Twenty-Year Survival of Patients Operated on for Non-Small-Cell Lung Cancer: The Impact of Tumor Stage and Patient-Related Parameters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Inclusions and General Management
2.2. Collected Data
2.3. Statistical Analysis
3. Results
3.1. Clinical Features
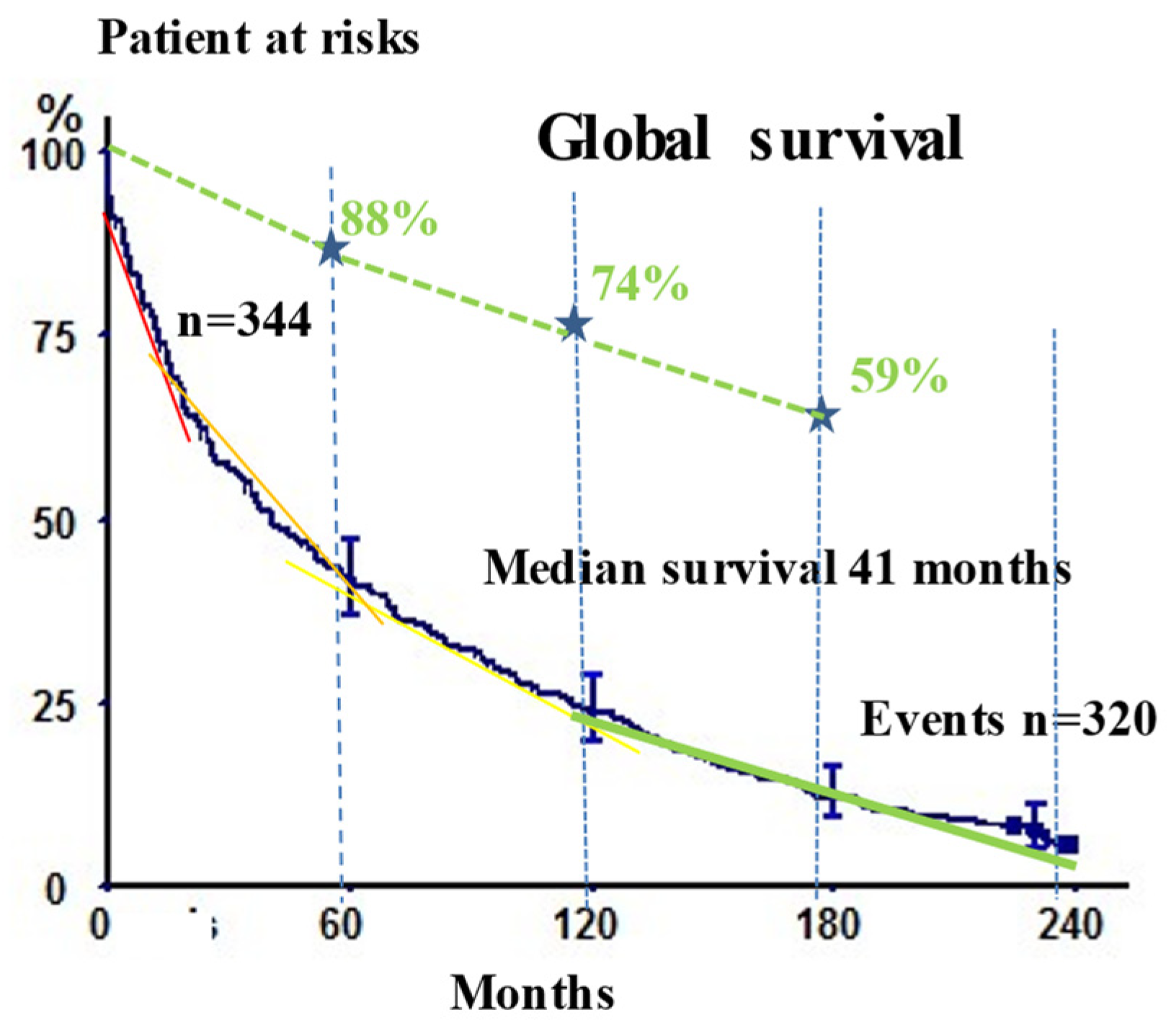
3.2. Long-Term Outcomes
3.2.1. Independent Risk Factors (Clinical and Biological) Affecting Long-Term Survival following Multivariate Cox Analyses
Independent Clinical Factors
Independent Risk Factors Affecting Very Late Mortality: Analysis of Clinical-pathological Parameters and Laboratory Parameters
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberg, A.J.; Ford, J.G.; Samet, J.M. American College of Chest Physicians. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007, 132, 29S–55S. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Youlden, D.R.; Cramb, S.; Baade, P. The International Epidemiology of Lung Cancer: Geographical Distribution and Secular Trends. J. Thorac. Oncol. 2008, 3, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Pandeya, N.; Byrnes, G.; Renehan, A.G.; Stevens, G.; Ezzati, M.; Ferlay, J.; Miranda, J.J.; Romieu, I.; Dikshit, R.; et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2014, 16, 36–46. [Google Scholar] [CrossRef]
- Custodio, A.B.; González-Larriba, J.L.; Bobokova, J.; Calles, A.; Álvarez, R.; Cuadrado, E.; Manzano, A.; Díaz-Rubio, E. Prognostic and Predictive Markers of Benefit from Adjuvant Chemotherapy in Early-Stage Non-small Cell Lung Cancer. J. Thorac. Oncol. 2009, 4, 891–910. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Yamada, Y.; Koh, E.; Yoshino, I.; Sekine, Y. Severity of Chronic Obstructive Pulmonary Disease and Its Relationship to Lung Cancer Prognosis after Surgical Resection. Thorac. Cardiovasc. Surg. 2012, 61, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Tewari, N.; Martin-Ucar, A.E.; Black, E.; Beggs, L.; Beggs, F.D.; Duffy, J.P.; Morgan, W.E. Nutritional status affects long term survival after lobectomy for lung cancer. Lung Cancer 2007, 57, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.; Nilsen, T.I.L.; Hatlen, E.; Sørensen, K.S.; Hole, T.; Haaverstad, R. Predictors of long time survival after lung cancer surgery: A retrospective cohort study. BMC Pulm. Med. 2008, 8, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Båtevik, R.; Grong, K.; Segadal, L.; Stangeland, L. The female gender has a positive effect on survival independent of background life expectancy following surgical resection of primary non-small cell lung cancer: A study of absolute and relative survival over 15 years. Lung Cancer 2005, 47, 173–181. [Google Scholar] [CrossRef]
- Xie, D.; Deschamps, C.; Shen, R.K.; Deng, B.; Wampfler, J.A.; Cassivi, S.D.; Nichols, F.C.; Allen, M.S.; Wigle, D.A.; Yang, P. Bilobectomy Versus Lobectomy for Non-Small Cell Lung Cancer: A Comparative Study of Outcomes, Long-Term Survival, and Quality of Life. Ann. Thorac. Surg. 2015, 100, 242–250. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Alifano, A.; Daffre, E.; Iannelli, A.; Brouchet, L.; Falcoz, P.E.; Le Pimpec-Barthes, F.; Bernard, A.; Pages, P.B.; Thomas, P.A.; Dahan, M.; et al. The reality of lung paradox: The impact of body mass index on mlong-term survival of resected lung cancer. A French Nationwide analysis form the epithor Database. Cancer J. 2021, 13, 4574. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shen, Y.; Tan, L.; Li, W. Prognostic Value of Sarcopenia in Lung Cancer: A Systematic Review and Meta-analysis. Chest 2019, 156, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Icard, P.; Schussler, O.; Loi, M.; Bobbio, A.; Lupo, A.M.; Wislez, M.; Iannelli, A.; Fournel, L.; Damotte, D.; Alifano, M. Pre-Disease and Pre-Surgery BMI, Weight Loss and Sarcopenia Impact Survival of Resected Lung Cancer Independently of Tumor Stage. Cancers 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, Z.; Huang, J.; Fan, J.; Du, H.; Liu, L.; Che, G. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: Does the ‘obesity paradox’ really exist? Eur. J. Cardiothorac. Surg. 2017, 51, 817–828. [Google Scholar] [CrossRef]
- Jiang, M.; Fares, A.F.; Shepshelovich, D.; Yang, P.; Christiani, D.; Zhang, J.; Shiraishi, K.; Ryan, B.M.; Chen, C.; Schwartz, A.G.; et al. The relationship between body-mass index and overall survival in non-small cell lung cancer by sex, smoking status, and race: A pooled analysis of 20,937 International lung Cancer consortium (ILCCO) patients. Lung Cancer 2020, 152, 58–65. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, S. Body mass index and lung cancer risk in never smokers: A meta-analysis. BMC Cancer 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [Green Version]
- Bugge, A.; Kongerud, J.; Brunborg, C.; Solberg, S.; Lund, M.B. Gender-specific survival after surgical resection for early stage non-small cell lung cancer. Acta Oncol. 2016, 56, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Onaitis, M.W.; Furnary, A.P.; Kosinski, A.S.; Kim, S.; Boffa, D.; Tong, B.C.; Cowper, P.; Jacobs, J.P.; Wright, C.D.; Putnam, J.B.; et al. Prediction of Long-Term Survival After Lung Cancer Surgery for Elderly Patients in The Society of Thoracic Surgeons General Thoracic Surgery Database. Ann. Thorac. Surg. 2017, 105, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Bugge, A.; Lund, M.B.; Brunborg, C.; Solberg, S.; Kongerud, J. Survival After Surgical Resection for Lung Cancer in Patients With Chronic Obstructive Pulmonary Disease. Ann. Thorac. Surg. 2016, 101, 2125–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghu, G.; Nyberg, F.; Morgan, G. The epidemiology of interstitial lung disease and its association with lung cancer. Br. J. Cancer 2004, 91, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Suda, T.; Naito, T.; Enomoto, N.; Hashimoto, D.; Fujisawa, T.; Nakamura, Y.; Inui, N.; Nakamura, H.; Chida, K. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology 2009, 14, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Watanabe, A.; Kondo, H.; Kanzaki, M.; Okubo, K.; Yokoi, K.; Matsumoto, K.; Marutsuka, T.; Shinohara, H.; Teramukai, S.; et al. Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J. Thorac. Cardiovasc. Surg. 2014, 149, 64–70.e2. [Google Scholar] [CrossRef] [Green Version]
- Strand, T.E.; Rostad, H.; Moller, B.; Norstein, J. Survival after resection for primary lung cancer: A population based study of 3211 resected patients. Thorax 2006, 61, 710–715. [Google Scholar] [CrossRef] [Green Version]
