DKK3, Downregulated in Invasive Epithelial Ovarian Cancer, Is Associated with Chemoresistance and Enhanced Paclitaxel Susceptibility via Inhibition of the β-Catenin-P-Glycoprotein Signaling Pathway
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemical Analysis
2.3. Cell Culture
2.4. Establishment of Paclitaxel-Resistant Cell Lines
2.5. Preparation of Recombinant Human DKK3
2.6. Preparation of Conditioned Medium
2.7. Three-Dimensional (3D) Spheroid Assay
2.8. Immunofluorescence Analysis
2.9. Western Blot Analysis
2.10. MTT Assay
2.11. Migration Assay
2.12. Chemicals and Antibodies
2.13. Statistical Analysis
3. Results
3.1. Aberrant Downregulation of DKK3 in Invasive Ovarian Carcinoma
3.2. Characteristics and Survival Analysis of 42 Patients with Serous Ovarian Carcinoma
3.3. DKK3 Loss Was Significantly Associated with Chemoresistant Ovarian Cancer
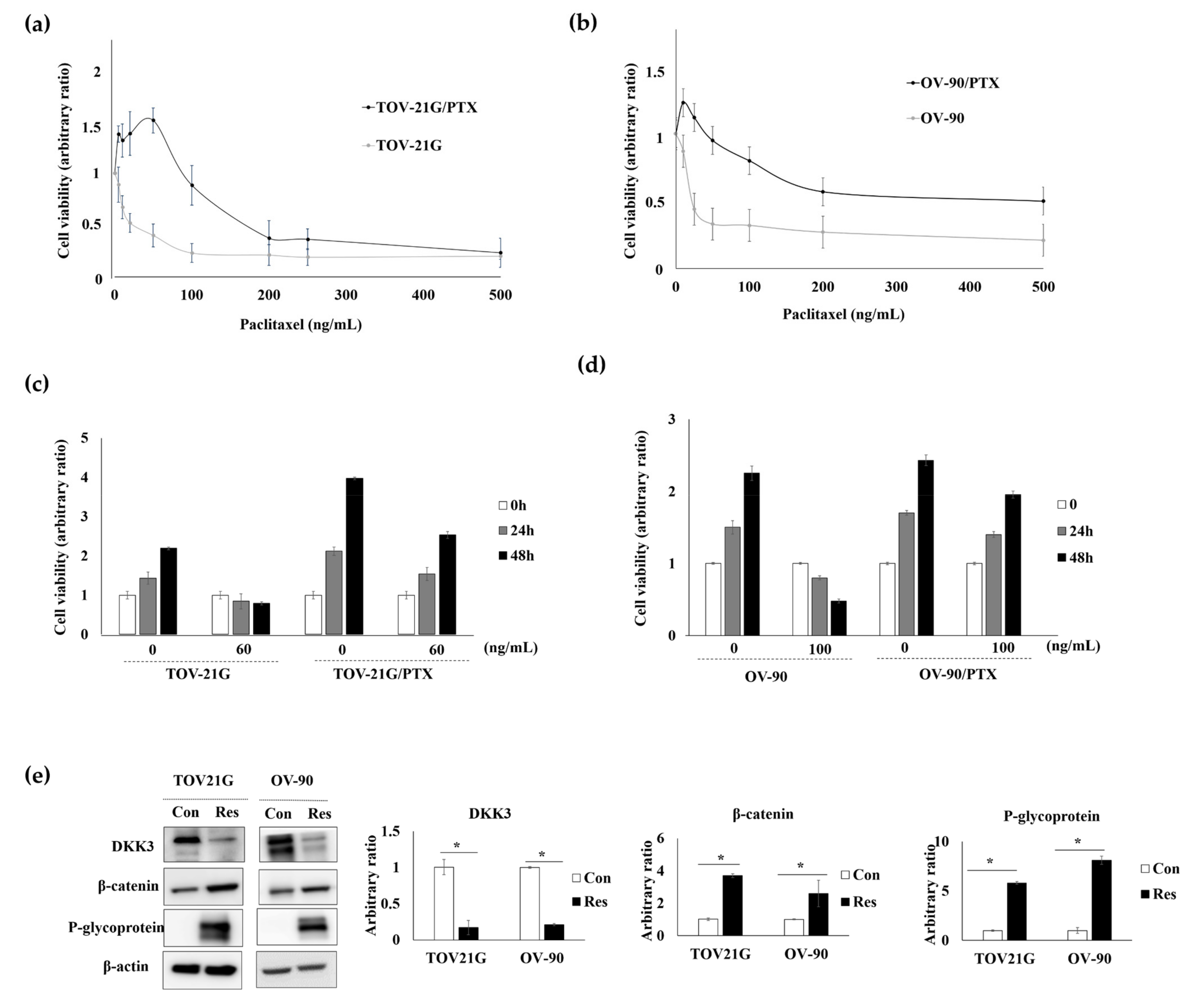
3.4. DKK3 Was Lost in Paclitaxel-Resistant Ovarian Cancer Cells
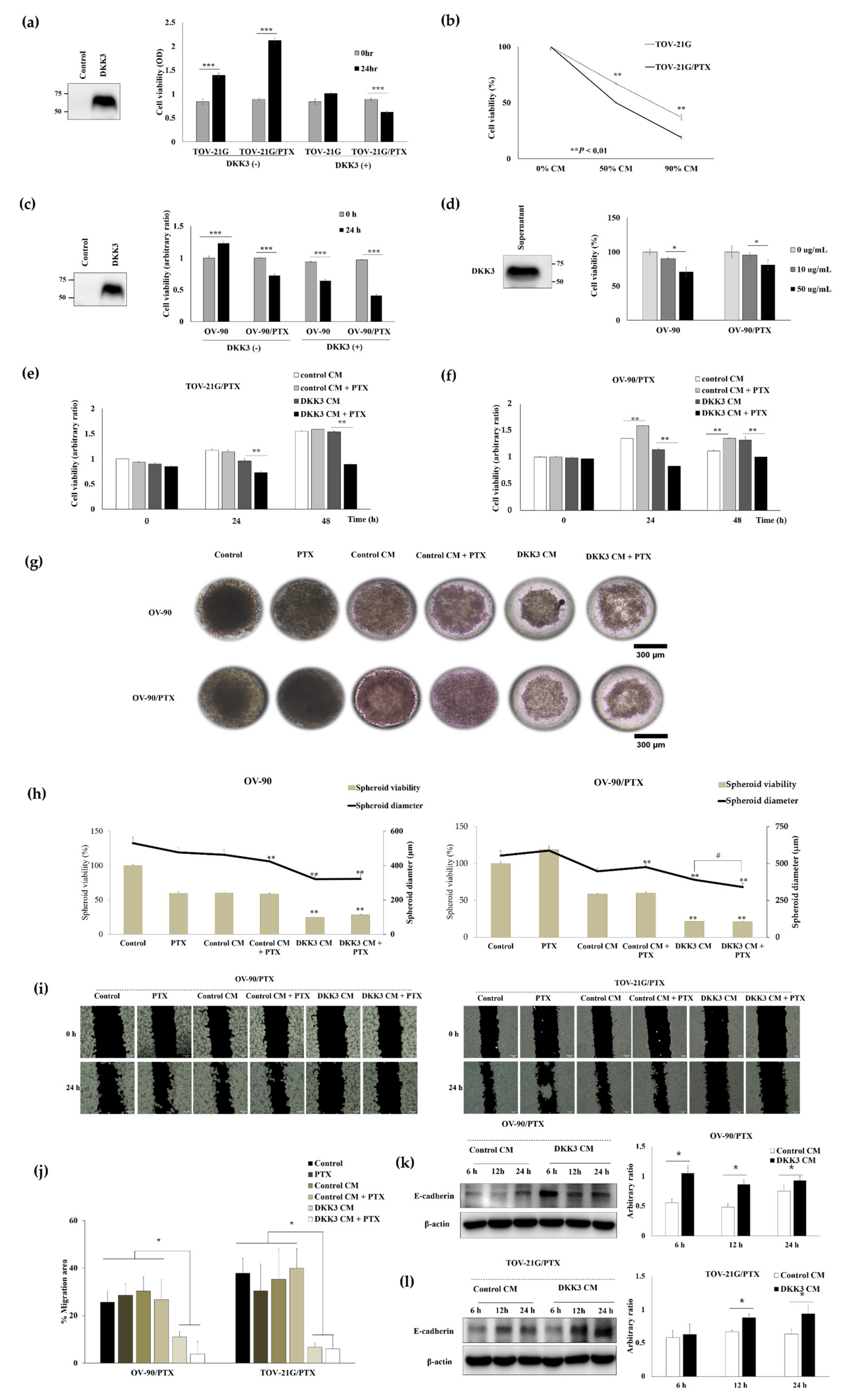
3.5. Secreted DKK3 Enhanced Paclitaxel Susceptibility of Ovarian Cancer Cells
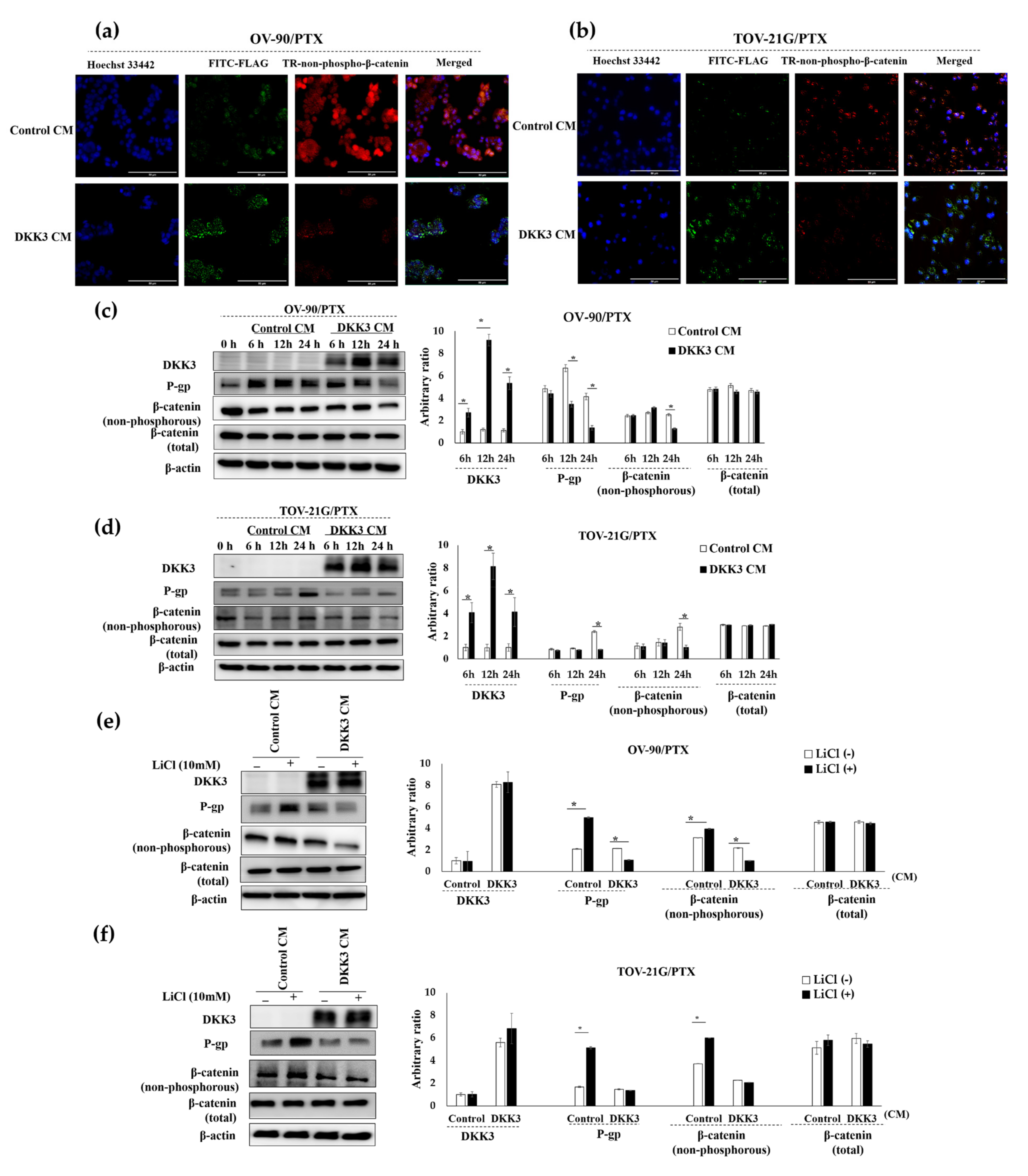
3.6. Secreted DKK3 Enhanced Paclitaxel Susceptibility of Ovarian Cancer Cells via Inhibition of β-Catenin-P-Glycoprotein Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.Y.; Kim, E.Y.; Jung, K.W.; Shin, A.; Chan, K.K.; Aoki, D.; Kim, J.W.; Low, J.J.; Won, Y.J. Trends in gynecologic cancer mortality in East Asian regions. J. Gynecol. Oncol. 2014, 25, 174–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.R.; Shih, I.