Grade 1–2 | 31–60 | 1–24 | 17–22 | 6–12.5–28 | 16–19 | ||||
| Grade 3–4 | 3–50 | 3–8 | |||||||
| Dry skin (%) | |||||||||
| Grade 1–2 | 9–38 | 8–65 | 6–59 | ||||||
| Grade 3–4 | 3–5 | ||||||||
| Pruritus (%) | |||||||||
| Grade 1–2 | 14–26 | 6–24 | 22 | 62.5 | 3 | ||||
| Grade 3–4 | 1–2 | 2.5 | |||||||
| Urticaria (%) | |||||||||
| Grade 1–2 | 15 | ||||||||
| Grade 3–4 | |||||||||
| Edema (%) | |||||||||
| Grade 1–2 | 10.5 | 37 | 33 | ||||||
| Grade 3–4 | 26 | 8 | 1 | ||||||
| Infection (%) | |||||||||
| Grade 1–2 | 3–17 | 1 | |||||||
| Grade 3–4 | 3–17 | 1 | |||||||
| Interstitial lung disease (%) | |||||||||
| Grade 1–2 | 8 | 1–6 | 3 | 5 | 1 | ||||
| Grade 3–4 | 5–10 | 2–4 | 6–9 | 1–6 | 2 | ||||
| Grade 5 | 2 | ||||||||
| Constipation (%) | |||||||||
| Grade 1–2 | 3–4 | 6.5 | 10 | ||||||
| Grade 3–4 | |||||||||
| Dehydration (%) | |||||||||
| Grade 1–2 | |||||||||
| Grade 3–4 | 3 | 1–6 | 6 | ||||||
| Alopecia (%) | |||||||||
| Grade 1–2 | 6–10 | 19 | 3 | 6 | |||||
| Grade 3–4 | |||||||||
| Pigmentation (%) | |||||||||
| Grade 1–2 | 21.6 | ||||||||
| Faintness (%) | |||||||||
| Grade 1–2 | 12 | 6 | |||||||
| Grade 3–4 | 1 | ||||||||
| Ventricular dysfunction (%) | |||||||||
| Grade 3–4 | 3 | ||||||||
| Prolonged QT interval (%) | |||||||||
| Grade 1–2 | 10 | ||||||||
| Grade 3–4 | 3 | ||||||||
| Hand-foot syndrome (%) | |||||||||
| Grade 1–2 | 27.5 | 21 | 20 | ||||||
| Grade 3–4 | 3 | 10 | |||||||
| Delirium (%) | |||||||||
| Grade 3–4 | 3 | ||||||||
| Dyspnea (%) | |||||||||
| All grades | 17.5 | ||||||||
| Grade 3–4 | 3 | 6 | |||||||
| Sinusitis (%) | |||||||||
| Grade 3–4 | 3 | ||||||||
| Fever (%) | |||||||||
| Grade 1–2 | 11 | 3–8 | |||||||
| Vision disturbances (%) | |||||||||
| Grade 1–2 | 31 | 31 | 9.7 | ||||||
| Conjunctivitis (%) | |||||||||
| Grade 3–4 | 1 | ||||||||
| Neuropathy (%) | |||||||||
| Grade 3–4 | 1 | ||||||||
| Erythema multiform (%) | |||||||||
| Grade 1–2 | 25 | ||||||||
| Grade 3–4 | 7.5 | ||||||||
| Dizziness (%) | |||||||||
| Grade 1–2 | 3 | ||||||||
| Grade 3–4 | 1 | ||||||||
| Proteinuria (%) | |||||||||
| Grade 1–2 | 20 | ||||||||
| Grade 3–4 | 8 | ||||||||
| Arterial hypertension (%) | |||||||||
| Grade 1–2 | 7 | 3–16 | |||||||
| Intracranial hemorrhage (%) | |||||||||
| Grade 1–2 | 4 | ||||||||
| Epistaxis (%) | |||||||||
| Grade 1–2 | 4 | ||||||||
| Gastrointestinal bleeding (%) | 8–12 | ||||||||
| Gastric perforation (%) | |||||||||
| Grade 3–4 | 4 | ||||||||
| Pneumothorax (%) | |||||||||
| Grade 3–4 | 4 | ||||||||
| Pneumonia (%) | |||||||||
| Grade 1–2 | 8 | ||||||||
| Cardiac toxicity (%) | |||||||||
| Grade 3–4 | 3 | 3 | |||||||
| Colonic perforation (%) | |||||||||
| Grade 3–4 | 3 | ||||||||
| Dysphonia (%) | |||||||||