- Khullar, O.V.; Gillespie, T.; Nickleach, D.C.; Liu, Y.; Higgins, K.; Ramalingam, S.; Lipscomb, J.; Fernandez, F.G. Socioeconomic Risk Factors for Long-Term Mortality after Pulmonary Resection for Lung Cancer: An Analysis of More than 90,000 Patients from the National Cancer Data Base. J. Am. Coll. Surg. 2014, 220, 156–168.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, F.G.; Furnary, A.P.; Kosinski, A.S.; Onaitis, M.W.; Kim, S.; Boffa, D.; Cowper, P.; Jacobs, J.P.; Wright, C.D.; Putnam, J.B., Jr. Lon-gitudinal Follow-up of Lung Cancer Resection From the Society of Thoracic Surgeons General Thoracic Surgery Database in Patients 65 Years and Older. Ann. Thorac. Surg. 2016, 101, 2067–2076. [Google Scholar] [CrossRef] [Green Version]
- Farjah, F.; Flum, D.R.; Varghese, T.K., Jr.; Symons, R.G.; Wood, D.E. Surgeon specialty and long-term survival after pulmonary re-section for lung cancer. Ann. Thorac. Surg. 2009, 87, 995–1004, discussion 1005–1006. [Google Scholar] [CrossRef]
- Schussler, O.; Alifano, M.; Dermine, H.; Strano, S.; Casetta, A.; Sepulveda, S.; Chafik, A.; Coignard, S.; Rabbat, A.; Regnard, J.F. Postop-erative pneumonia after major lung resection. Am. J. Respir. Crit. Care. Med. 2006, 173, 1161–1169. [Google Scholar] [CrossRef]
- Schussler, O.; Dermine, H.; Alifano, M.; Casetta, A.; Coignard, S.; Roche, N.; Strano, S.; Meunier, A.; Salvi, M.; Magdeleinat, P.; et al. Should We Change Antibiotic Prophylaxis for Lung Surgery? Postoperative Pneumonia Is the Critical Issue. Ann. Thorac. Surg. 2008, 86, 1727–1733. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.; Yatabe, Y.; Powell, C.A.; Beer, D.; Riely, G.; Garg, K.; et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: International Multidisciplinary Classification of Lung Adenocarcinoma: Executive Summary. Proc. Am. Thorac. Soc. 2011, 8, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Alifano, M.; Mansuet-Lupo, A.; Lococo, F.; Roche, N.; Bobbio, A.; Canny, E.; Schussler, O.; Dermine, H.; Régnard, J.-F.; Burroni, B.; et al. Systemic Inflammation, Nutritional Status and Tumor Immune Microenvironment Determine Outcome of Resected Non-Small Cell Lung Cancer. PLoS ONE 2014, 9, e106914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobin, L.H.; Compton, C.C. TNM seventh edition: What’s new, what’s changed: Communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer 2010, 116, 5336–5339. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strano, S.; Lupo, A.; Lococo, F.; Schussler, O.; Loi, M.; Younes, M.; Bobbio, A.; Damotte, D.; Regnard, J.-F.; Alifano, M. Prognostic Significance of Vascular and Lymphatic Emboli in Resected Pulmonary Adenocarcinoma. Ann. Thorac. Surg. 2013, 95, 1204–1210. [Google Scholar] [CrossRef]
- Vestbo, J.; Prescott, E.; Lange, P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pul-monary disease morbidity. Copenhagen City Heart Study Group. Am. J. Respir. Crit. Care. Med. 1996, 153, 1530–1535. [Google Scholar] [CrossRef]
- Morel, H.; Raynard, B.; D’Arlhac, M.; Hauss, P.-A.; Lecuyer, E.; Oliviero, G.; Marty, C.; Gury, J.-P.; Asselain, B.; Grivaux, M.; et al. Prediagnosis weight loss, a stronger factor than BMI, to predict survival in patients with lung cancer. Lung Cancer 2018, 126, 55–63. [Google Scholar] [CrossRef]
- Kesimer, M.; Ford, A.A.; Ceppe, A.; Radicioni, G.; Cao, R.; Davis, C.W.; Doerschuk, C.M.; Alexis, N.E.; Anderson, W.H.; Henderson, A.G.; et al. Airway Mucin Concentration as a Marker of Chronic Bronchitis. N. Engl. J. Med. 2017, 377, 911–922. [Google Scholar] [CrossRef]
