A Comprehensive Commentary on the Multilocular Cystic Renal Neoplasm of Low Malignant Potential: A Urologist’s Perspective
Abstract
:Simple Summary
Abstract
1. Introduction
2. Clinical Characteristics
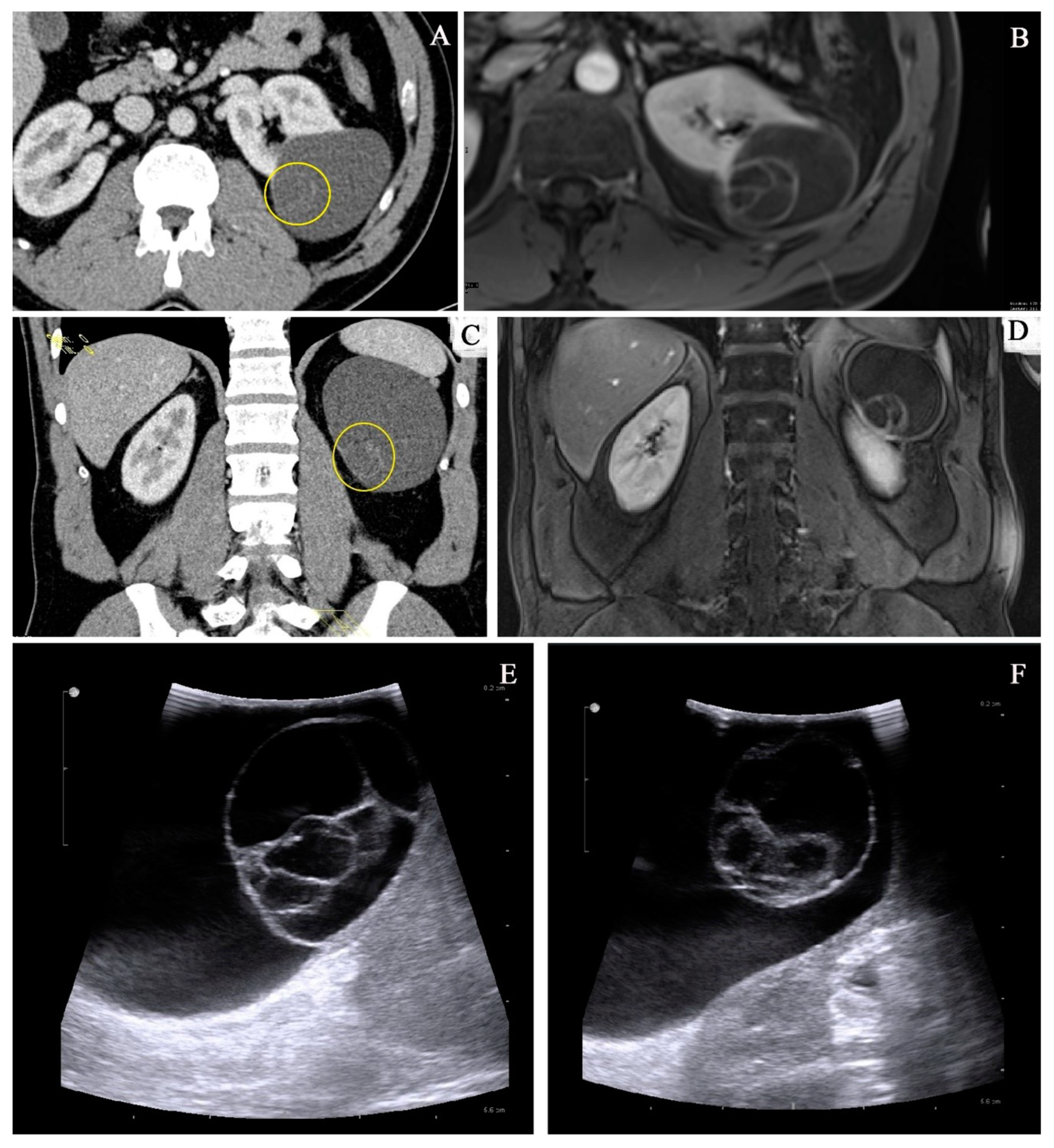
3. Imaging Studies
3.1. Bosniak Classification
3.2. Differential Diagnostics
4. Therapeutic Management
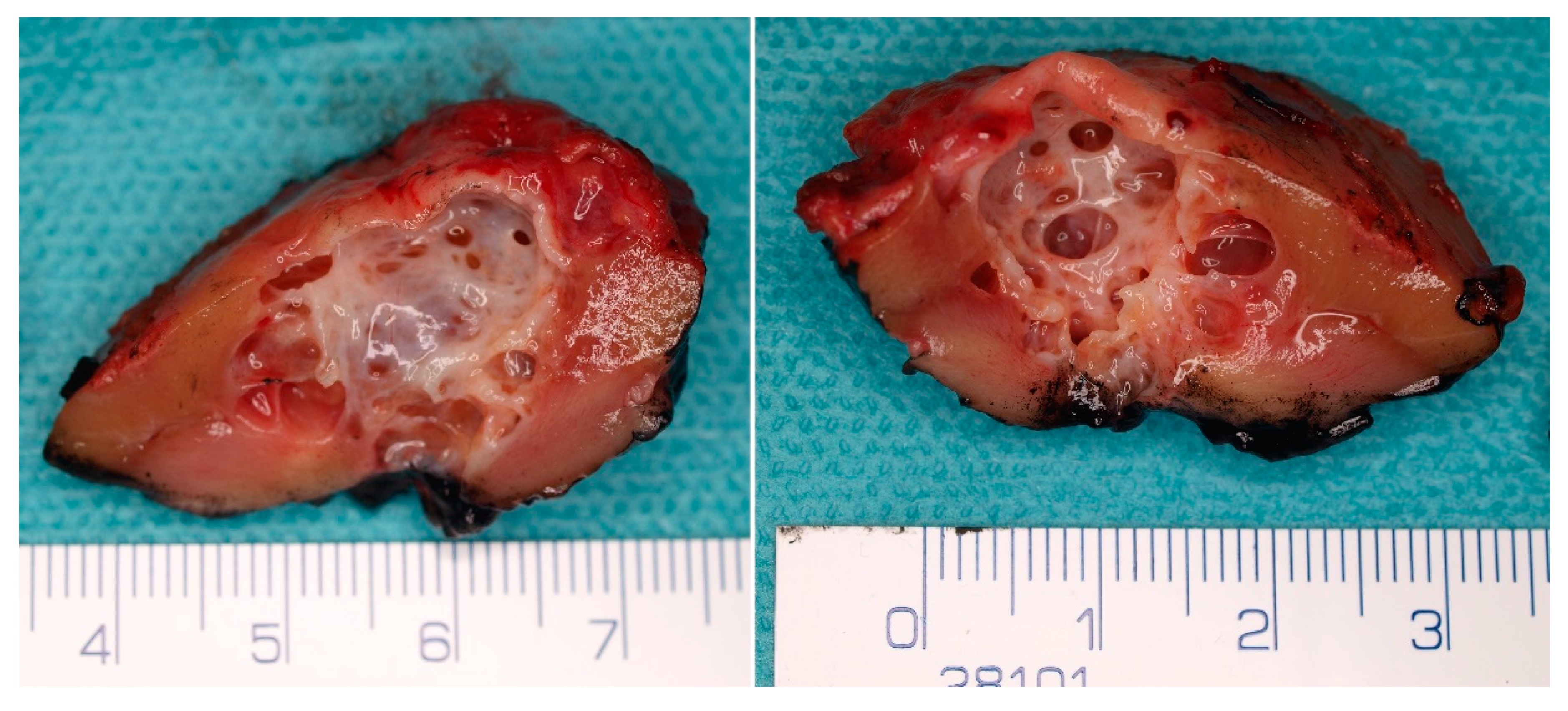
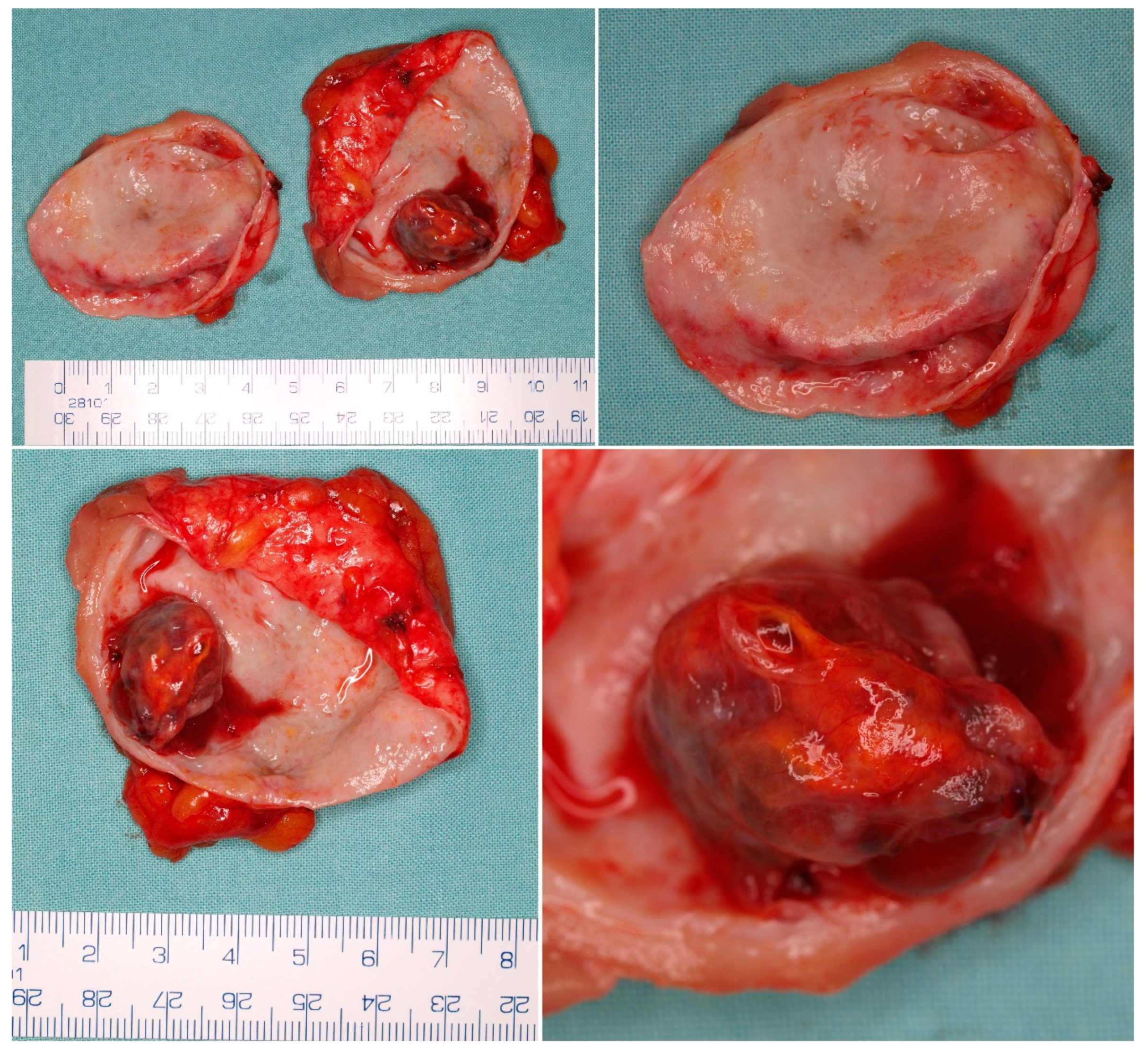
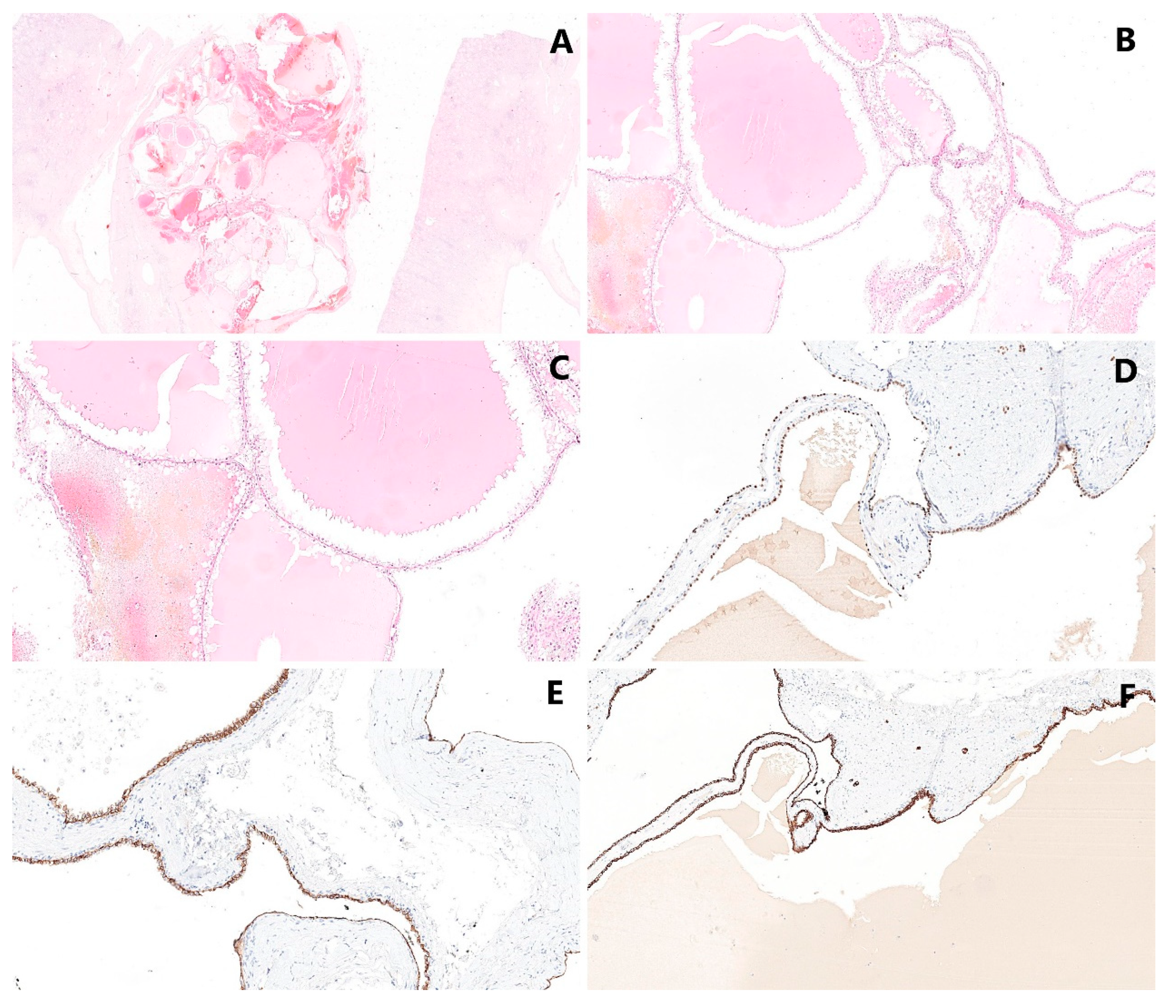
5. Pathological Findings
5.1. Macroscopic Findings
5.2. Microscopic Findings
5.3. Immunohistochemical Findings
5.4. Molecular Genetic Findings
6. Prognosis
7. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Lewis, R.H.; Clark, M.A.; Dobson, C.L.; O’Connell, K.J. Multilocular cystic renal adenocarcinoma arising in a solitary kidney. J Urol. 1982, 127, 314–316. [Google Scholar] [CrossRef]
- Murad, T.; Komaiko, W.; Oyasu, R.; Bauer, K. Multilocular cystic renal cell carcinoma. Am. J. Clin. Pathol. 1991, 95, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Corica, F.A.; Iczkowski, K.A.; Cheng, L.; Zincke, H.; Blute, M.L.; Wendel, A.; Sebo, T.J.; Neumann, R.; Botswick, D.G. Cystic renal cell carcinoma is cured by resection: A study of 24 cases with long-term followup. J. Urol. 1999, 161, 408–411. [Google Scholar] [CrossRef]
- Kristiansen, G.; Delahunt, B.; Srigley, J.R.; Lüders, C.; Lunkenheimer, J.M.; Gevensleben, H.; Thiesler, T.; Montironi, R.; Egevad, L. Vancouver classification of renal tumors: Recommendations of the 2012 consensus conference of the International Society of Urological Pathology (ISUP). Der Pathol. 2015, 36, 310–316. [Google Scholar]
- Srigley, J.R.; Delahunt, B.; Eble, J.N.; Egevad, L.; Epstein, J.I.; Grignon, D.; Hes, O.; Moch, H.; Montironi, R.; Tickoo, S.T.; et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am. J. Surg. Pathol. 2013, 37, 1469–1489. [Google Scholar] [CrossRef]
- Bhatt, J.R.; Jewett, M.A.; Richard, P.O.; Kawaguchi, S.; Timilshina, N.; Evans, A.; Alibhai, S.; Finelli, A. Multilocular Cystic Renal Cell Carcinoma: Pathological T Staging Makes No Difference to Favorable Outcomes and Should be Reclassified. J. Urol. 2016, 196, 1350–1355. [Google Scholar] [CrossRef]
- Chen, S.; Jin, B.; Xu, L.; Fu, G.; Meng, H.; Liu, B.; Li, J.; Xia, D. Cystic renal cell carcinoma: A report of 67 cases including 4 cases with concurrent renal cell carcinoma. BMC Urol. 2014, 14, 87. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Chen, J.; Jiang, Y.; Ning, X.; Peng, S.; Wang, J.; He, Q.; Yang, X.; Gong, K. Multilocular Cystic Renal Cell Neoplasm of Low Malignant Potential: A Series of 76 Cases. Clin Genitourin. Cancer 2016, 14, e553–e557. [Google Scholar] [CrossRef]