-M. Ovarian cancer. Annu. Rev. Pathol. 2009, 4, 287–313. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Miyazaki, M.; Sakaguchi, M.; Inoue, Y.; Namba, M. A REIC gene shows down-regulation in human immortalized cells and human tumor-derived cell lines. Biochem. Biophys. Res. Commun. 2000, 268, 20–24. [Google Scholar] [CrossRef]
- Hsieh, S.Y.; Hsieh, P.S.; Chiu, C.T.; Chen, W.Y. Dickkopf-3/REIC functions as a suppressor gene of tumor growth. Oncogene 2004, 23, 9183–9189. [Google Scholar] [CrossRef] [Green Version]
- Lorsy, E.; Topuz, A.S.; Geisler, C.; Stahl, S.; Garczyk, S.; von Stillfried, S.; Hoss, M.; Gluz, O.; Hartmann, A.; Knüchel, R.; et al. Loss of Dickkopf 3 Promotes the Tumorigenesis of Basal Breast Cancer. PLoS ONE 2016, 11, e0160077. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, S.; Xia, P.; Zheng, H.C. Down-regulated REIC expression in lung carcinogenesis: A molecular target for gene therapy. Histol. Histopathol. 2018, 33, 691–704. [Google Scholar] [CrossRef]
- Hirata, T.; Watanabe, M.; Kaku, H.; Kobayashi, Y.; Yamada, H.; Sakaguchi, M.; Takei, K.; Huh, N.H.; Nasu, Y.; Kumon, H. REIC/Dkk-3-encoding adenoviral vector as a potentially effective therapeutic agent for bladder cancer. Int. J. Oncol. 2012, 41, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Gondkar, K.; Patel, K.; Patil Okaly, G.V.; Nair, B.; Pandey, A.; Gowda, H.; Kumar, P. Dickkopf Homolog 3 (DKK3) Acts as a Potential Tumor Suppressor in Gallbladder Cancer. Front. Oncol. 2019, 9, 1121. [Google Scholar] [CrossRef]