- Mytelka, D.S.; Li, L.; Benoit, K. Post-diagnosis weight loss as a prognostic factor in non-small cell lung cancer. J. Cachex- Sarcopenia Muscle 2017, 9, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Gallup, M.; Zlock, L.; Basbaum, C.; Finkbeiner, W.E.; McNamara, N.A. Cigarette smoke disrupts the integrity of airway adherens junctions through the aberrant interaction of p120-catenin with the cytoplasmic tail of MUC1. J. Pathol. 2012, 229, 74–86. [Google Scholar] [CrossRef]
- Lappi-Blanco, E.; Mäkinen, J.M.; Lehtonen, S.; Karvonen, H.; Sormunen, R.; Laitakari, K.; Johnson, S.; Mäkitaro, R.; Bloigu, R.; Kaarteenaho, R. Mucin-1 correlates with survival, smoking status, and growth patterns in lung adenocarcinoma. Tumor Biol. 2016, 37, 13811–13820. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Busquets, S.; Lopez-Soriano, F.J. Cancer cachexia, a clinical challenge. Curr. Opin. Oncol. 2019, 31, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, J.; Van Der Valk, S.W.; Vos, H.L.; Sonnenberg, A.; Hilkens, J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J. Cell Biol. 1995, 129, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, H.; Carraway, K.L. MUC1 and MUC4: Switching the Emphasis from Large to Small. Cancer Biother. Radiopharm. 2011, 26, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Icard, P.; Shulman, S.; Farhat, D.; Steyaert, J.M.; Alifano, M.; Lincet, H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat. 2018, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Merikallio, H.; Kaarteenaho, R.; Linden, S.; Padra, M.; Karimi, R.; Li, C.X.; Lappi-Blanco, E.; Wheelock, A.M.; Skold, M.C. Smok-ing-associated increase in mucins 1 and 4 in human airways. Respir. Res. 2020, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Qi, Y.; Xu, X.; Jiang, H.; Li, Z.; Yang, Q.; Zhang, C.; Zhang, K.; Chen, R.; Wang, J.; et al. Sputum mucin 1 is increased during the acute phase of chronic obstructive pulmonary disease exacerbation. J. Thorac. Dis. 2017, 9, 1873–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.J. Chronic obstructive pulmonary disease. N. Engl. J. Med. 2000, 343, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Houghton, A.M. Mechanistic links between COPD and lung cancer. Nat. Cancer 2013, 13, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.; Teixeira, A.L.; Coelho, A.; Araújo, A.; Medeiros, R. The Role of Inflammation in Lung Cancer. Inflamm. Cancer 2014, 816, 1–23. [Google Scholar] [CrossRef]
- Bremnes, R.M.; Al-Shibli, K.; Donnem, T.; Sirera, R.; Al-Saad, S.; Andersen, S.; Stenvold, H.; Camps, C.; Busund, L.T. The role of tu-mor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: Emphasis on non-small cell lung cancer. J. Thorac. Oncol. 2010, 6, 824–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, T.; Toyazaki, T.; Chiba, N.; Ueda, Y.; Gotoh, M. Prognostic value of body mass index and change in body weight in postoperative outcomes of lung cancer surgery. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 560–566. [Google Scholar] [CrossRef]
- Katsui, K.; Ogata, T.; Watanabe, K.; Yoshio, K.; Kuroda, M.; Yamane, M.; Hiraki, T.; Kiura, K.; Toyooka, S.; Kanazawa, S. Sarcopenia is related to poor prognosis in patients after trimodality therapy for locally advanced non-small cell lung cancer. Int. J. Clin. Oncol. 2021, 26, 1450–1460. [Google Scholar] [CrossRef]




| Variable | n (%) or Mean ± SD |
|---|---|
| Total | n = 345 |
| Sex | |
