MicroRNA-375-3p Suppresses Upper Tract Urothelial Carcinoma Cell Migration and Invasion via Targeting Derlin-1
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Tissues and Clinicopathological Data
2.2. Immunohistochemical Analysis of Derlin-1
2.3. Estimation of Immunohistochemical Staining
2.4. Cell Culture
2.5. RNA Isolation and Real-Time PCR Analyses
2.6. Cell Transfection
2.7. Western Blot Analysis
2.8. Cell Proliferation Assay
2.9. Wound Healing Assay
2.10. Cell Invasion Assays
2.11. Dual-Luciferase Reporter Assay
2.12. Statistical Analysis
3. Results
3.1. Clinical Significance of Derlin-1 in Patients of UTUC
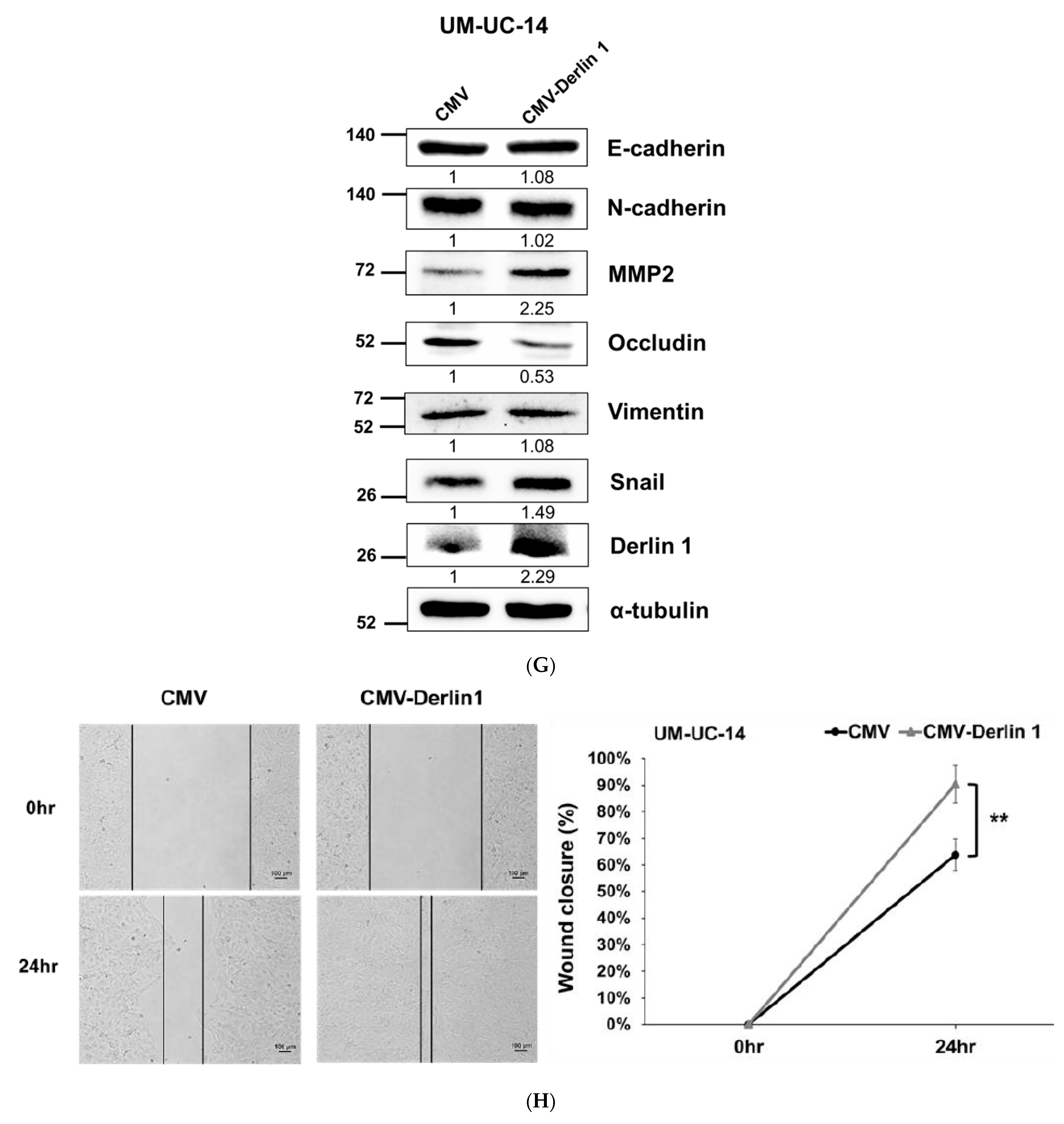
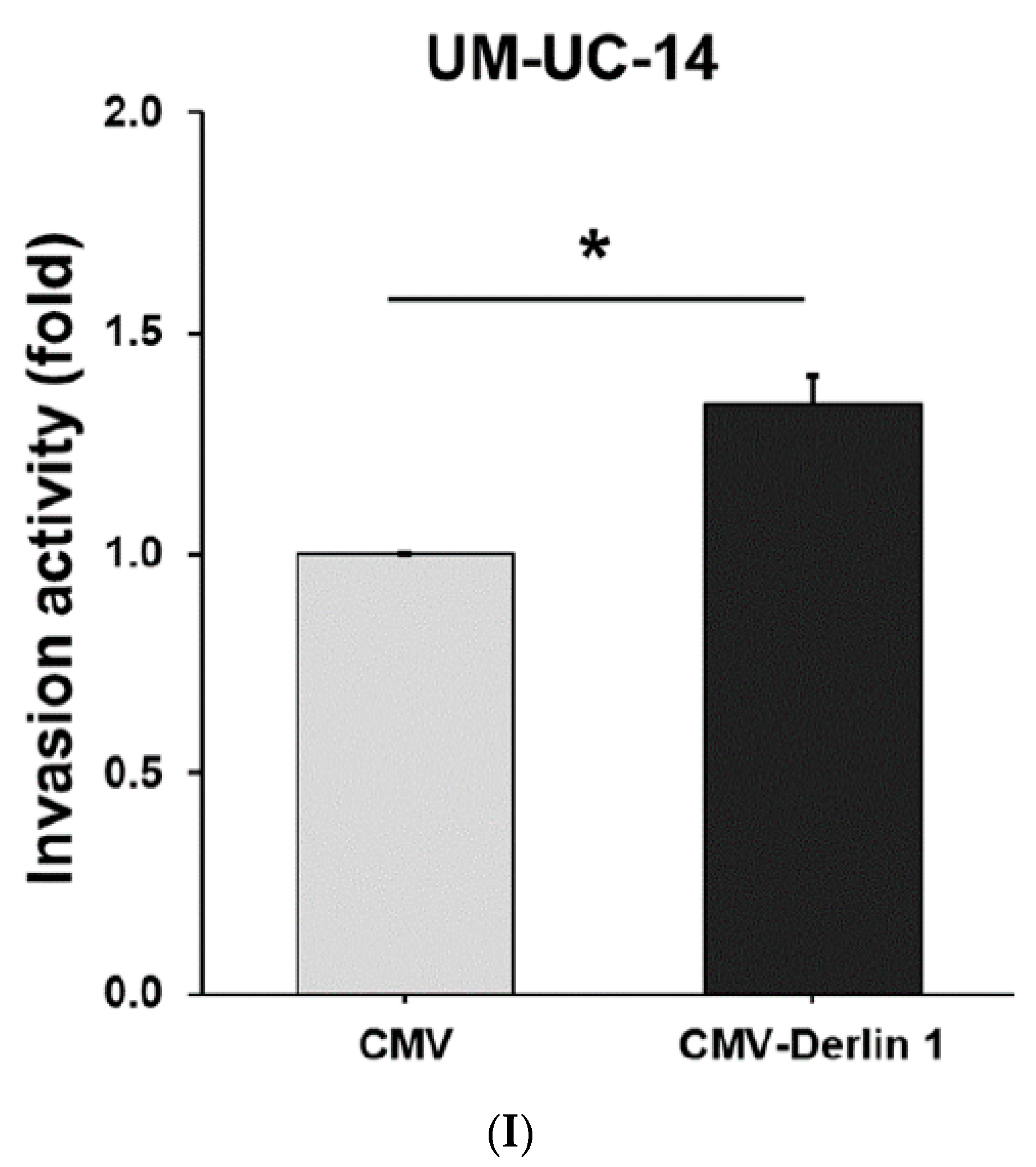
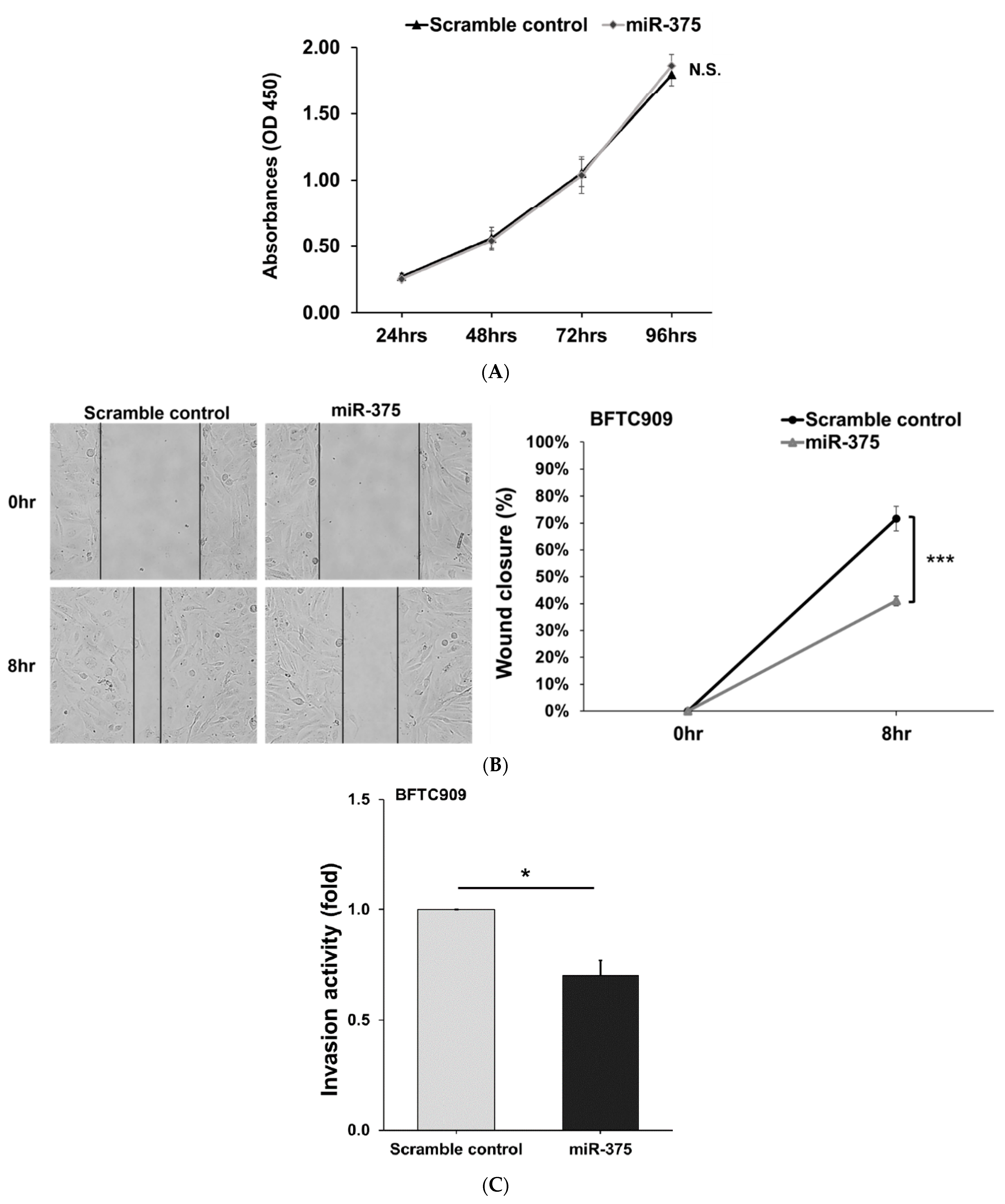
3.2. Derlin-1 Is Overexpressed in UTUC Cell Lines and Enhances Migration and Invasion
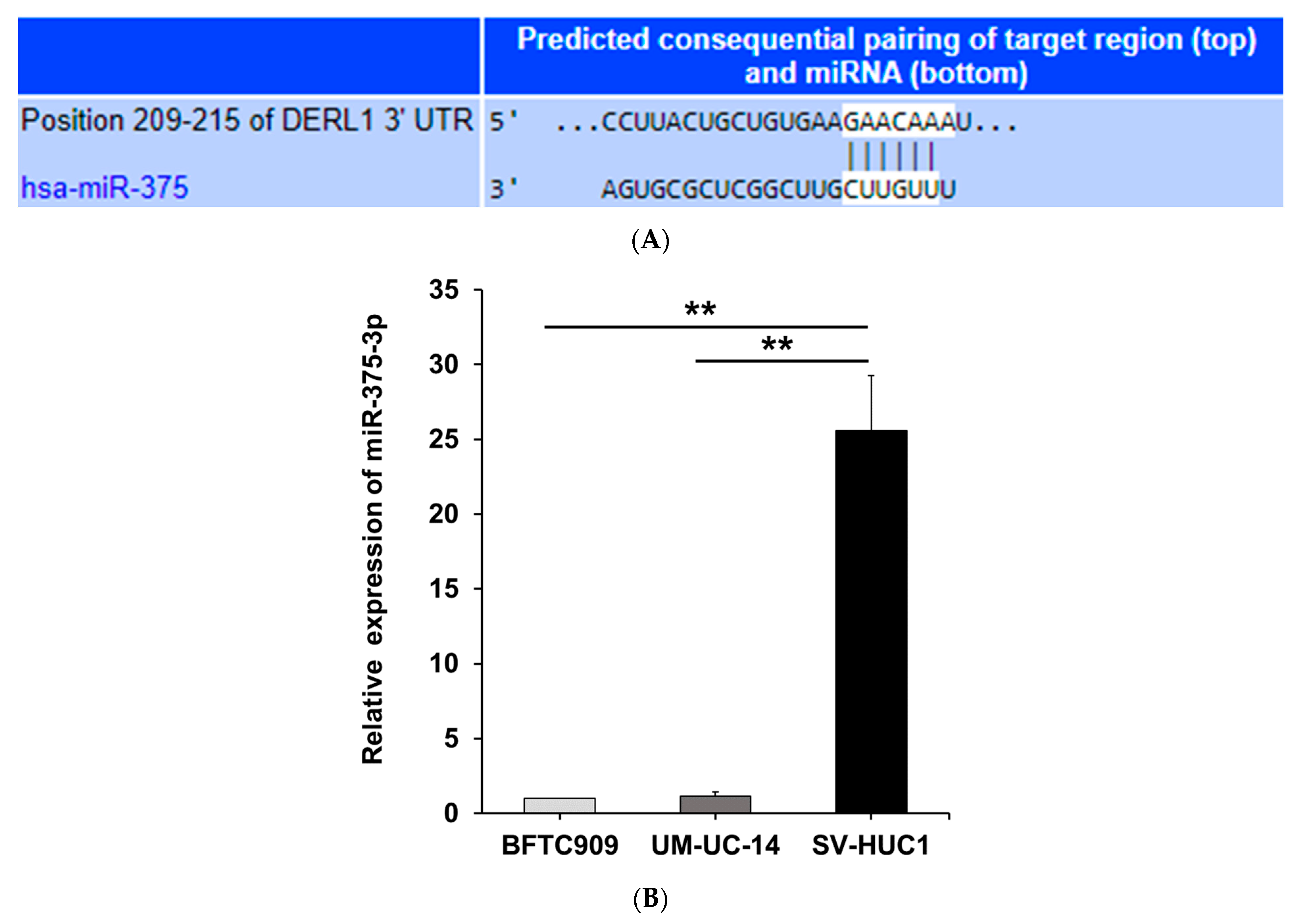
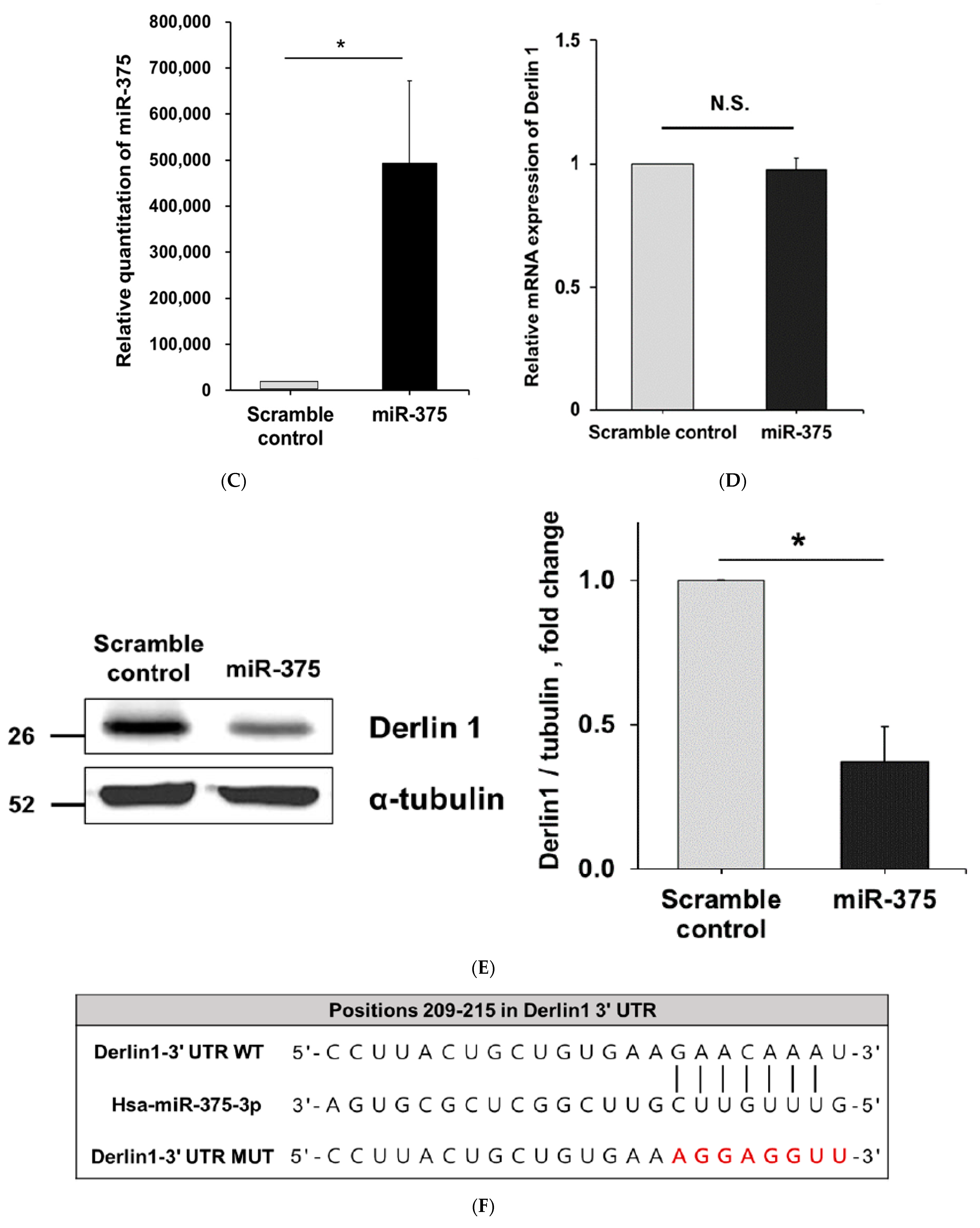
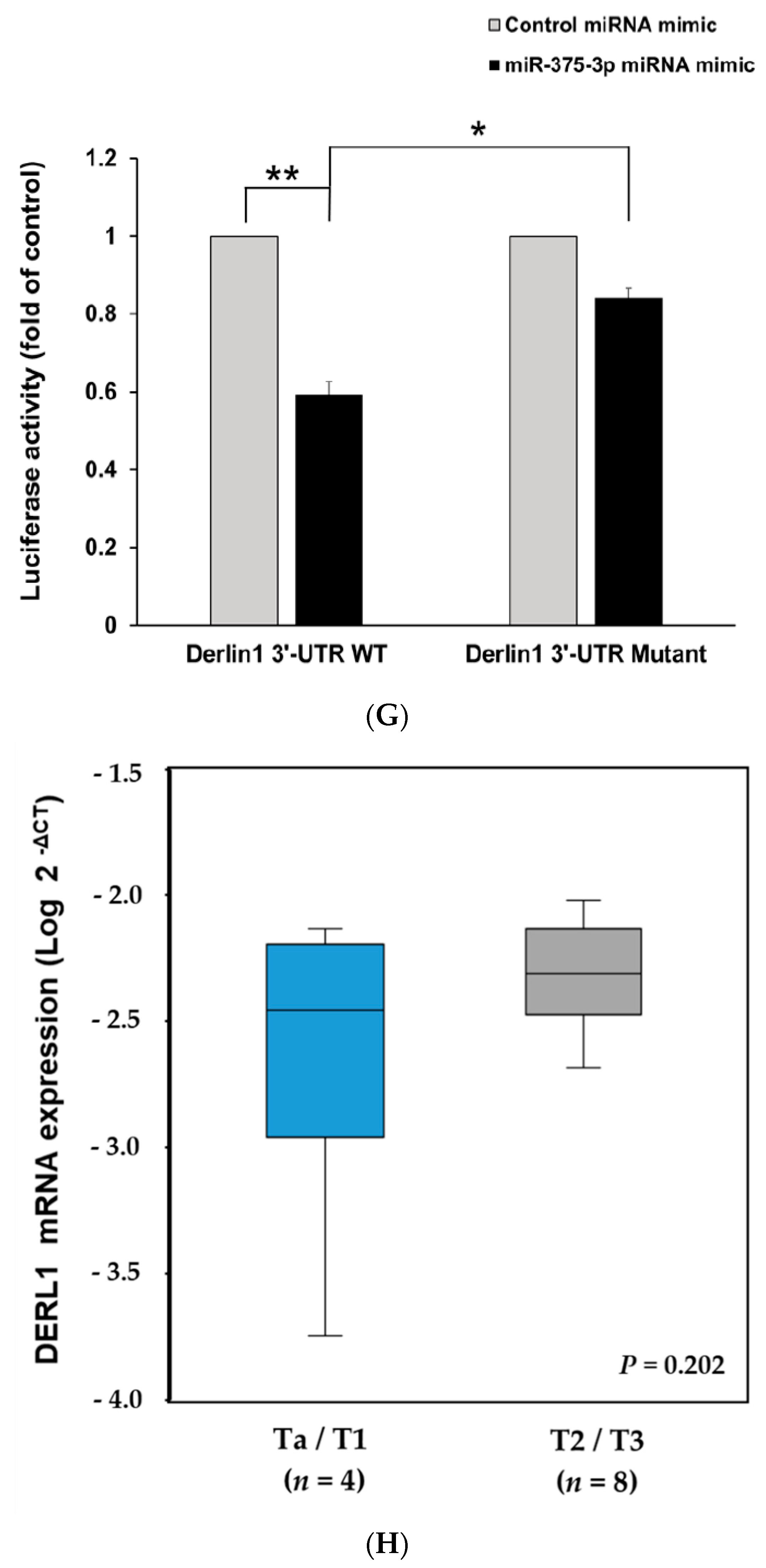
3.3. MiR-375-3p Downregulates Derlin-1 Protein Expression via Directly Targeting Derlin-1 in BFTC909 Cells
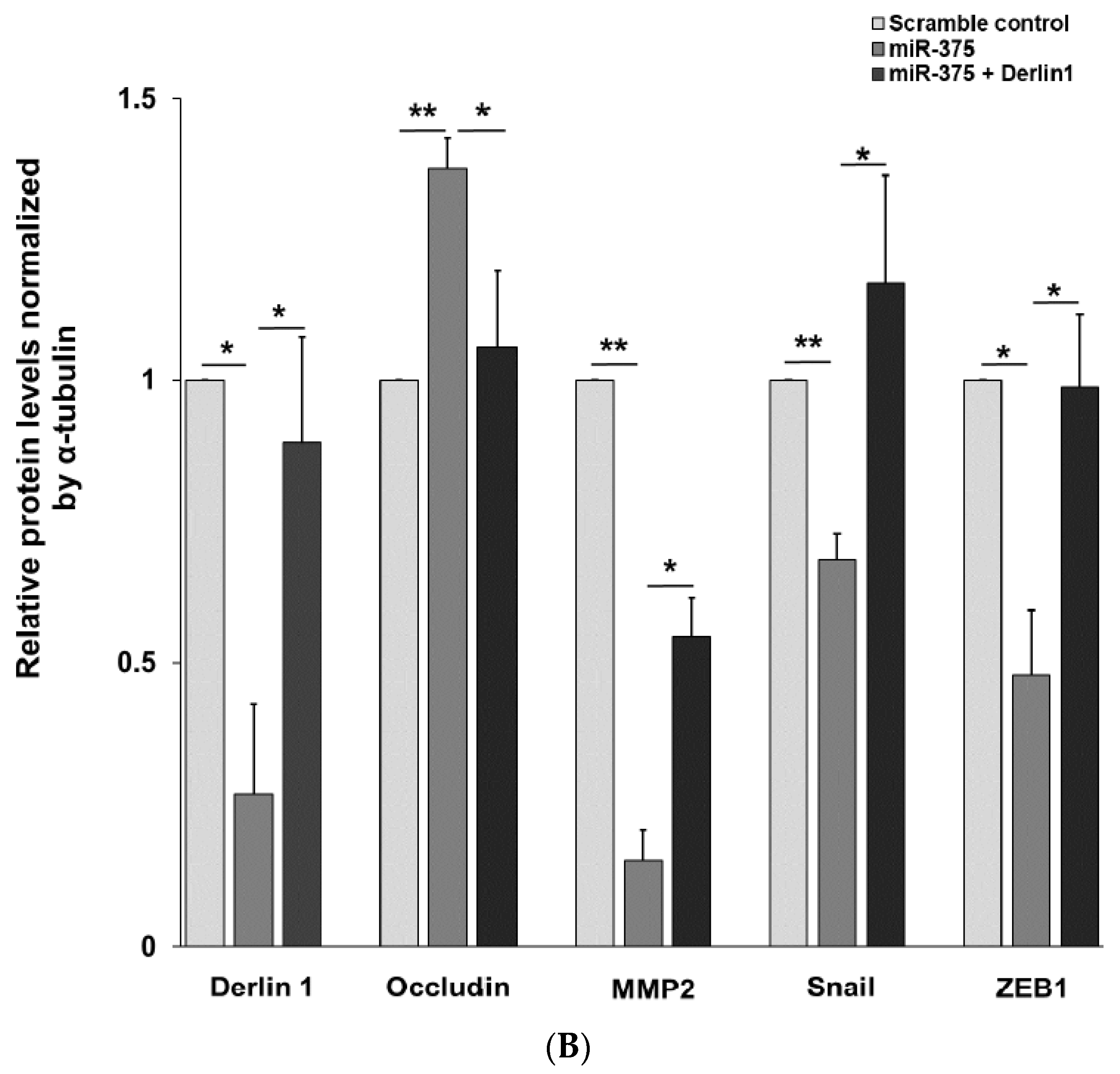
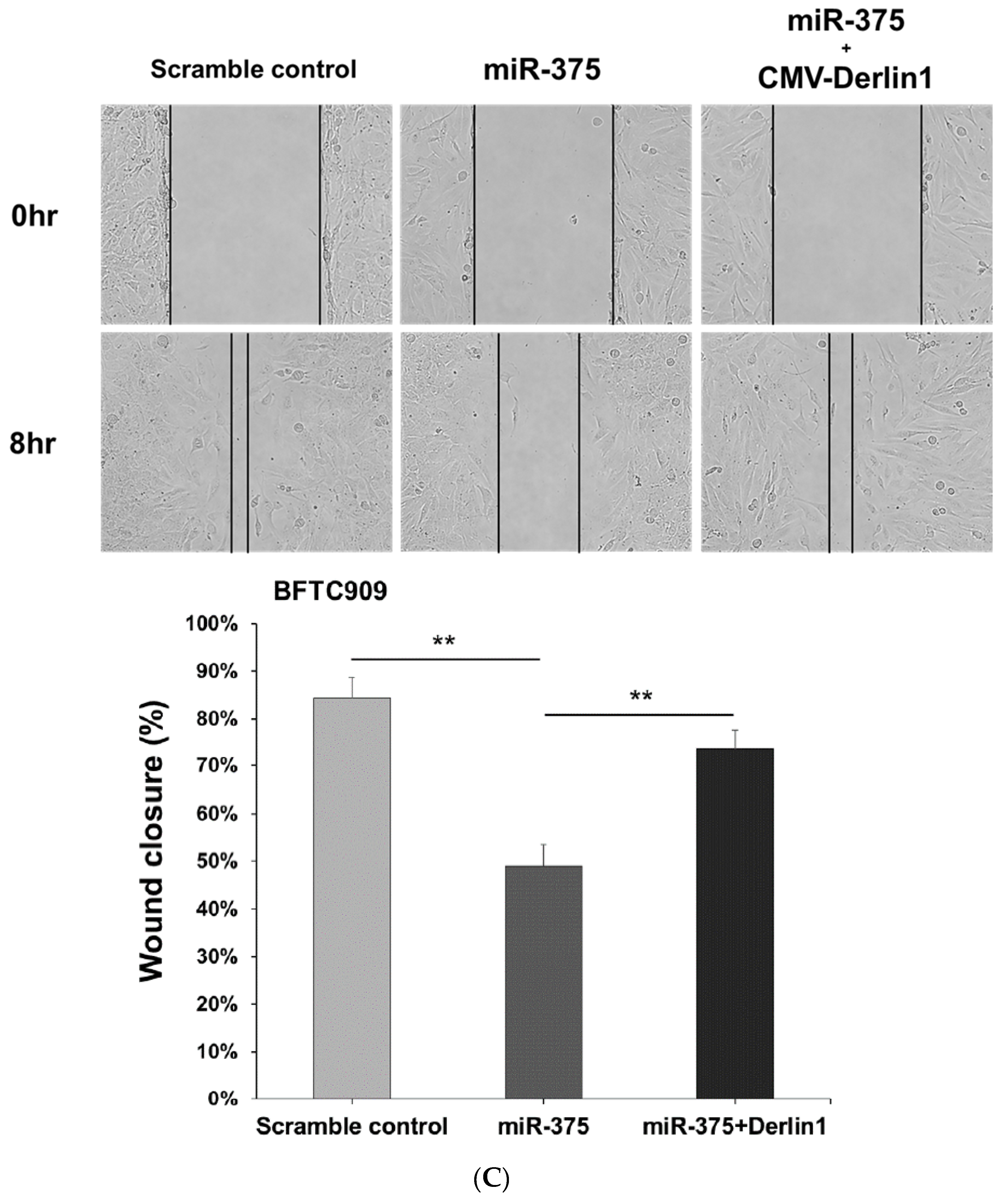
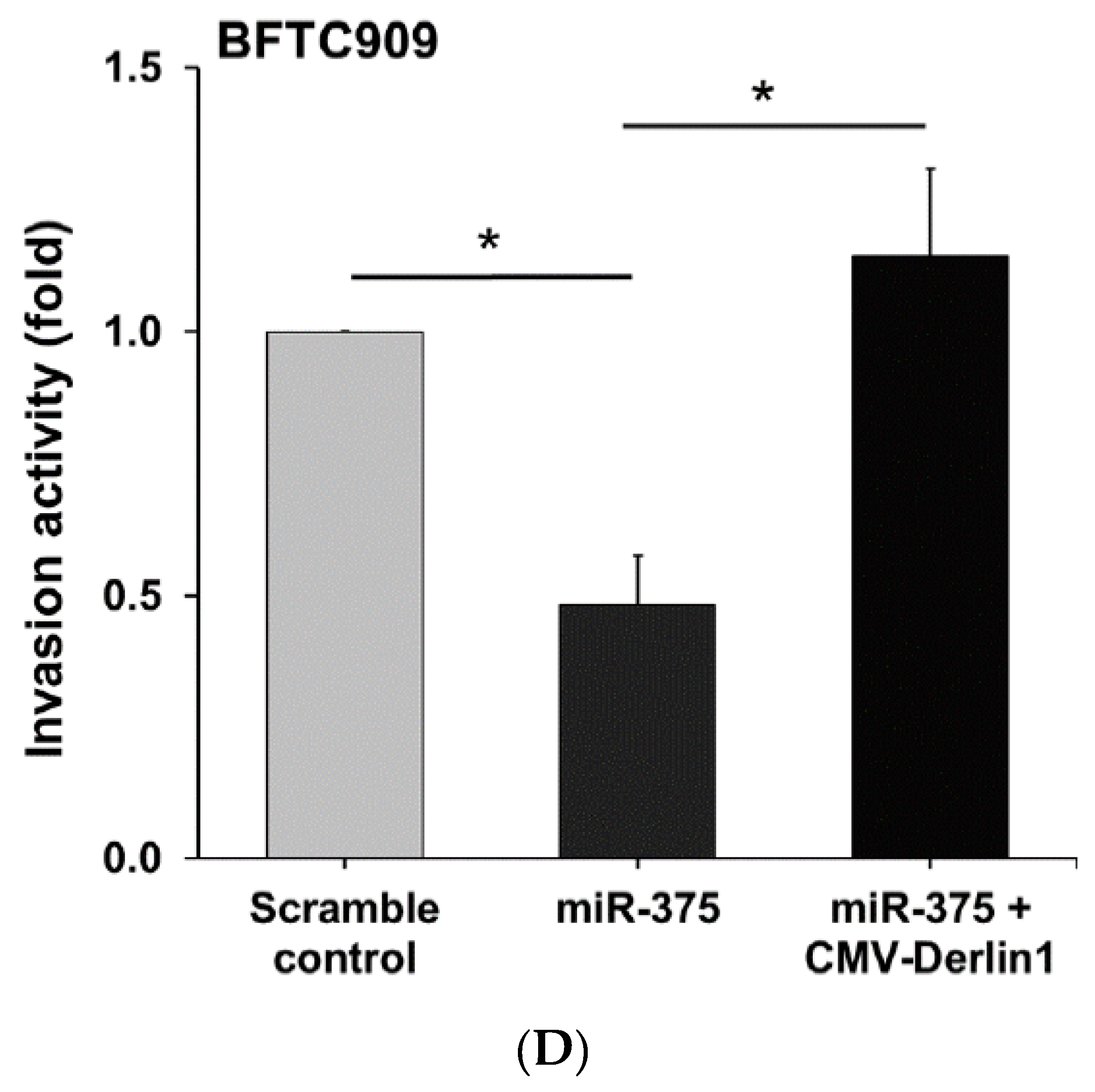
3.4. MiR-375-3p Inhibits Cell Migration and Invasion by Blocking EMT via Reducing Derlin-1 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Soria, F.; Shariat, S.F.; Lerner, S.P.; Fritsche, H.M.; Rink, M.; Kassouf, W.; Spiess, P.E.; Lotan, Y.; Ye, D.; Fernández, M.I.; et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J. Urol. 2017, 35, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Chen, K.K.; Yen, C.C.; Wang, W.S.; Chang, Y.H.; Huang, W.J.; Fan, F.S.; Chiou, T.J.; Liu, J.H.; Chen, P.M. Unusually high incidence of upper urinary tract urothelial carcinoma in Taiwan. Urology 2002, 59, 681–687. [Google Scholar] [CrossRef]
- Shariat, S.F.; Favaretto, R.L.; Gupta, A.; Fritsche, H.M.; Matsumoto, K.; Kassouf, W.; Walton, T.J.; Tritschler, S.; Baba, S.; Matsushita, K.; et al. Gender differences in radical nephroureterectomy for upper tract urothelial carcinoma. World J. Urol. 2011, 29, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Fang, D.; Su, X.; Bao, Z.; Cao, Z.; Jafri, S.M.; Xiong, G.; Zhang, L.; Hutchinson, R.; Sagalowsky, A.; et al. A Multi-Institutional Comparison of Clinicopathological Characteristics and Oncologic Outcomes of Upper Tract Urothelial Carcinoma in China and the United States. J. Urol. 2017, 197, 1208–1213. [Google Scholar] [CrossRef]