- Suzigan, S.; López-Beltrán, A.; Montironi, R.; Drut, R.; Romero, A.; Hayashi, T.; Gentili, A.L.C.; Fonseca, P.S.P.; deTorres, I.; Billis, A.; et al. Multilocular cystic renal cell carcinoma: A report of 45 cases of a kidney tumor of low malignant potential. Am. J. Clin. Pathol. 2006, 125, 217–222. [Google Scholar] [CrossRef]
- Tretiakova, M.; Mehta, V.; Kocherginsky, M.; Minor, A.; Shen, S.S.; Sirintrapun, S.J.; Yao, J.L.; Alvarado-Cabrero, I.; Antic, T.; Eggener, S.; et al. Predominantly cystic clear cell renal cell carcinoma and multilocular cystic renal neoplasm of low malignant potential form a low-grade spectrum. Virchows Arch. 2018, 473, 85–93. [Google Scholar] [CrossRef]
- Westerman, M.E.; Cheville, J.C.; Lohse, C.M.; Sharma, V.; Boorjian, S.A.; Leibovich, B.C.; Thompson, R.H. Long-Term Outcomes of Patients with Low Grade Cystic Renal Epithelial Neoplasms. Urology 2019, 133, 145–150. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Nassir, A.; Jollimore, J.; Gupta, R.; Bell, D.; Norman, R. Multilocular cystic renal cell carcinoma: A series of 12 cases and review of the literature. Urology 2002, 60, 421–427. [Google Scholar] [CrossRef]
- Agarwal, S.; Agrawal, U.; Mohanty, N.K.; Saxena, S. Multilocular cystic renal cell carcinoma: A case report of a rare entity. Arch. Pathol. Lab. Med. 2011, 135, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Sabhiki, A.; Abrari, A.; Sachdev, R.; Chawla, A.; Vaidya, A. Multilocular cystic renal cell carcinoma: A diagnostic rarity. Indian J. Pathol. Microbiol. 2008, 51, 457–458. [Google Scholar] [PubMed]
- Gong, K.; Zhang, N.; He, Z.; Zhou, L.; Lin, G.; Na, Y. Multilocular cystic renal cell carcinoma: An experience of clinical management for 31 cases. J. Cancer Res. Clin. Oncol. 2008, 134, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Curry, N.S.; Cochran, S.T.; Bissada, N.K. Cystic renal masses: Accurate Bosniak classification requires adequate renal CT. AJR Am. J. Roentgenol. 2000, 175, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Bosniak, M.A. The current radiological approach to renal cysts. Radiology 1986, 158, 1–10. [Google Scholar] [CrossRef]
- Bosniak, M.A. Diagnosis and management of patients with complicated cystic lesions of the kidney. AJR Am. J. Roentgenol. 1997, 169, 819–821. [Google Scholar] [CrossRef] [Green Version]