| Female, n0 (%) | 54 (15.7) |
| Men, n0 (%) | 291 (84.3) |
| Age, mean (SD) yrs | 62.1 (10.5) |
| Smoking | |
| Past or current smoker, n0 (%) | 319 (93.3) |
| Smoking cessation before surgery, n0 (%) | 182 (57.4) |
| Cumulative smoking: packs/year index, mean (SD) | 44.9 (23.6) |
| <20 pks/yr., n0 (%) | 21 (7.1) |
| 20–50 pks/yr., n0 (%) | 155 (86.4) |
| 50–100 pks/yr., n0 (%) | 109 (36.9) |
| 100 ≤ pks/yr., n0 (%) | 10 (3.4) |
| Comorbid illnesses and respiratory status | |
| Alcohol abuse, n0 (%) | 89 (26.0) |
| Diabetes mellitus, n0 (%) | 42 (13.2) |
| Chronic bronchitis, n0 (%) | 219 (64.0) |
| COPD | 149 (44.5) |
| GOLD COPD | |
| GOLD 1 (80% ≤ FEV1), n0 (%) | 46 (31) |
| GOLD 2 (50% ≤ FEV1 < 80%), n0 (%) | 90 (60.8) |
| GOLD 3 (30% ≤ FEV1 < 50%), n0 (%) | 12 (8.1) |
| GOLD 4 (FEV1 < 30%), n0 (%) | 0 |
| FEV1 (% predicted), mean (SD) | 83 (19.7) |
| FEV1/FVC (%), mean (SD) | 70 (13.5) |
| FEV1 (% predicted) | |
| 80% ≤ FEV1, n0 (%) | 184 (53.9) |
| 70% ≤ FEV1 < 80%, n0 (%) | 81 (23.7) |
| 60% ≤ FEV1 < 70%, n0 (%) | 45 (13.2) |
| 50% ≤ FEV1 < 60% | 20 (5.8) |
| FEV1 < 50%, n0 (%) | 11 (3.2) |
| Surgical procedures | |
| Lobectomy, n0 (%) | 242 (70.1) |
| Bilobectomy, n0 (%) | 20 (5.8) |
| Pneumonectomy, n0 (%) | 83 (24.1) |
| Parietectomy, n0 (%) | 19 (5.5) |
| Preoperative treatments | |
| Chemotherapy, n0 (%) | 88 (22.9) |
| Radiotherapy, n0 (%) | 14 (4.1) |
| Histological types | |
| Adenocarcinoma, n0 (%) | 160 (47.9) |
| Squamous cell carcinoma, n0 (%) | 133 (39.8) |
| Large-cell carcinoma, n0 (%) | 25 (7.5) |
| Others +, n0 (%) | 16 (4.8) |
| Pathological stage and tumor characteristics | |
| Pathological stages (4 classes) | |
| I, n0 (%) | 138 (41.4) |
| II, n0 (%) | 72 (21.6.) |
| III, n0 (%) | 109 (32.7) |
| IV, n0 (%) | 14 (4.2) |
| Pathological stages (8 classes) | |
| IA, n0 (%) | 69 (20.7) |
| IB, n0 (%) | 48 (14.4) |
| IIA, n0 (%) | 45 (13.5) |
| IIB, n0 (%) | 44 (13.2) |
| IIIA, n0 (%) | 100 (30.0) |
| IIIB, n0 (%) | 19 (5.7) |
| IIIC, n0 (%) | 0 |
| IV, n0 (%) | 8 (2.4) |
| Morphomics and nutritional parameters | |
| Height, mean (SD) cm | 170 cm (160–180) |
| Weight, mean (SD) kg | 73.0 kg (57.8–88.2) |
| BMI | |
| Pre-surgery body mass index (BMI), mean (SD) | 24.4 (4.4) |
| BMI < 18.5, n0 (%) | 37 (10.4) |
| 18.5 ≤ BMI < 25, n0 (%) | 181 (51.2) |
| 25 ≤ BMI, n0 (%) | 135 (38.4) |
| % decrease in body weight at surgery, mean (SD) | 4.0 (5.9) |
| Increased body weight, n0 (%) | 11 (3.3%) |
| Stable or decreased body weight < 5%, n0 (%) | 208 (62.5%) |
| 5% ≤ Decreased body weight < 10%, n0 (%) | 55 (16.5) |
| 10% ≤ Decreased body weight, n0 (%) | 59 (17.7) |
| Biological variable | |
| Nutritional variable | |
| Prealbumin, mean (SD) mg/mL | 275 (92) |
| Prealbumin < 275 mg/mL, n0 (%) | 203 (59.0) |
| 275 mg/mL ≤ prealbumin, n0 (%) | 141 (41.0) |
| Inflammatory variable | |
| CRP, mean (SD) mg/mL | 22.8 (43) |
| CRP ≤ 3 mg/mL, n0 (%) | 173 (50.3) |
| 3 mg/mL< CRP, n0 (%) | 171 (49.7) |
| Univariate | |||||
|---|---|---|---|---|---|
| Variable |
5-Year
Survival Rate (95%CI) |
10-Year
Survival Rate (95% CI) |
15-Year
Survival Rate (95% CI) |
20-Year
Survival Rate (95% CI) | p |
| Global survival | 41.9 (36.7–47.1) | 23.8 (19.6–28.6) | 12.2 (9.1–16.0) | 5.7 (3.4–9.3) | |
| Sex | |||||
| Men | 40.7 (35.2–46.4) | 23.1 (18.6–28.3) | 10.3 (7.3–14.4) | 4.1 (2.0–7.9) | |
| Women | 46.3 (33.7–59.4) | 27.8 (17.6–40.8) | 22.2 (13.2–34.9) | 14.3 (6.7–27.6) | 0.059 |