- Lughezzani, G.; Jeldres, C.; Isbarn, H.; Sun, M.; Shariat, S.F.; Alasker, A.; Pharand, D.; Widmer, H.; Arjane, P.; Graefen, M.; et al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: A population-based study of 2299 patients. Eur. J. Cancer 2009, 45, 3291–3297. [Google Scholar] [CrossRef]
- Aboumohamed, A.A.; Krane, L.S.; Hemal, A.K. Oncologic Outcomes Following Robot-Assisted Laparoscopic Nephroureterectomy with Bladder Cuff Excision for Upper Tract Urothelial Carcinoma. J. Urol. 2015, 194, 1561–1566. [Google Scholar] [CrossRef]
- Hassler, M.R.; Bray, F.; Catto, J.W.F.; Grollman, A.P.; Hartmann, A.; Margulis, V.; Matin, S.F.; Roupret, M.; Sfakianos, J.P.; Shariat, S.F.; et al. Molecular Characterization of Upper Tract Urothelial Carcinoma in the Era of Next-generation Sequencing: A Systematic Review of the Current Literature. Eur. Urol. 2020, 78, 209–220. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, X.; Cobb, G.P.; Anderson, T.A. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007, 302, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Kudo, M.; Huang, X.; Sui, H.; Tian, H.; Croce, C.M.; Cui, R. Frontiers of MicroRNA Signature in Non-small Cell Lung Cancer. Front. Cell Dev. Biol. 2021, 9, 643942. [Google Scholar] [CrossRef]
- Falzone, L.; Grimaldi, M.; Celentano, E.; Augustin, L.S.A.; Libra, M. Identification of Modulated MicroRNAs Associated with Breast Cancer, Diet, and Physical Activity. Cancers 2020, 12, 2555. [Google Scholar] [CrossRef] [PubMed]
- Neaga, A.; Bagacean, C.; Tempescul, A.; Jimbu, L.; Mesaros, O.; Blag, C.; Tomuleasa, C.; Bocsan, C.; Gaman, M.; Zdrenghea, M. MicroRNAs Associated With a Good Prognosis of Acute Myeloid Leukemia and Their Effect on Macrophage Polarization. Front. Immunol. 2020, 11, 582915. [Google Scholar] [CrossRef]
- Falzone, L.; Lupo, G.; La Rosa, G.R.M.; Crimi, S.; Anfuso, C.D.; Salemi, R.; Rapisarda, E.; Libra, M.; Candido, S. Identification of Novel MicroRNAs and Their Diagnostic and Prognostic Significance in Oral Cancer. Cancers 2019, 11, 610. [Google Scholar] [CrossRef] [Green Version]