- Ryu, S.W.; Kim, J.H.; Kim, M.K.; Lee, Y.J.; Park, J.S.; Park, H.M.; Kim, D.H.; Lee, S.H.; Lee, E.J. Reduced expression of DKK3 is associated with adverse clinical outcomes of uterine cervical squamous cell carcinoma. Int. J. Gynecol. Cancer 2013, 23, 134–140. [Google Scholar] [CrossRef]
- Xi, M.; Cheng, L.; Hua, W.; Zhou, Y.L.; Gao, Q.L.; Yang, J.X.; Qi, S.Y. MicroRNA-95-3p promoted the development of prostatic cancer via regulating DKK3 and activating Wnt/β-catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1002–1011. [Google Scholar] [CrossRef]
- Lee, E.J.; Nguyen, Q.T.T.; Lee, M. Dickkopf-3 in Human Malignant Tumours: A Clinical Viewpoint. Anticancer Res. 2020, 40, 5969–5979. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, M.; Cao, B.; Sheng, X.; Li, P. Methylation of the DKK3 promoter is associated with poor prognosis in patients with cervical adenocarcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 788–794. [Google Scholar]
- Fong, D.; Hermann, M.; Untergasser, G.; Pirkebner, D.; Draxl, A.; Heitz, M.; Moser, P.; Margreiter, R.; Hengster, P.; Amberger, A. Dkk-3 expression in the tumor endothelium: A novel prognostic marker of pancreatic adenocarcinomas. Cancer Sci. 2009, 100, 1414–1420. [Google Scholar] [CrossRef]
- Lee, E.J.; Jo, M.; Rho, S.B.; Park, K.; Yoo, Y.N.; Park, J.; Chae, M.; Zhang, W.; Lee, J.H. Dkk3, downregulated in cervical cancer, functions as a negative regulator of beta-catenin. Int. J. Cancer 2009, 124, 287–297. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, M.K.; Chi, K.C.; Kim, J.H.; Lee, S.H.; Lee, E.J. Aberrant loss of dickkopf-3 in gastric cancer: Can it predict lymph node metastasis preoperatively? World J. Surg. 2015, 39, 1018–1025. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, W.; Xu, X.Y.; Nie, X.C.; Yang, X.; Xing, Y.N.; Yu, M.; Liu, Y.P.; Takano, Y.; Zheng, H.C. The clinicopathological significance of REIC expression in colorectal carcinomas. Histol. Histopathol. 2012, 27, 735–743. [Google Scholar] [CrossRef]
- Kim, B.R.; Lee, E.J.; Seo, S.H.; Lee, S.H.; Rho, S.B. Dickkopf-3 (DKK-3) obstructs VEGFR-2/Akt/mTOR signaling cascade by interacting of β2-microglobulin (β2M) in ovarian tumorigenesis. Cell. Signal. 2015, 27, 2150–2159. [Google Scholar] [CrossRef]
- Takata, A.; Terauchi, M.; Hiramitsu, S.; Uno, M.; Wakana, K.; Kubota, T. Dkk-3 induces apoptosis through mitochondrial and Fas death receptor pathways in human mucinous ovarian cancer cells. Int. J. Gynecol. Cancer 2015, 25, 372–379. [Google Scholar] [CrossRef]
- You, A.; Fokas, E.; Wang, L.F.; He, H.; Kleb, B.; Niederacher, D.; Engenhart-Cabillic, R.; An, H.X. Expression of the Wnt antagonist DKK3 is frequently suppressed in sporadic epithelial ovarian cancer. J. Cancer Res. Clin. Oncol. 2011, 137, 621–627. [Google Scholar] [CrossRef]
- Zheng, H.C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef] [Green Version]
- Phatak, V.; von Grabowiecki, Y.; Janus, J.; Officer, L.; Behan, C.; Aschauer, L.; Pinon, L.; Mackay, H.; Zanivan, S.; Norman, J.C.; et al. Mutant p53 promotes RCP-dependent chemoresistance coinciding with increased delivery of P-glycoprotein to the plasma membrane. Cell Death Dis. 2021, 12, 207. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Dai, F.; Zhang, L.; Yuan, M.; Yang, D.; Liu, S.; Cheng, Y. miR-21 Induces Chemoresistance in Ovarian Cancer Cells via Mediating the Expression and Interaction of CD44v6 and P-gp. Onco Targets Ther. 2021, 14, 325–336. [Google Scholar] [CrossRef]
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacotherapy of Cancers. Front. Oncol. 2020, 10, 576559. [Google Scholar] [CrossRef]
- Bebawy, M.; Sze, D.M. Targeting P-glycoprotein for effective oral anti-cancer chemotherapeutics. Curr. Cancer Drug Targets 2008, 8, 47–52. [Google Scholar] [CrossRef]
- Lai, J.I.; Tseng, Y.J.; Chen, M.H.; Huang, C.F.; Chang, P.M. Clinical Perspective of FDA Approved Drugs With P-Glycoprotein Inhibition Activities for Potential Cancer Therapeutics. Front. Oncol. 2020, 10, 561936. [Google Scholar] [CrossRef]
- Shen, D.Y.; Zhang, W.; Zeng, X.; Liu, C.Q. Inhibition of Wnt/β-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. Cancer Sci. 2013, 104, 1303–1308. [Google Scholar] [CrossRef]
- Vang, R.; Shih Ie, M.; Kurman, R.J. Ovarian low-grade and high-grade serous carcinoma: Pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv. Anat. Pathol. 2009, 16, 267–282. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, S.; Ahn, J.; Park, J.; Ryu, B.-Y.; Park, J.Y. Membrane-bottomed microwell array added to Transwell insert to facilitate non-contact co-culture of spermatogonial stem cell and STO feeder cell. Biofabrication 2020, 12, 045031. [Google Scholar] [CrossRef]