| Patient age | |||||
| 19–49 | 45.1 (32.3–58.6) | 23.5 (14.0–36.8) | 17.6 (9.6–30.2) | 12.5 (5.7–25.3) | |
| 50–59 | 46.6 (37.2–56.1) | 29.1 (21.2–38.5) | 17.5 (11.3–25.9) | 9.7 (4.8–18.5) | |
| 60–69 | 41.8 (33.4–50.7) | 24.6 (17.8–32.9) | 9.0 (5.1–15.4) | 3.2 (1.1–9.1) | |
| 70–84 | 35.1 (26.2–45.2) | 16.0 (9.9–24.7) | 3.2 (1.1–8.9) | 1.1 (0.2–5.8) | 0.0042 |
| Smoking | |||||
| Never smoked | 43.5 (25.6–63.2) | 34.8 (18.8–55.1) | 20.7 (6.9–37.1) | 13.0 (4.5–32.1) | |
| Past or | 42.1 (36.8–47.6) | 23.0 (18.6–27.9) | 10.1 (7.2–13.9) | 5.3 (3.0–9.0) | 0.24 |
| Current smoker | |||||
| Smoking cessation before surgery | |||||
| Yes | 42.0 (35.0–49.3) | 23.2 (17.6–29.9) | 12.2 (8.2–17.7) | 6.9 (3.7–12.5) | |
| No | 41.5 (33.5–49.9) | 22.2 (16.0–29.9) | 10.4 (6.3–16.7) | 3.0 (0.9–8.9) | 0.39 |
| Cum. Smok. | |||||
| <20 p./yr | 41.3 (34.4–48.5) | 27.2 (21.3–34.0) | 16.8 (12.1–22.9) | 9.5 (5.7–15.4) | |
| 20–50 p./yr | 43.4 (38.2–48.8) | 24.7 (20.3–29.6) | 14.8 (11.3–19.0) | 6.9 (3.7–12.7) | |
| 50–100 p./yr | 40.3 (33.5–47.5) | 20.4 (15.3–26.8) | 11.3 (7.5–16.6) | 4.6 (1.9–10.3) | |
| 100 p./yr≤ | 25.0 (8.9–53.3) | 16.7 (4.7–44.8) | 8.3 (1.5–35.9) | 0 | 0.33 |
| Com. Illness. Resp. status | |||||
| Alcohol | |||||
| No | 44.4 (38.4–50.6) | 26.6 (21.5–32.7) | 14.3 (10.5–19.5) | 7.5 (4.7–11.8) | |
| Yes | 34.8 (25.7–45.7) | 15.7 (9.6–24.7) | 6.7 (3.1–13.9) | 4.5 (1.7–10.9) | 0.062 |
| Diabetes mel. | |||||
| No | 42.9 (37.2–48.8) | 24.0 (19.3–29.9) | 13.1 (9.6–17.6) | 6.1 (3.5–10.1) | |
| Yes | 31.0 (19.0–46.0) | 19.0 (9.9–33.3) | 4.8 (1.3–15.8) | 2.4 (0.4–12.3) | 0.31 |
| Chron. bronch. | |||||
| No | 46.3 (37.8–55.1) | 26.8 (19.8–35.3) | 17.1 (11.4–24.7) | 9.9 (5.6–16.8) | |
| Yes | 39.0 (32.7–45.6) | 22.5 (17.4–28.4) | 9.2 (6.0–13.8) | 3.0 (1.1–8.3) | 0.027 |
| COPD | |||||
| No | 43.9 (37.0–51.0) | 22.8 (17.3–29.2) | 13.2 (9.1–18.8) | 7.9 (4.6–12.9) | |
| yes | 38.8 (31.4–46.7) | 24.3 (18.2–31.7) | 11.2 (7.1–17.2) | 2.5 (0.6–10.1) | 0.40 |
| COPD | |||||
| Mild | 41.5 (29.2–54.9) | 24.5 (14.9–37.5) | 15.1 (7.8–27.0) | 9.4 (4.1–20.2) | |
| Severe | 42.1 (33.1–51.5) | 29.9 (22.0–39.1) | 15.9 (10.1–23.9) | 4.0 (0.9–15.9) | 0.97 |
| FEV1 (% pred.) | |||||
| 80%≤ | 46.9 (40.1–53.9) | 27.6 (21.7–34.2) | 17.9 (13.1–23.8) | 13.6 (9.4–19.1) | |
| 70–80% | 40.4 (31.0–50.5) | 28.7 (20.5–38.6) | 14.9 (9.1–23.4) | 7.0 (2.8–16.2) | |
| 60–70% | 39.3 (27.5–52.3) | 30.4 (19.9–43.3) | 14.3 (7.4–25.7) | 10.7 (5.0–21.4) | |
| 50–60% | 34.8 (18.8–55.1) | 14.5 (6.9–37.1) | 13.0 (4.5–32.1) | 8.7 (2.4–26.8) | |
| <50% | 42.1 (23.1–63.7) | 21.1 (8.5–43.3) | 17.1 (5.5–37.0) | 15.8 (5.5–37.5) | 0.86 |
| Preoperative treat. | |||||
| Chemotherapy | |||||
| No | 47.4 (41.3–53.6) | 26.3 (21.2–32.0) | 13.5 (9.8–18.3) | 5.9 (3.3–10.4) | |
| Yes | 26.1 (18.0–36.1) | 17.0 (10.6–26.2) | 8.0 (3.9–15.5) | 5.7 (2.4–12.6) | 0.0076 |
| Radiotherapy | |||||
| No | 41.8 (36.6–47.2) | 24.3 (19.9–29.2) | 12.6 (9.4–16.6) | 6.1 (3.6–9.8) | |
| Yes | 28.6 (11.7–54.6) | 7.1 (1.2–31.4) | 0 | 0 | 0.071 |
| Resection type | |||||
| Lobectomy | 46.9 (40.7–53.1) | 27.0 (21.7–32.9) | 14.5 (10.6–19.5) | 7.5 (4.4–12.3) | |
| Bilobectomy | 40.0 (21.9–61.3) | 30.0 (14.5–51.9) | 10.0 (2.8–30.1) | 5.0 (0.9–23.6) | |
| Pneumonec. | 24.1 (16.1–34.3) | 10.8 (5.8–19.3) | 3.6 (1.2–10.1) | 1.2 (0.2–6.5) | 0.0000067 |
| Pathological stage | |||||