- Falzone, L.; Scola, L.; Zanghì, A.; Biondi, A.; Di Cataldo, A.; Libra, M.; Candido, S. Integrated analysis of colorectal cancer microRNA datasets: Identification of microRNAs associated with tumor development. Aging 2018, 10, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Korotaeva, A.; Mansorunov, D.; Apanovich, N.; Kuzevanova, A.; Karpukhin, A. MiRNA Expression in Neuroendocrine Neoplasms of Frequent Localizations. Non-Coding RNA 2021, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Crimi, S.; Falzone, L.; Gattuso, G.; Grillo, C.M.; Candido, S.; Bianchi, A.; Libra, M. Droplet Digital PCR Analysis of Liquid Biopsy Samples Unveils the Diagnostic Role of hsa-miR-133a-3p and hsa-miR-375-3p in Oral Cancer. Biology 2020, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, L.; Ingelmo-Torres, M.; Mallofré, C.; Lozano, J.J.; Verhasselt-Crinquette, M.; Leroy, X.; Colin, P.; Comperat, E.; Roupret, M.; Alcaraz, A.; et al. Prognostic value of microRNA expression pattern in upper tract urothelial carcinoma. BJU Int. 2014, 113, 813–821. [Google Scholar] [CrossRef] [Green Version]
- Browne, B.M.; Stensland, K.D.; Patel, C.K.; Sullivan, T.; Burks, E.J.; Canes, D.; Raman, J.D.; Warrick, J.; Reiger-Christ, K.M. MicroRNA Expression Profiles in Upper Tract Urothelial Carcinoma Differentiate Tumor Grade, Stage, and Survival: Implications for Clinical Decision-Making. Urology 2019, 123, 93–100. [Google Scholar] [CrossRef]
- Lilley, B.N.; Ploegh, H.L. A membrane protein required for dislocation of misfolded proteins from the ER. Nature 2004, 429, 834–840. [Google Scholar] [CrossRef]
- Ye, Y.; Shibata, Y.; Yun, C.; Ron, D.; Rapoport, T.A. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 2004, 429, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Tian, L.; Huang, S.; Zhang, J.; Zhao, B. Derlin-1 Promotes the Progression of Human Hepatocellular Carcinoma via the Activation of AKT Pathway. OncoTargets Ther. 2020, 13, 5407–5417. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hua, H.; Ran, Y.; Zhang, H.; Liu, W.; Yang, Z.; Jiang, Y. Derlin-1 is overexpressed in human breast carcinoma and protects cancer cells from endoplasmic reticulum stress-induced apoptosis. Breast Cancer Res. 2008, 10, R7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, M.; Zhang, J.; Jiang, J. Overexpression of Derlin-1 is Associated with Poor Prognosis in Patients with Non-small Cell Lung Cancer. Ann. Clin. Lab. Sci. 2018, 48, 29–34. [Google Scholar]