- Bosniak, M.A. The use of the Bosniak classification system for renal cysts and cystic tumors. J. Urol. 1997, 157, 1852–1853. [Google Scholar] [CrossRef]
- Israel, G.M.; Bosniak, M.A. An update of the Bosniak renal cyst classification system. Urology 2005, 66, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Hora, M.; Hes, O.; Michal, M.; Boudova, L.; Chudacek, Z.; Kreuzberg, B.; Klecka, J. Extensively cystic renal neoplasms in adults (Bosniak classification II or III)-possible common histological diagnoses: Multilocular cystic renal cell carcinoma, cystic nephroma, and mixed epithelial and stromal tumor of the kidney. Int. Urol. Nephrol. 2005, 37, 743–750. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Shim, M.; Jeong, I.G.; Song, C.; Kim, J.K.; Ro, J.Y.; Hong, J.H.; Ahn, H.; Kim, C.S. Multilocular cystic renal cell carcinoma: Clinicopathological features and preoperative prediction using multiphase computed tomography. BJU Int. 2011, 108, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Pitra, T.; Pivovarcikova, K.; Tupy, R.; Alaghehbandan, R.; Barakova, T.; Travnicek, I.; Prochazkova, K.; Klatte, T.; Chlosta, P.; Hes, O.; et al. Magnetic resonance imaging as an adjunct diagnostic tool in computed tomography defined Bosniak IIF-III renal cysts: A multicenter study. World J. Urol. 2018, 36, 905–911. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Reis, R.B.; Kajiwara, P.P.; Silva, G.E.; Elias, J.; Muglia, V.F. MRI evaluation of complex renal cysts using the Bosniak classification: A comparison to CT. Abdom Radiol. 2016, 41, 2011–2019. [Google Scholar] [CrossRef]
- Clevert, D.A.; Minaifar, N.; Weckbach, S.; Jung, E.M.; Stock, K.; Reiser, M.; Staehler, M. Multislice computed tomography versus contrast-enhanced ultrasound in evaluation of complex cystic renal masses using the Bosniak classification system. Clin. Hemorheol. Microcirc. 2008, 39, 171–178. [Google Scholar] [CrossRef]
- Graumann, O.; Osther, S.S.; Karstoft, J.; Hørlyck, A.; Osther, P.J. Bosniak classification system: A prospective comparison of CT, contrast-enhanced US, and MR for categorizing complex renal cystic masses. Acta Radiol. 2015, 57, 1409–1417. [Google Scholar] [CrossRef]
- Ignee, A.; Straub, B.; Brix, D.; Schuessler, G.; Ott, M.; Dietrich, C.F. The value of contrast enhanced ultrasound (CEUS) in the characterisation of patients with renal masses. Clin. Hemorheol. Microcirc. 2010, 46, 275–290. [Google Scholar] [CrossRef]
- Rübenthaler, J.; Bogner, F.; Reiser, M.; Clevert, D.A. Contrast-Enhanced Ultrasound (CEUS) of the Kidneys by Using the Bosniak Classification. Ultraschall Med. 2016, 37, 234–251. [Google Scholar] [CrossRef]