| I | 50.9 (44.4–57.4) | 29.7 (24.1–36.0) | 16.7 (12.3–22.1) | 7.8 (4.4–13.2) | |
| II | 26.1 (20.2–32.9) | 14.4 (10.0–20.3) | 6.1 (3.4–10.6) | 5.0 (2.6–9.2) | |
| III | 25.4 (18.5–33.8) | 13.1 (8.2–20.2) | 3.3 (1.3–8.1) | 1.6 (0.4–5.8) | |
| IV | 28.6 (11.7–54.6) | 14.3(4.0–39.9) | 7.1 (1.3–31.4) | 0 | 0.00000042 |
| Mediastinal pathological stage | |||||
| IA | 68.1 (56.4–77.9) | 40.6 (29.8–52.3) | 23.2 (14.8–34.4) | 6.5 (1.6–23.4) | |
| IB | 64.6 (50.4–76.6) | 33.3 (21.7–47.5) | 16.7 (8.7–29.6) | 4.2 (0.9–14.4) | |
| IIA | 37.8 (25.1–52.4) | 15.6 (7.7–28.8) | 13.3 (6.2–26.2) | 8.9 (3.0–23.3) | |
| IIB | 29.5 (18.2–44.2) | 20.5 (11.1–34.5) | 6.8 (2.3–18.2) | 6.8 (2.3–18.2) | |
| IIIA | 25.3 (17.7–34.6) | 12.1 (7.0–20.0) | 3.0 (1.0–8.5) | 2.0 (0.6–7.0) | |
| IIIB | 21.1 (8.5–43.3) | 10.5 (2.9–31.4) | 0 | 0 | |
| IV | 12.5 (2.2–47.0) | 0 | 0 | 0 | <0.0000001 |
| Histopath. type | |||||
| Adenocarci. | 43.4 (35.9–51.2) | 22.6 (16.8–29.7) | 11.9 (7.8–17.9) | 6.0 (3.0–11.4) | |
| Squam. cell carcinoma | 42.1 (34.0–50.6) | 26.3 (19.6–34.4) | 10.5 (6.4–16.9) | 1.9 (0.4–8.4) | |
| Large-cell carc. | 26.0(17.2–47.5) | 28.0(14.2–47.5) | 8.0 (2.2–25.0) | 8.0 (2.2–24.9) | |
| Others + | 37.5 (18.5–61.4) | 31.2 (14.2–55.6) | 25.0 (10.2–49.5) | 25.0 (10.2–49.5) | 0.33 |
| Morphomics | |||||
| Nutritionals par. | |||||
| Presurgical BMI | |||||
| BMI < 18.5 | 29.7 (17.4–45.7) | 13.5 (5.9–27.9) | 10.8 (4.2–24.7) | 4.1 (0.8–17.9) | |
| 18.5 ≤ BMI < 25 | 38.1 (31.3–45.3) | 19.9 (14.7–26.3) | 8.3 (5.0–13.2) | 2.4 (0.7–7.1) | |
| 25 ≤ BMI | 49.6 (41.3–57.9) | 30.4 (23.2–38.5) | 16.3 (11.0–23.4) | 11.1 (6.8–17.5) | 0.0090 |
| Body weight | |||||
| at surg. % var. | |||||
| increased | 60 (31.2–83.3) | 20 (5.6–50.9) | 10 (1.8–40.4) | 10 (1.7–40.4) | |
| Stable. or | |||||
| decreas. < 5% | 46.9 (40.2–53.6) | 29.0 (23.2–35.5) | 14.5 (10.3–19.9) | 8.1 (4.7–13.4) | |
| 5 ≤ decreas. < 10 | 34.5 (23.3–47.7) | 21.8 (12.9–34.3) | 9.1 (3.9–19.6) | 5.5 (1.9–14.8) | |
| 10% ≤ decreas. | 27.1 (17.4–39.6) | 11.9 (5.9–22.5) | 6.8 (2.6–16.2) | 1.7 (0.3–9.0) | 0.0034 |
| Biological variables | |||||
| Nutrition varia. | |||||
| prealbumin | |||||
| preal. < 275 mg/mL | 47.5 (39.4–55.7) | 29.1 (22.2–37.0) | 14.2 (9.3–20.9) | 6.7 (3.4–12.7) | |
| 275 ≤ prelab. | 36.7 (30.4–43.5) | 20.3 (15.4–26.2) | 10.1 (6.7–15.0) | 5.3 (2.7–10.1) | 0.065 |
| Inflammatory | |||||
| CRP ≤ 3 mg/mL | 45.7 (38.3–53.4) | 26.8 (20.6–34.1) | 14.6 (10.0–20.8) | 10.1 (6.3–15.8) | |
| 3 mg/mL < CRP | 37.8 (31.0–45.0) | 21.1 (15.7–27.6) | 10.0 (6.4–15.2) | 2.4 (0.7–7.0) | 0.049 |
| Variable | Relative Risk (RR) | 95% CI of RR | p |
|---|---|---|---|
| p = 0.000075 model | |||
| Number of patients at multivariate n = 276 | |||
| Model 1 | |||
| Pathological stages (7 classes) | |||
| IA | 1 | ||
| IB | 1.12 | (1.04–1.20) | |
| IIA | 1.25 | (1.08–1.44) | |
| IIB | 1.39 | (1.12–1.72) | |
| IIIA | 1.55 | (1.17–2.07)) | |
| IIIB | 1.73 | (1.21–2.48) | |
| IV | 1.93 | (1.26–2.97) | 0.0026 |
| Age (years) | |||
| <50 yrs | 1 | ||
| 50 yrs–60 yrs | 1.21 | (1.06–1.37) | |
| 60 yrs–70 yrs | 1.46 | (1.13–1.89) | |
| 70 yrs ≤ | 1.77 | (1.20–2.60) | 0.004 |
| Cumulative smoking | |||
| <20 p./yr | 1 | ||
| 20 p./yr–50 p./yr | 1.21 | (1.00–1.47) | |
| 50 p./yr–100 p./yr | 1.47 | (1.01–2.15) | |
| 100 p./yr≤ | 1.79 | (1.01–3.16) | 0.045 |
| BMI (kg/m2) | |||
| BMI < 18.5 | 1 | ||
| 18.5 ≤ BMI < 25 | 0.82 | (0.67–1.00) | |