- Pi, L.; Zhu, G.; She, L.; Wei, M.; Liu, G.; Chen, C.; Hu, D.; Peng, F.; Tan, H.; Liu, Y.; et al. Elevated expression of Derlin-1 associates with unfavorable survival time of squamous cell carcinoma of the head and neck and promotes its malignance. J. Cancer 2017, 8, 2336–2345. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; He, X.; Jiang, Z.; Wang, X.; Ma, L.; Liu, L.; Wang, X.; Fan, Z.; Su, D. Derlin-1 is overexpressed in human colon cancer and promotes cancer cell proliferation. Mol. Cell Biochem. 2015, 408, 205–213. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, C.; Zhang, Z.; Liu, W.; Xu, H.; Wang, H.; Wang, Y.; Zhang, W.; Wang, S.L. High Expression of Derlin-1 Is Associated with the Malignancy of Bladder Cancer in a Chinese Han Population. PLoS ONE 2016, 11, e0168351. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Wei, K.; Qin, Z.; Liu, W.; Shao, C.; Wang, C.; Ma, L.; Xie, M.; Shu, Y.; Shen, H. MiR-598 Suppresses Invasion and Migration by Negative Regulation of Derlin-1 and Epithelial-Mesenchymal Transition in Non-Small Cell Lung Cancer. Cell Physiol. Biochem. 2018, 47, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Hsu, W.C.; Li, W.M.; Lee, Y.C.; Huang, A.M.; Chang, L.L.; Lin, H.H.; Wu, W.J.; Li, C.C.; Liang, P.I.; Ke, H.L. MicroRNA-145 suppresses cell migration and invasion in upper tract urothelial carcinoma by targeting ARF6. FASEB J. 2020, 34, 5975–5992. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Ran, Y.; Hu, H.; Hu, D.; Zhou, Z.; Sun, Y.; Yu, L.; Sun, L.; Pan, J.; Liu, J.; Liu, T.; et al. Derlin-1 is overexpressed on the tumor cell surface and enables antibody-mediated tumor targeting therapy. Clin. Cancer Res. 2008, 14, 6538–6545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verfaillie, T.; Garg, A.D.; Agostinis, P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013, 332, 249–264. [Google Scholar] [CrossRef]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Alasiri, G.; Fan, L.Y.; Zona, S.; Goldsbrough, I.G.; Ke, H.L.; Auner, H.W.; Lam, E.W. ER stress and cancer: The FOXO forkhead transcription factor link. Mol. Cell Endocrinol. 