- Shan, K.; Fu, A.B.D.L.; Liu, N.; Cai, Q.; Fu, Q.; Liu, L.; Sun, X.; Zhang, Z. Contrast-enhanced Ultrasound (CEUS) vs contrast-enhanced computed tomography for multilocular cystic renal neoplasm of low malignant potential: A retrospective analysis for diagnostic performance study. Medicine 2020, 99, e23110. [Google Scholar] [CrossRef]
- Hindman, N.M. Imaging of Cystic Renal Masses. Radiol. Clin. N. Am. 2017, 55, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Robbin, M.L.; Lockhart, M.E.; Barr, R.G. Renal imaging with ultrasound contrast: Current status. Radiol. Clin. N. Am. 2003, 41, 963–978. [Google Scholar] [CrossRef]
- Cantisani, V.; Bertolotto, M.; Clevert, D.A.; Correas, J.M.; Drudi, F.M.; Fischer, T.; Gilja, O.H.; Granata, A.; Graumann, O.; Harvey, C.J.; et al. EFSUMB 2020 Proposal for a Contrast-Enhanced Ultrasound-Adapted Bosniak Cyst Categorization-Position Statement. Ultraschall Med. 2021, 42, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Edney, E.; Davenport, M.S.; Curci, N.; Schieda, N.; Krishna, S.; Hindman, N.; Silverman, S.G.; Pedrosa, I. Bosniak classification of cystic renal masses, version 2019: Interpretation pitfalls and recommendations to avoid misclassification. Abdom. Radiol. 2021, 46, 2699–2711. [Google Scholar] [CrossRef] [PubMed]
- Schieda, N.; Davenport, M.S.; Krishna, S.; Edney, E.A.; Pedrosa, I.; Hindman, N.; Baroni, R.H.; Curci, N.E.; Shinagare, A.; Silverman, S.G. Bosniak Classification of Cystic Renal Masses, Version 2019: A Pictorial Guide to Clinical Use. Radiographics 2022, 42, E33. [Google Scholar] [CrossRef]
- Silverman, S.G.; Pedrosa, I.; Ellis, J.H.; Hindman, N.M.; Schieda, N.; Smith, A.D.; Remer, E.M.; Shinagare, A.B.; Curci, N.E.; Raman, S.S.; et al. Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology 2019, 292, 475–488. [Google Scholar] [CrossRef] [Green Version]
- Sevcenco, S.; Spick, C.; Helbich, T.H.; Heinz, G.; Shariat, S.F.; Klingler, H.C.; Rauchenwald, M.; Baltzer, P.A. Malignancy rates and diagnostic performance of the Bosniak classification for the diagnosis of cystic renal lesions in computed tomography—A systematic review and meta-analysis. Eur. Radiol. 2017, 27, 2239–2247. [Google Scholar] [CrossRef] [Green Version]
- Schoots, I.G.; Zaccai, K.; Hunink, M.G.; Verhagen, P.C.M.S. Bosniak Classification for Complex Renal Cysts Reevaluated: A Systematic Review. J. Urol. 2017, 198, 12–21. [Google Scholar] [CrossRef]
- Israel, G.M.; Bosniak, M.A. Follow-up CT of moderately complex cystic lesions of the kidney (Bosniak category IIF). AJR Am. J. Roentgenol. 2003, 181, 627–633. [Google Scholar] [CrossRef]