- Umesha, S.; Monahar, B.; Naidu, K.A. Microencapsulation of α-linolenic acid-rich garden cress seed oil: Physical characteristics and oxidative stability. Eur. J. Lipid Sci. Technol. 2013, 115, 1474–1482. [Google Scholar] [CrossRef]
- Niehrs, C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006, 25, 7469–7481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Van Roy, F.; Berx, G. The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 2008, 65, 3756–3788. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, Y.; Watanabe, M.; Sadahira, T.; Ariyoshi, Y.; Kobayashi, Y.; Araki, M.; Wada, K.; Ochiai, K.; Li, S.A.; Nasu, Y. Overexpression of REIC/Dkk-3 suppresses the expression of CD147 and inhibits the proliferation of human bladder cancer cells. Oncol. Lett. 2017, 14, 3223–3228. [Google Scholar] [CrossRef] [Green Version]
- Mori, A.; Watanabe, M.; Sadahira, T.; Kobayashi, Y.; Ariyoshi, Y.; Ueki, H.; Wada, K.; Ochiai, K.; Li, S.A.; Nasu, Y. The Downregulation of the Expression of CD147 by Tumor Suppressor REIC/Dkk-3, and Its Implication in Human Prostate Cancer Cell Growth Inhibition. Acta Med. Okayama 2017, 71, 135–142. [Google Scholar] [CrossRef]
- Yue, W.; Sun, Q.; Dacic, S.; Landreneau, R.J.; Siegfried, J.M.; Yu, J.; Zhang, L. Downregulation of Dkk3 activates beta-catenin/TCF-4 signaling in lung cancer. Carcinogenesis 2008, 29, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Abarzua, F.; Sakaguchi, M.; Takaishi, M.; Nasu, Y.; Kurose, K.; Ebara, S.; Miyazaki, M.; Namba, M.; Kumon, H.; Huh, N.-H. Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer Res. 2005, 65, 9617–9622. [Google Scholar] [CrossRef] [Green Version]