2018, 462, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.Z.; Wang, Y.; Tang, Z.P.; Fu, L.; Li, Q.C.; Wang, E.D.; Wang, E.H. Derlin-1 is overexpressed in non-small cell lung cancer and promotes cancer cell invasion via EGFR-ERK-mediated up-regulation of MMP-2 and MMP-9. Am. J. Pathol 2013, 182, 954–964. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Cai, H.; Hong, Y.; Li, Y.; Su, D.; Fan, Z. Long non-coding RNA MIAT promotes growth and metastasis of colorectal cancer cells through regulation of miR-132/Derlin-1 pathway. Cancer Cell Int. 2018, 18, 59. [Google Scholar] [CrossRef]
- Li, D.; Shi, M.; Ji, H.; Chen, G.; Jiang, H.; Wang, Z. MicroRNA-181d is a tumor suppressor in human esophageal squamous cell carcinoma inversely regulating Derlin-1. Oncol. Rep. 2016, 36, 2041–2048. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.A.; Jewell, M.L.; Kanke, M.; Francisco, A.; Sritharan, R.; Turnham, R.E.; Lee, S.; Kastenhuber, E.R.; Wauthier, E.; Guy, C.D.; et al. MicroRNA-375 Suppresses the Growth and Invasion of Fibrolamellar Carcinoma. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 803–817. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Ye, M.L.; Zhang, Y.P.; Li, W.J.; Li, M.T.; Wang, H.Z.; Qiu, X.; Xu, Y.; Yin, J.W.; Hu, Q.; et al. MicroRNA-375-3p enhances chemosensitivity to 5-fluorouracil by targeting thymidylate synthase in colorectal cancer. Cancer Sci. 2020, 111, 1528–1541. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, T.; Sun, F.F.; Feng, J.Q.; Peng, J.J.; Li, H.; Wang, C.; Wang, D.; Liu, Y.; Bai, Y.D.; et al. MicroRNA-375 represses tumor angiogenesis and reverses resistance to sorafenib in hepatocarcinoma. Cancer Gene Ther. 2021, 28, 126–140. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Xu, M.; Liu, X.; Pan, B.; Qin, J.; Xu, T.; Zeng, K.; Pan, Y.; He, B.; et al. miR-375-3p suppresses tumorigenesis and partially reverses chemoresistance by targeting YAP1 and SP1 in colorectal cancer cells. Aging 2019, 11, 7357–7385. [Google Scholar] [CrossRef]