- Weibl, P.; Hora, M.; Kollarik, B.; Shariat, S.F.; Klatte, T. Management, pathology and outcomes of Bosniak category IIF and III cystic renal lesions. World J. Urol. 2015, 33, 295–300. [Google Scholar] [CrossRef]
- Weibl, P.; Hora, M.; Kollarik, B.; Kalusova, K.; Pitra, T.; Remzi, M.; Hübner, W.; Balzer, P.; Klatte, T. A practical guide and decision-making protocol forthe management of complex renal cystic masses. Arab. J. Urol. 2017, 15, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bensalah, K.; Dabestani, S.; Montes, S.F.; Gilles, R.H.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur. Urol. 2019, 75, 799–810. [Google Scholar] [CrossRef]
- Singhai, A.; Babu, S.; Verma, N.; Singh, V. Multilocular cystic renal cell carcinoma: A rare entity. BMJ Case Rep. 2013. [Google Scholar] [CrossRef]
- Kuroda, N.; Ohe, C.; Mikami, S.; Inoue, K.; Nagashima, Y.; Cohen, R.J.; Pan, C.-C.; Michal, M.; Hes, O. Multilocular cystic renal cell carcinoma with focus on clinical and pathobiological aspects. Histol. Histopathol. 2012, 27, 969–974. [Google Scholar] [PubMed]
- Montironi, R.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M. Words of wisdom: Re: Multilocular cystic renal cell carcinoma with focus on clinical and pathobiological aspects. Eur. Urol. 2013, 63, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.R.; Halat, S.; Eble, J.N.; Grignon, D.J.; Lopez-Beltran, A.; Montironi, R.; Tan, P.-H.; Wang, M.; Maclennan, G.T.; Baldridge, L.A.; et al. Multilocular cystic renal cell carcinoma: Similarities and differences in immunoprofile compared with clear cell renal cell carcinoma. Am. J. Surg. Pathol. 2012, 36, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- von Teichman, A.; Compérat, E.; Behnke, S.; Storz, M.; Moch, H.; Schraml, P. VHL mutations and dysregulation of pVHL- and PTEN-controlled pathways in multilocular cystic renal cell carcinoma. Mod. Pathol. 2011, 24, 571–578. [Google Scholar] [CrossRef]
- Li, G.; Bilal, I.; Gentil-Perret, A.; Feng, G.; Zhao, A.; Peoc'h, M.; Genin, C.; Tostain, J.; Gigante, M. CA9 as a molecular marker for differential diagnosis of cystic renal tumors. Urol. Oncol. 2012, 30, 463–468. [Google Scholar] [CrossRef]
- Gonzalez, M.L.; Alaghehbandan, R.; Pivovarcikova, K.; Michalova, K.; Rogala, J.; Martinek, P.; Foix, M.P.; Mundo, E.C.; Comperat, E.; Ulamec, M.; et al. Reactivity of CK7 across the spectrum of renal cell carcinomas with clear cells. Histopathology 2019, 74, 608–617. [Google Scholar] [CrossRef]