- Mühlmann, G.; Untergasser, G.; Zitt, M.; Zitt, M.; Maier, H.; Mikuz, G.; Kronberger, I.E.; Haffner, M.C.; Gunsilius, E.; Öfner, D. Immunohistochemically detectable dickkopf-3 expression in tumor vessels predicts survival in gastric cancer. Virchows Archiv 2010, 456, 635–646. [Google Scholar] [CrossRef]
- Jiang, T.; Huang, L.; Wang, S.; Zhang, S. Clinical significance of serum Dkk-3 in patients with gynecological cancer. J. Obstet. Gynaecol. Res. 2010, 36, 769–773. [Google Scholar] [CrossRef]
- Tanaka, E.; Uchida, D.; Shiraha, H.; Kato, H.; Ohyama, A.; Iwamuro, M.; Watanabe, M.; Kumon, H.; Okada, H. Promising Gene Therapy Using an Adenovirus Vector Carrying REIC/Dkk-3 Gene for the Treatment of Biliary Cancer. Curr. Gene Ther. 2020, 20, 64–70. [Google Scholar] [CrossRef]
- Sawahara, H.; Shiraha, H.; Uchida, D.; Kato, H.; Kato, R.; Oyama, A.; Nagahara, T.; Iwamuro, M.; Horiguchi, S.; Tsutsumi, K.; et al. Promising therapeutic efficacy of a novel reduced expression in immortalized cells/dickkopf-3 expressing adenoviral vector for hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2017, 32, 1769–1777. [Google Scholar] [CrossRef]
- Araki, K.; Yamamuro, N.; Tomonobu, N.; Kumon, H. REIC/Dkk-3 Gene Therapy Induces Immunogenic Cell Death in a Mouse Model of Malignant Mesothelioma. Anticancer Res. 2021, 41, 4837–4855. [Google Scholar] [CrossRef]