- Wei, L.J.; Bai, D.M.; Wang, Z.Y.; Liu, B.C. MicroRNA-375 accelerates the invasion and migration of colorectal cancer through targeting RECK. Eur. Rev. Med. Pharm. Sci 2019, 23, 4738–4745. [Google Scholar] [CrossRef]
- Falzone, L.; Candido, S.; Salemi, R.; Basile, M.S.; Scalisi, A.; McCubrey, J.A.; Torino, F.; Signorelli, S.S.; Montella, M.; Libra, M. Computational identification of microRNAs associated to both epithelial to mesenchymal transition and NGAL/MMP-9 pathways in bladder cancer. Oncotarget 2016, 7, 72758–72766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashrafizadeh, M.; Hushmandi, K.; Hashemi, M.; Akbari, M.E.; Kubatka, P.; Raei, M.; Koklesova, L.; Shahinozzaman, M.; Mohammadinejad, R.; Najafi, M.; et al. Role of microRNA/Epithelial-to-Mesenchymal Transition Axis in the Metastasis of Bladder Cancer. Biomolecules 2020, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Kikuchi, E.; Mikami, S.; Miyajima, A.; Shirotake, S.; Ishida, M.; Okada, Y.; Oya, M. Expression of snail in upper urinary tract urothelial carcinoma: Prognostic significance and implications for tumor invasion. Clin. Cancer Res. 2010, 16, 5814–5823. [Google Scholar] [CrossRef] [Green Version]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 2015, 14, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Ikenouchi, J.; Matsuda, M.; Furuse, M.; Tsukita, S. Regulation of tight junctions during the epithelium-mesenchyme transition: Direct repression of the gene expression of claudins/occludin by Snail. J. Cell Sci. 2003, 116, 1959–1967. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, Y.; Matyunina, L.V.; Akbar, A.; McDonald, J.F. The ability of miRNAs to induce mesenchymal-to-epithelial transition (MET) in cancer cells is highly dependent upon genetic background. Cancer Lett. 2020, 480, 15–23. [Google Scholar] [CrossRef] [PubMed]
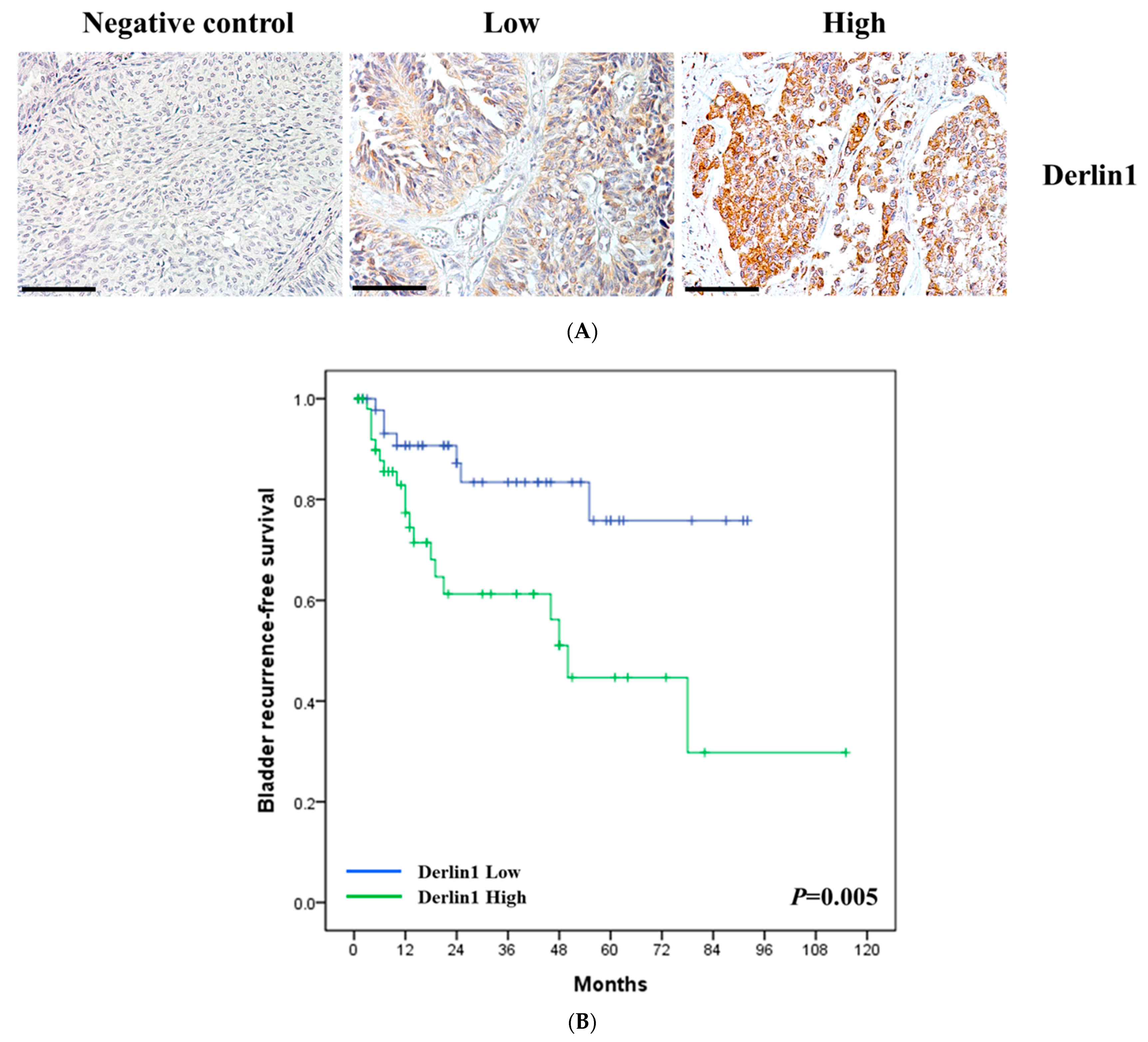
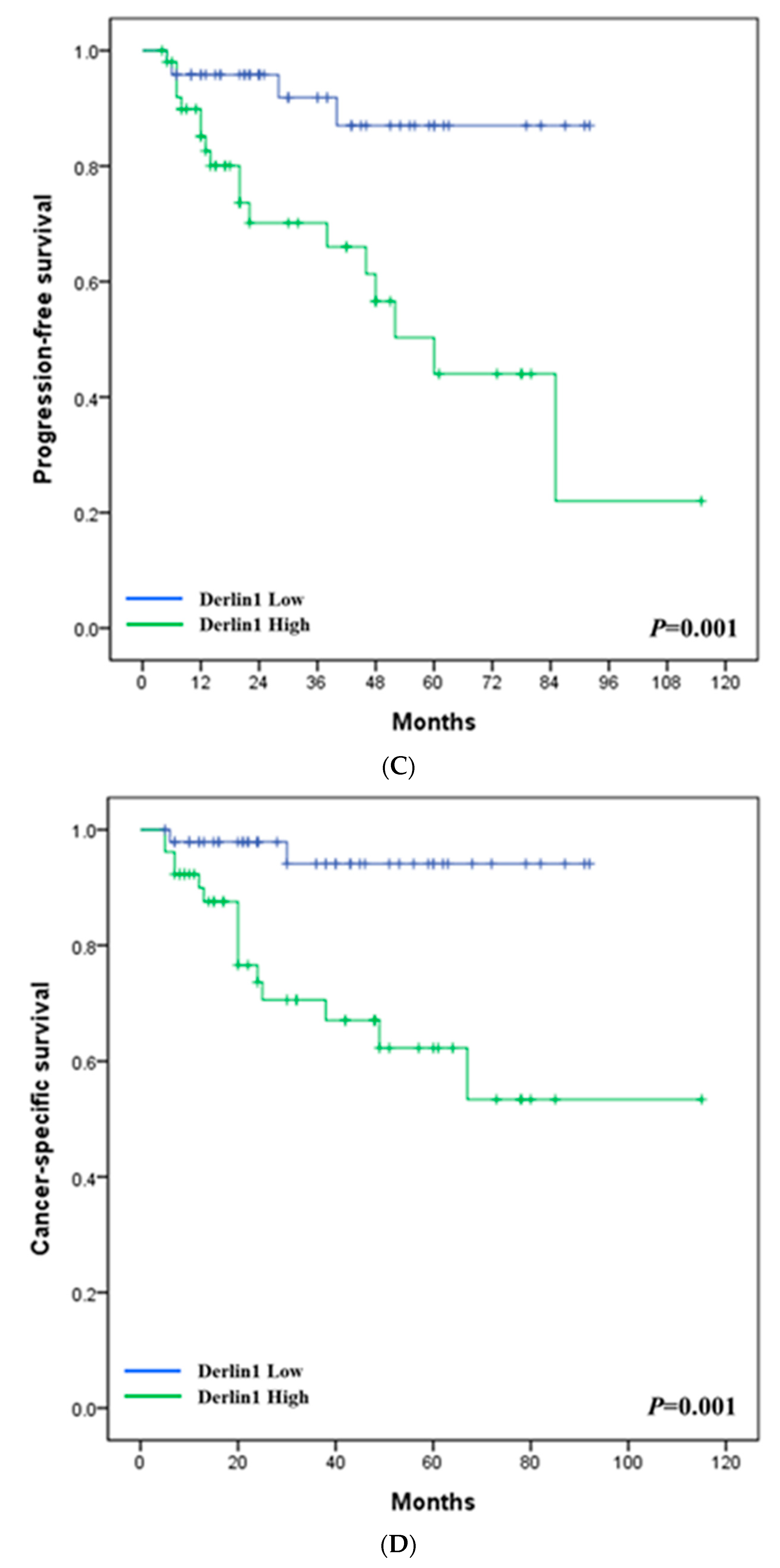
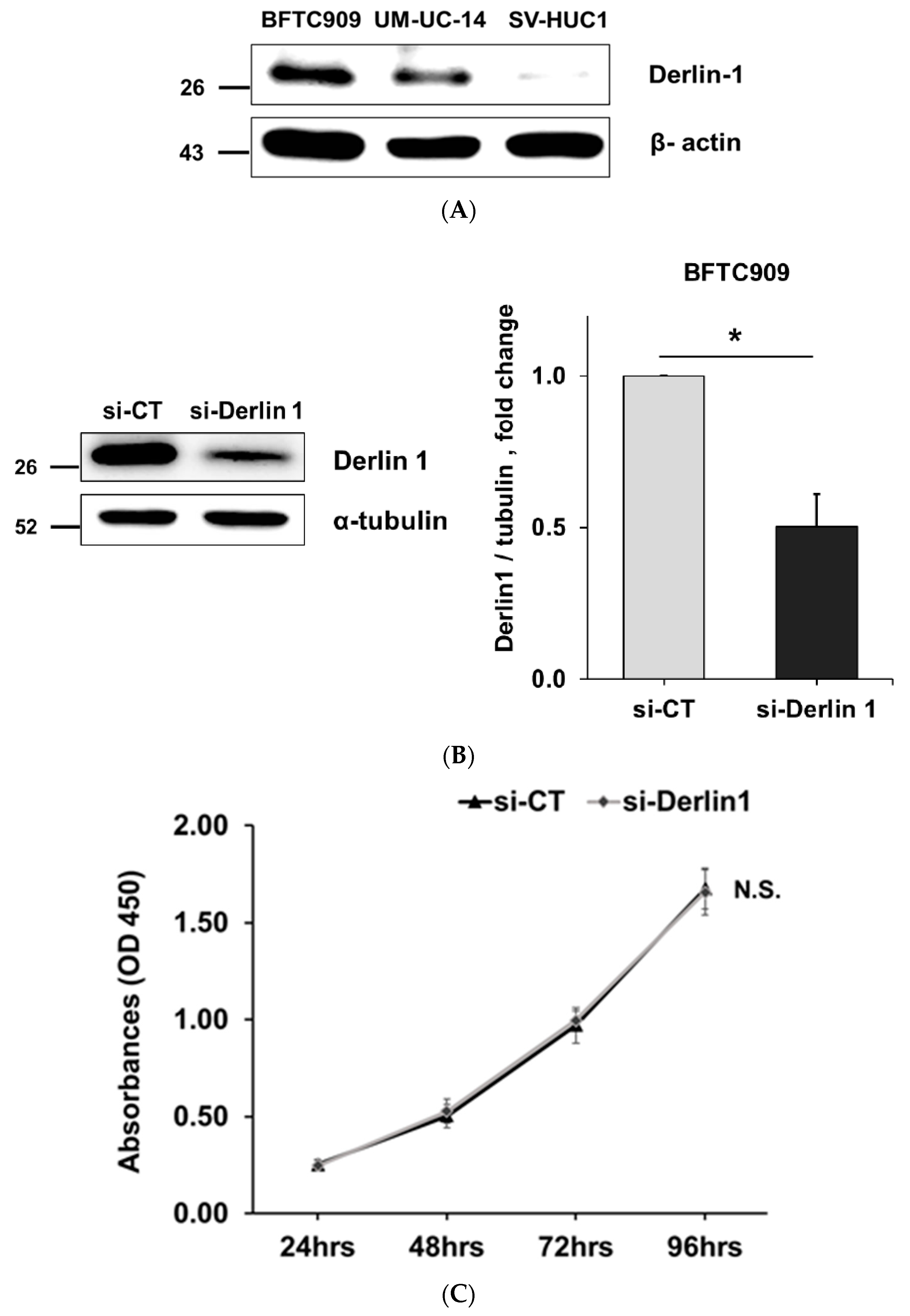
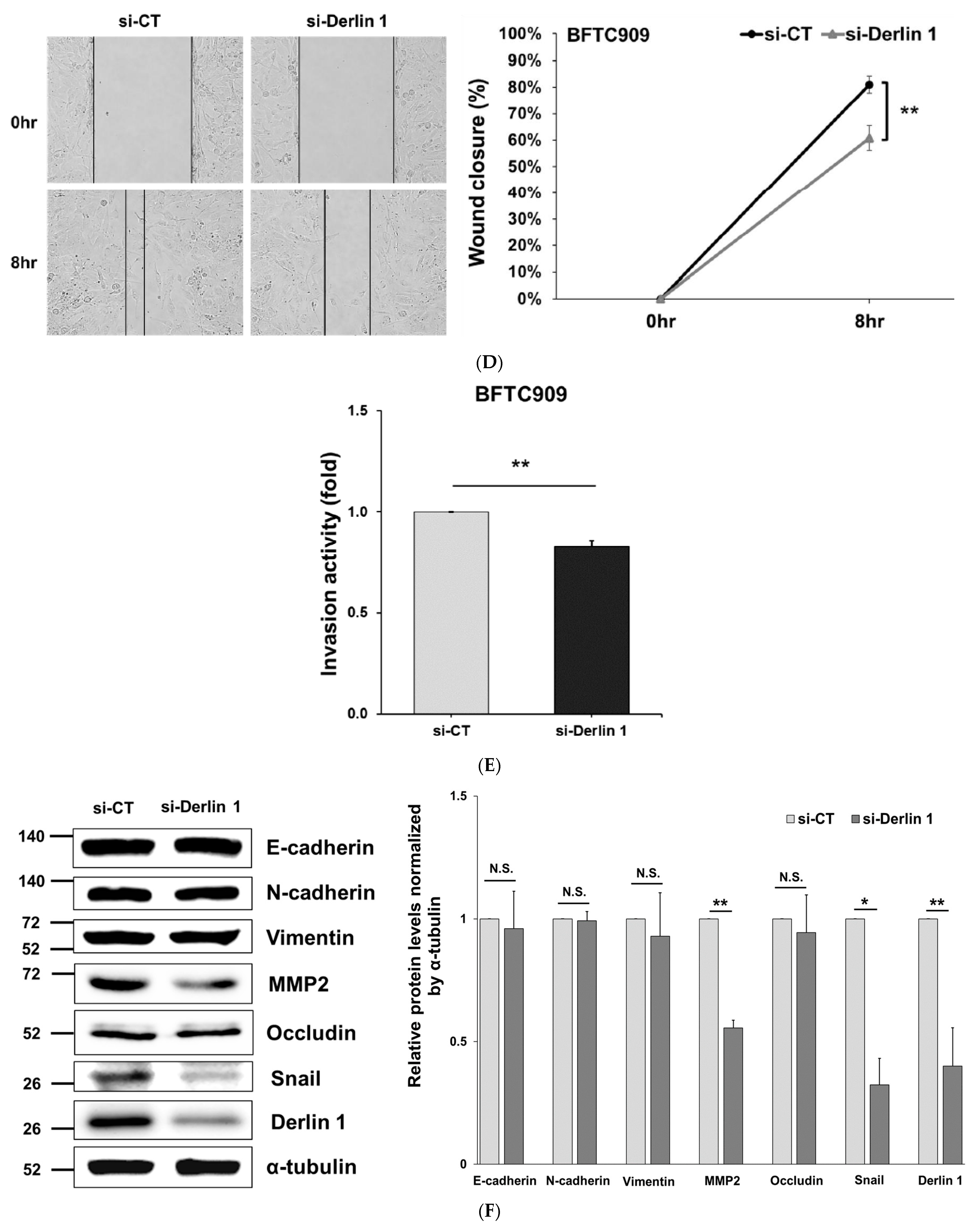



| Derlin-1 | |||||||
|---|---|---|---|---|---|---|---|
| Low | High | ||||||
| Variables | Item | Patient No. | No. | % | No. | % | p Value |
| Overall | 100 | 48 | 48 | 52 | 52 | ||
| Stage | I/II | 61 | 35 | 72.9 | 26 | 50 | 0.019 # |
| III/IV | 39 | 13 | 27.1 | 26 | 50 | ||