- Ostler, D.A.; Prieto, V.G.; Reed, J.A.; Deavers, M.T.; Lazar, A.J.; Ivan, D. Adipophilin expression in sebaceous tumors and other cutaneous lesions with clear cell histology: An immunohistochemical study of 117 cases. Mod. Pathol. 2010, 23, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Park, B.; Joo, J.; Joung, J.Y.; Seo, H.K.; Lee, K.H.; Park, W.S.; Chung, J. Retrospective analysis of 25 immunohistochemical tissue markers for differentiating multilocular cystic renal neoplasm of low malignant potential and multicystic renal cell carcinoma. Histol. Histopathol. 2018, 33, 589–596. [Google Scholar]
- Halat, S.; Eble, J.N.; Grignon, D.J.; Lopez-Beltran, A.; Montironi, R.; Tan, P.H.; Wang, M.; Zhang, S.; MacLennan, G.T.; Cheng, L. Multilocular cystic renal cell carcinoma is a subtype of clear cell renal cell carcinoma. Mod. Pathol. 2010, 23, 931–936. [Google Scholar] [CrossRef]
- Raspollini, M.R.; Castiglione, F.; Martignoni, G.; Cheng, L.; Montironi, R.; Lopez-Beltran, A. Unlike in clear cell renal cell carcinoma, KRAS is not mutated in multilocular cystic clear cell renal cell neoplasm of low potential. Virchows Arch. 2015, 467, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, W.S.; Chung, J. SETD2, GIGYF2, FGFR3, BCR, KMT2C, and TSC2 as candidate genes for differentiating multilocular cystic renal neoplasm of low malignant potential from clear cell renal cell carcinoma with cystic change. Investig. Clin. Urol. 2019, 60, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Delahunt, B.; Cheville, J.C.; Martignoni, G.; Humphrey, P.A.; Magi-Galluzzi, C.; McKenney, J.; Egevad, L.; Algaba, F.; Moch, H.; Grignon, D.J.; et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am. J. Surg. Pathol. 2013, 37, 1490–1504. [Google Scholar] [CrossRef] [Green Version]
- Eble, J.N.; Bonsib, S.M. Extensively cystic renal neoplasms: Cystic nephroma, cystic partially differentiated nephroblastoma, multilocular cystic renal cell carcinoma, and cystic hamartoma of renal pelvis. Semin. Diagn. Pathol. 1998, 15, 2–20. [Google Scholar] [PubMed]
- Eble, J.N.; Sauter, G.; Epstein, J.I.; Sesterhenn, I.A. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs; IARC Press: Lyon, France, 2004. [Google Scholar]
- Aubert, S.; Zini, L.; Delomez, J.; Biserte, J.; Lemaitre, L.; Leroy, X. Cystic renal cell carcinomas in adults. Is preoperative recognition of multilocular cystic renal cell carcinoma possible? J. Urol. 2005, 174, 2115–2119. [Google Scholar] [CrossRef]