| Grade 1–2 | 3 | ||||||||
| Endobronchial cavitation (%) | 3 | ||||||||
| Hemorrhages (%) | |||||||||
| Grade 1–2 | 3 | ||||||||
| Grade 3–4 | 3 |
| Molecules | Afatinib | Gefitinib | Osimertinib | Crizotinib | Ceritinib | Alectinib | Erlotinib | Combination of TKI and Chemotherapy |
|---|---|---|---|---|---|---|---|---|
| Publications | Tanaka 2018 [26], Imai 2018 [27], Corre 2018 [36], Minegishi 2021 [28] | Douillard 2014 [52], Inomata 2016 [58], Tateishi 2013 [29], Fujita 2012 [30], Morikawa 2015 [31], Maemondo 2012 [32], Takahashi 2014 [33], Kuwako 2015 [34], Asami 2011 [35], Corre 2018 [36], Wu 2015 [59], Kobayashi 2011 [67] | Furuta 2018 [37], Nakao 2020 [38], Nakao 2019 [60], Auliac 2019 [39], Kato 2019 [40] | Bedas 2019 [55] | Bedas 2019 [55] | Bedas 2019 [55] | Minemura 2015 [61], Stinchcombe 2011 [76], Rossi 2010 [62], Yoshioka 2014 [63], Merimsky 2012 [72], Kurishima 2013 [73], Brueckl 2018 [64], Corre 2018 [36], Heigener 2014 [68], Chen 2012 [69], Inoue 2015 [42], Yamada 2016 [65] | Aoshima 2020 [66], Tam 2013 [74] |
| Median duration of treatment (months) | 4.0 (1–69) | 1.6–8.0 | 15.0 ± 9 | 4.2 | 5.8 | 5.0 | 1–39 1–9 1.7–6.2 | 10.4 |
| Dose reduction (%) | 47.5–89 | 20–45 | 19–39 | 21 | 60 | 44 | 7–56 | 64 |
| Treatment discontinuation due to toxicity (%) | 5–21 | 3–52 | 9–28 | 21 | 60 | 4–45 | ||
| Dose reduction due to toxicity (%) | 17 | 9–28 | 7–56 | 64 |
| Molecules | Afatinib | Gefitinib | Osimertinib | Crizotinib | Ceritinib | Alectinib | Erlotinib | Combination of TKI |
|---|---|---|---|---|---|---|---|---|
| Publications | Wu 2018 [44] | Wu 2018 [44] | Quoix 2013 [71] | Gridelli 2011 [70] | ||||
| Median duration of treatment (months) | 12 | 12 | 2.0–2.2 | |||||
| Dose reduction (%) | 42 | |||||||
| Treatment discontinuation due to toxicity (%) | 9 | 14 | 12 | 21 | ||||
| Dose reduction due to toxicity (%) | 42 | 29 |
| Publication | Treatment | Median Age (Years) | Comorbidities-Charlson Scale (CCI) or Frailty Scales (%) | Quality of Life | CGA |
|---|---|---|---|---|---|
| Aoshima 2020 [66] | Erlotinib 150 mg/day + bevacizumab | 80 | CCI = 1: 36%, CCI = 2: 4%, CCI ≥ 3: 8% | ||
| Stinchcombe 2011 [76] | Erlotinib 100 mg/day + chemotherapy or erlotinib alone (150 mg/day) or chemotherapy alone | 76 | CIRS-G frailty scale | No differences in quality of life | |
| Morikawa 2015 [31] | Gefitinib 250 mg/day or carboplatin/paclitaxel | 75 | No differences in the quality of life domains of pain and dyspnea, anxiety, and daily functioning between <70 and >70 years groups | ||
| Takahashi 2014 [33] | Gefitinib 250 mg/day | 79.5 | Shortness of breath and cough improved significantly after 4 weeks of treatment | ||