| 25 ≤ BMI | 0.67 | (0.45–0.99) | 0.046 |
| Chronic bronchitis | 0.20 | ||
| COPD yes/no | 0.36 | ||
| Sexe | 0.28 | ||
| Weight loss | |||
| <5% | |||
| 5–10% | |||
| 10%≤ | 0.41 | ||
| Diabete mellitus | |||
| Yes/no | 0.44 | ||
| Type of resection | |||
| Pneumonec./bilob. or lob. | 0.60 | ||
| Histological type | |||
| Aden./Squa./Lar.cell/others | 0.62 | ||
| Alcohol abuse | |||
| Yes/no | 0.74 | ||
| COPD GOLD | 0.96 | ||
| Past or current smoker | |||
| Yes/no | 0.99 | ||
| Variable | Relative Risk (RR) | 95% CI of RR | p |
| Model 2 | |||
| p = 0.00000059 model | |||
| Number of patients at multivariate n = 276 | |||
| FEV1 preoperative (%) | |||
| <50% | 1 | ||
| 50–60% | 0.76 | (0.66–0.86) | |
| 60–70% | 0.57 | (0.44–0.74) | |
| 70–80% | 0.43 | (0.29–0.63) | |
| 80%≤ | 0.33 | (0.19–0.54) | 0.000019 |
| COPD | |||
| GOLD1 | 1 | ||
| GOLD2 | 0.67 | (0.50–0.90) | |
| GOLD3 | 0.45 | (0.25–0.81) | 0.0079 |
| Pathological stages (7 classes) | |||
| IA | 1 | ||
| IB | 1.14 | (1.06–1.22) | |
| IIA | 1.29 | (1.12–1.49) | |
| IIB | 1.47 | (1.18–1.83) | |
| IIIA | 1.67 | (1.24–2.23) | |
| IIIB | 1.89 | (1.31–2.73) | |
| IV | 2.15 | (1.39–3.34) | 0.00063 |
| Chronic bronchitis | |||
| No | 1 | ||
| Yes | 1.40 | (1.03–1.90) | |
| Age (years) | 0.03 | ||
| <50 yrs | 1 | ||
| 50 yrs–60 yrs | 1.18 | (1.04–1.35) | |
| 60 yrs–70 yrs | 1.40 | (1.08–1.83) | |
| 70 yrs< | 1.65 | (1.11–2.47) | 0.013 |
| BMI kg/m2 | |||
| BMI < 18.5 | 1 | ||
| 18.5 ≤ BMI < 25 | 0.79 | (0.65–0.96) | |
| 25 ≤ BMI | 0.62 | (0.42–0.93) | 0.02 |
| Cumulative smoking | |||
| <20 p./yr | 1 | ||
| 20 p./yr–50 p./yr | 1.18 | (0.98–1.43) | |
| 50 p./yr–100 p./yr | 1.39 | (0.95–2.04) | |
| 100 p./yr≤ | 1.65 | (0.93–2.91) | 0.087 |
| Weight loss | |||
| <5% | |||
| 5–10% | |||
| 10%≤ | 0.20 | ||
| Histological type | |||
| Aden./Squa./Lar.cell/others | 0.32 | ||
| Sexe | 0.39 | ||
| Diabete mellitus | |||
| Yes/not | 0.44 | ||
| Type of resection | |||
| Pneumonec./bilob. or lob. | 0.65 | ||
| COPD yes/no | 0.73 | ||
| Past or current smoker | |||
| Yes/no | 0.87 | ||
| Alcohol abuse | |||
| Yes/no | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schussler, O.; Bobbio, A.; Dermine, H.; Lupo, A.; Damotte, D.; Lecarpentier, Y.; Alifano, M. Twenty-Year Survival of Patients Operated on for Non-Small-Cell Lung Cancer: The Impact of Tumor Stage and Patient-Related Parameters. Cancers 2022, 14, 874. https://doi.org/10.3390/cancers14040874
Schussler O, Bobbio A, Dermine H, Lupo A, Damotte D, Lecarpentier Y, Alifano M. Twenty-Year Survival of Patients Operated on for Non-Small-Cell Lung Cancer: The Impact of Tumor Stage and Patient-Related Parameters. Cancers. 2022; 14(4):874. https://doi.org/10.3390/cancers14040874
Chicago/Turabian StyleSchussler, Olivier, Antonio Bobbio, Hervé Dermine, Audrey Lupo, Diane Damotte, Yves Lecarpentier, and Marco Alifano. 2022. "Twenty-Year Survival of Patients Operated on for Non-Small-Cell Lung Cancer: The Impact of Tumor Stage and Patient-Related Parameters" Cancers 14, no. 4: 874. https://doi.org/10.3390/cancers14040874
APA StyleSchussler, O., Bobbio, A., Dermine, H., Lupo, A., Damotte, D., Lecarpentier, Y., & Alifano, M. (2022). Twenty-Year Survival of Patients Operated on for Non-Small-Cell Lung Cancer: The Impact of Tumor Stage and Patient-Related Parameters. Cancers, 14(4), 874. https://doi.org/10.3390/cancers14040874