- Hindman, N.M.; Bosniak, M.A.; Rosenkrantz, A.B.; Lee-Felker, S.; Melamed, J. Multilocular cystic renal cell carcinoma: Comparison of imaging and pathologic findings. AJR Am. J. Roentgenol. 2012, 198, W20–W26. [Google Scholar] [CrossRef]
- Raspollini, M.R.; Montagnani, I.; Montironi, R.; Cheng, L.; Martignoni, G.; Minervini, A.; Serni, S.; Nicita, G.; Carini, M.; Lopez-Beltran, A. A contemporary series of renal masses with emphasis on recently recognized entities and tumors of low malignant potential: A report based on 624 consecutive tumors from a single tertiary center. Pathol. Res. Pract. 2017, 213, 804–808. [Google Scholar] [CrossRef]
- Rübenthaler, J.; Paprottka, K.J.; Marcon, J.; Reiser, M.; Clevert, D.A. MRI and contrast enhanced ultrasound (CEUS) image fusion of renal lesions. Clin. Hemorheol. Microcirc. 2016, 64, 457–466. [Google Scholar] [CrossRef]
- Sanz, E.; Hevia, V.; Gómez, V.; Álvarez, S.; Fabuel, J.J.; Martínez, L.; Rodriguez-Patrón, R.; González-Gordaliza, C.; Burgos, F.-J. Renal Complex Cystic Masses: Usefulness of Contrast-Enhanced Ultrasound (CEUS) in Their Assessment and Its Agreement with Computed Tomography. Curr. Urol. Rep. 2016, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Katabathina, V.S.; Garg, D.; Prasad, S.R.; Vikram, R. Cystic renal neoplasms and renal neoplasms associated with cystic renal diseases in adults: Cross-sectional imaging findings. J. Comput. Assist. Tomogr. 2012, 36, 659–668. [Google Scholar] [CrossRef] [PubMed]
| Entity in Differential Diagnosis | Clinical Characteristics | Imaging Studies | Macroscopic Findings | Microscopic Findings | Immunoprofile | Molecular Genetic Findings |
|---|---|---|---|---|---|---|
| MCRNLMP | Indolent behavior, frequently incidental finding, no clinical symptoms. | Mostly Bosniak III on CT/MRI | Variably large non-communicating cysts, no solid component | Cystic spaces lined by clear cells lining, low grade nuclei (WHO/ISUP grade 1–2), no expansile/solid nodular growth, no necrosis, no vascular invasion, no sarcomatoid changes | PAX8 +, CANH +, CK7 +, AMACR −, ER −, PR − | Chromosome 3p deletion, VHL mutation |
| Renalcorticalcyst | Benign, symptoms only in big size lesion | Bosniak I or II on CT | Usually unilocular, thin-walled cortical cyst | Cystic space lined by single layer of cuboidal/flattened cells/atrophic epithelium | PAX8 − | No specific changes |