| Miyamoto 2020 [41] | Erlotinib 50 mg/day | 80 | CCI ≥ 6 was the cut off for frailty | ||
| Corre 2018 [36] | Gefitinib or erlotinib or afatinib | 83.9 | CGA was performed for 35% of patients | ||
| Inoue 2015 [42] | Erlotinib 150 mg/day | 80 | CCI 1–2: 44% CCI ≥ 3: 6% | ||
| Chen 2012 [69] | Erlotinib 150 mg/day or vinorelbine | 77 | Patients in the erlotinib arm had significantly better physical well-being than patients in the vinorelbine arm | ||
| Quoix 2013 [71] | Erlotinib 150 mg/day (second line) | CCI ≤ 2: 68% | MMSE < 24: 52% ADL < 6: 49% BMI < 21: 61.5% | ||
| Tam 2013 [74] | Erlotinib or gefitinib (first or second line) | 73 | CCI = 1: 18%, CCI = 2: 9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greillier, L.; Gauvrit, M.; Paillaud, E.; Girard, N.; Montégut, C.; Boulahssass, R.; Wislez, M.; Pamoukdjian, F.; Corre, R.; Cabart, M.; et al. Targeted Therapy for Older Patients with Non-Small Cell Lung Cancer: Systematic Review and Guidelines from the French Society of Geriatric Oncology (SoFOG) and the French-Language Society of Pulmonology (SPLF)/French-Language Oncology Group (GOLF). Cancers 2022, 14, 769. https://doi.org/10.3390/cancers14030769
Greillier L, Gauvrit M, Paillaud E, Girard N, Montégut C, Boulahssass R, Wislez M, Pamoukdjian F, Corre R, Cabart M, et al. Targeted Therapy for Older Patients with Non-Small Cell Lung Cancer: Systematic Review and Guidelines from the French Society of Geriatric Oncology (SoFOG) and the French-Language Society of Pulmonology (SPLF)/French-Language Oncology Group (GOLF). Cancers. 2022; 14(3):769. https://doi.org/10.3390/cancers14030769
Chicago/Turabian StyleGreillier, Laurent, Manon Gauvrit, Elena Paillaud, Nicolas Girard, Coline Montégut, Rabia Boulahssass, Marie Wislez, Frédéric Pamoukdjian, Romain Corre, Mathilde Cabart, and et al. 2022. "Targeted Therapy for Older Patients with Non-Small Cell Lung Cancer: Systematic Review and Guidelines from the French Society of Geriatric Oncology (SoFOG) and the French-Language Society of Pulmonology (SPLF)/French-Language Oncology Group (GOLF)" Cancers 14, no. 3: 769. https://doi.org/10.3390/cancers14030769
APA StyleGreillier, L., Gauvrit, M., Paillaud, E., Girard, N., Montégut, C., Boulahssass, R., Wislez, M., Pamoukdjian, F., Corre, R., Cabart, M., Caillet, P., Belaroussi, Y., Frasca, M., Noize, P., Wang, P., Mebarki, S., Mathoulin-Pelissier, S., & Couderc, A.-L. (2022). Targeted Therapy for Older Patients with Non-Small Cell Lung Cancer: Systematic Review and Guidelines from the French Society of Geriatric Oncology (SoFOG) and the French-Language Society of Pulmonology (SPLF)/French-Language Oncology Group (GOLF). Cancers, 14(3), 769. https://doi.org/10.3390/cancers14030769