| Grade | Low | 22 | 13 | 27.1 | 9 | 17.3 | 0.238 # |
| High | 78 | 35 | 72.9 | 43 | 82.7 | ||
| Gender | Female | 58 | 28 | 58.3 | 30 | 57.7 | 0.948 # |
| Male | 42 | 20 | 41.7 | 22 | 42.3 | ||
| Age (years) | <65 | 34 | 17 | 35.4 | 17 | 32.7 | 0.774 # |
| ≥65 | 66 | 31 | 64.6 | 35 | 67.3 | ||
| Tumor size (cm) | <2 | 20 | 12 | 25 | 8 | 15.4 | 0.230 # |
| ≥2 | 80 | 36 | 75 | 44 | 84.6 | ||
| LN Metastasis | No | 87 | 44 | 91.7 | 43 | 82.7 | 0.182 ## |
| Yes | 13 | 4 | 30.8 | 9 | 17.3 | ||
| Location | Renal pelvis | 50 | 22 | 45.8 | 28 | 53.8 | 0.643 # |
| Ureter | 39 | 21 | 43.8 | 18 | 34.6 | ||
| Both | 11 | 5 | 10.4 | 6 | 11.5 | ||
| Tumor number | Single | 89 | 43 | 89.6 | 46 | 88.5 | 1.000 # |
| Multiple | 11 | 5 | 10.4 | 6 | 11.5 | ||
| Distant Metastasis | Negative | 82 | 44 | 91.7 | 38 | 73.1 | 0.019 ## |
| Positive | 18 | 4 | 8.3 | 14 | 26.9 | ||
| Bladder recurrence | Negative | 74 | 41 | 85.4 | 33 | 63.5 | 0.012 # |
| Positive | 26 | 7 | 14.6 | 19 | 36.5 | ||
| Local recurrence | Negative | 78 | 44 | 91.7 | 34 | 65.4 | 0.002 ## |
| Positive | 22 | 4 | 8.3 | 18 | 34.6 | ||
| Cancer Death | No | 83 | 46 | 95.8 | 37 | 71.2 | 0.001 ## |
| Yes | 17 | 2 | 4.2 | 15 | 28.8 | ||
| Variables | Item | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| Stage | III/IV | 1.2 | (0.54, 2.66) | 0.661 | - | - | - |
| I/II | 1 | - | |||||
| Grade | High | 0.91 | (0.37, 2.28) | 0.845 | - | - | - |
| Low | 1 | - | |||||
| Gender | Male | 1.21 | (0.56, 2.61) | 0.63 | - | - | - |
| Female | 1 | - | |||||
| Age (years) | ≥65 | 1.75 | (0.76, 4.07) | 0.191 | 1.98 | (0.84, 4.67) | 0.117 |
| <65 | 1 | 1 | |||||
| Tumor number | Multiple | 1.97 | (0.58, 6.65) | 0.275 | 2.97 | (0.83, 10.60) | 0.093 |
| Single | 1 | 1 | |||||
| Derlin-1 | High | 3.27 | (1.37, 7.80) | 0.008 | 3.62 | (1.50, 8.76) | 0.004 |
| Low | 1 | 1 | |||||
| Variables | Item | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| Stage | III/IV | 6.72 | (2.41, 18.68) | <0.001 | 4.81 | (1.65, 14.06) | 0.004 |
| I/II | 1 | 1 | |||||
| Grade | High | 6.86 | (0.92, 51.13) | 0.06 | 4.42 | (0.56, 34.60) | 0.157 |
| Low | 1 | 1 | |||||
| Gender | Male | 0.8 | (0.34, 1.91) | 0.618 | - | - | - |
| Female | 1 | - | |||||
| Age (years) | ≥65 | 1.33 | (0.54, 3.26) | 0.539 | - | - | - |