| CCRCC with cystic changes (or regressive changes) | Malignant lesion with favorable behavior compared with CCRCC | Bosniak III or IV on CT/MRI | Solid component, necrosis, hemorrhage may be present | Composed of cells with clear cytoplasm and distinct membrane, solid nodule present at least focally; necrosis, vascular invasion, and sarcomatoid changes may be present, even high-grade feature | PAX8 +, CANH +, CD10 +, AE1/3 +, Vimentin +, CK7 +/− (usually −/focally), AMACR −(usually), TFE3 −, HMB45 −, Melan A − | Chromosome 3p deletion, VHL mutation, VHL promoter methylation |
| MEST | Usually perimenopausal women, benign with possible rare malignant transformation | Bosniak III or IV on CT/MRI | Solitary, well circumscribed (unencapsulated), mixture of solid and cystic areas | Stromal (collagenous/edematous/spindle/ovarian-like) and epithelial (cysts of various size with flat/cuboidal/columnar/hobnail epithelial lining) component | PAX8 + (epithelium), ER + (stroma), PR + (stroma), inhibin + (stroma), HMB45 −, Melan A − | No specific changes |
| MiT family RCC (some variant of Xp11 translocation RCC [33] | Malignant, rare entity | Mostly Bosniak III or IV on CT/MRI | Multicystic mass, with a circumscribed appearance | Well-delimited, multilocular cystic lesion with thin membranous and fibrous septa, lined by a single layer of cell with clear to eosinophilic cytoplasm, WHO/ISUP grade 1/2 nuclei, no solid nodule | Cytokeratins +/−, TFE3 +, PAX8 +, CANH − | TFE3 gene rearrangements (MED15-TFE3 gene fusion) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitra, T.; Pivovarcikova, K.; Alaghehbandan, R.; Bartos Vesela, A.; Tupy, R.; Hora, M.; Hes, O. A Comprehensive Commentary on the Multilocular Cystic Renal Neoplasm of Low Malignant Potential: A Urologist’s Perspective. Cancers 2022, 14, 831. https://doi.org/10.3390/cancers14030831
Pitra T, Pivovarcikova K, Alaghehbandan R, Bartos Vesela A, Tupy R, Hora M, Hes O. A Comprehensive Commentary on the Multilocular Cystic Renal Neoplasm of Low Malignant Potential: A Urologist’s Perspective. Cancers. 2022; 14(3):831. https://doi.org/10.3390/cancers14030831
Chicago/Turabian StylePitra, Tomas, Kristyna Pivovarcikova, Reza Alaghehbandan, Adriena Bartos Vesela, Radek Tupy, Milan Hora, and Ondrej Hes. 2022. "A Comprehensive Commentary on the Multilocular Cystic Renal Neoplasm of Low Malignant Potential: A Urologist’s Perspective" Cancers 14, no. 3: 831. https://doi.org/10.3390/cancers14030831
APA StylePitra, T., Pivovarcikova, K., Alaghehbandan, R., Bartos Vesela, A., Tupy, R., Hora, M., & Hes, O. (2022). A Comprehensive Commentary on the Multilocular Cystic Renal Neoplasm of Low Malignant Potential: A Urologist’s Perspective. Cancers, 14(3), 831. https://doi.org/10.3390/cancers14030831