| <65 | 1 | - | |||||
| Tumor number | Multiple | 2.16 | (0.63, 7.47) | 0.222 | 2.78 | (0.76, 10.14) | 0.121 |
| Single | 1 | 1 | |||||
| Derlin-1 | High | 5.07 | (1.71, 15.00) | 0.003 | 4.68 | (1.52, 14.44) | 0.007 |
| Low | 1 | 1 | |||||
| Variables | Item | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| Stage | III/IV | 7.07 | (2.22, 22.51) | 0.001 | 5.65 | (1.75, 18.26) | 0.004 |
| I/II | 1 | 1 | |||||
| Grade | High | 2.38 | (0.54, 10.40) | 0.25 | 1.52 | (0.30, 7.75) | 0.615 |
| Low | 1 | 1 | |||||
| Gender | Male | 1.11 | (0.43, 2.88) | 0.837 | - | - | - |
| Female | 1 | - | |||||
| Age (years) | ≥65 | 1.94 | (0.63, 5.97) | 0.246 | 2.92 | (0.87, 9.77) | 0.082 |
| <65 | 1 | 1 | |||||
| Tumor number | Multiple | 2.54 | (0.73, 8.87) | 0.145 | 2.98 | (0.80, 11.12) | 0.104 |
| Single | 1 | 1 | |||||
| Derlin-1 | High | 7.81 | (1.78, 34.16) | 0.006 | 6.24 | (1.39, 27.97) | 0.017 |
| Low | 1 | 1 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jhan, J.-H.; Hsu, W.-C.; Lee, Y.-C.; Li, W.-M.; Huang, A.-M.; Lin, H.-H.; Wang, C.-S.; Wu, Y.-R.; Li, C.-C.; Wu, W.-J.; et al. MicroRNA-375-3p Suppresses Upper Tract Urothelial Carcinoma Cell Migration and Invasion via Targeting Derlin-1. Cancers 2022, 14, 880. https://doi.org/10.3390/cancers14040880
Jhan J-H, Hsu W-C, Lee Y-C, Li W-M, Huang A-M, Lin H-H, Wang C-S, Wu Y-R, Li C-C, Wu W-J, et al. MicroRNA-375-3p Suppresses Upper Tract Urothelial Carcinoma Cell Migration and Invasion via Targeting Derlin-1. Cancers. 2022; 14(4):880. https://doi.org/10.3390/cancers14040880
Chicago/Turabian StyleJhan, Jhen-Hao, Wei-Chi Hsu, Yi-Chen Lee, Wei-Ming Li, A-Mei Huang, Hui-Hui Lin, Chien-Sheng Wang, Yi-Ru Wu, Ching-Chia Li, Wen-Jeng Wu, and et al. 2022. "MicroRNA-375-3p Suppresses Upper Tract Urothelial Carcinoma Cell Migration and Invasion via Targeting Derlin-1" Cancers 14, no. 4: 880. https://doi.org/10.3390/cancers14040880
APA StyleJhan, J.-H., Hsu, W.-C., Lee, Y.-C., Li, W.-M., Huang, A.-M., Lin, H.-H., Wang, C.-S., Wu, Y.-R., Li, C.-C., Wu, W.-J., & Ke, H.-L. (2022). MicroRNA-375-3p Suppresses Upper Tract Urothelial Carcinoma Cell Migration and Invasion via Targeting Derlin-1. Cancers, 14(4), 880. https://doi.org/10.3390/cancers14040880