- Shi, X.; Dou, Y.; Zhou, K.; Huo, J.; Yang, T.; Qin, T.; Liu, W.; Wang, S.; Yang, D.; Chang, L. Targeting the Bcl-2 family and P-glycoprotein reverses paclitaxel resistance in human esophageal carcinoma cell line. Biomed. Pharmacother. 2017, 90, 897–905. [Google Scholar] [CrossRef]
- Fujihara, T.; Mizobuchi, Y.; Nakajima, K.; Kageji, T.; Matsuzaki, K.; Kitazato, K.T.; Otsuka, R.; Hara, K.; Mure, H.; Okazaki, T.; et al. Down-regulation of MDR1 by Ad-DKK3 via Akt/NFκB pathways augments the anti-tumor effect of temozolomide in glioblastoma cells and a murine xenograft model. J. Neurooncol. 2018, 139, 323–332. [Google Scholar] [CrossRef]
- Kawasaki, K.; Watanabe, M.; Sakaguchi, M.; Ogasawara, Y.; Ochiai, K.; Nasu, Y.; Doihara, H.; Kashiwakura, Y.; Huh, N.H.; Kumon, H.; et al. REIC/Dkk-3 overexpression downregulates P-glycoprotein in multidrug-resistant MCF7/ADR cells and induces apoptosis in breast cancer. Cancer Gene Ther. 2009, 16, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Winston, J.T.; Strack, P.; Beer-Romero, P.; Chu, C.Y.; Elledge, S.J.; Harper, J.W. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999, 13, 270–283. [Google Scholar] [CrossRef]
- Lee, E.-J.; Cho, M.; Rho, S.B.; Park, J.P.; Chae, D.-A.; Nguyen, Q.T.T. β-TrCP1-variant 4, a novel splice variant of β-TrCP1, is a negative regulator of β-TrCP1-variant 1 in β-catenin degradation. Biochem. Biophys. Res. Commun. 2021, 542, 9–16. [Google Scholar] [CrossRef]
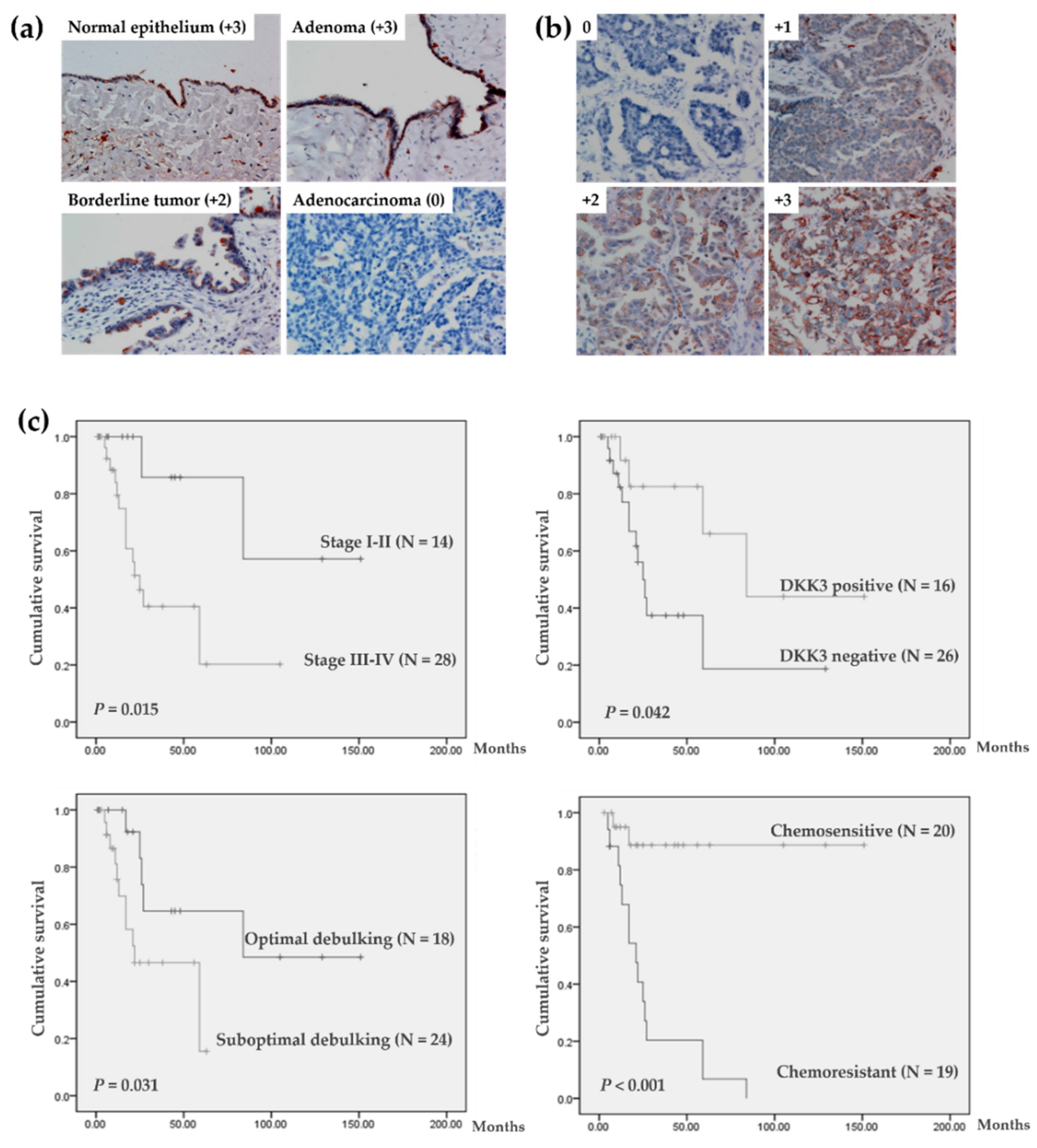
| Immunoreactivity, N (%) | ||||||
|---|---|---|---|---|---|---|
| 0 | +1 | +2 | +3 | Total | p-Value | |
| Normal epithelium | 0 | 2 (13.3) | 6 (40) | 7 (46.7) | 15 (100) | <0.001 * |
| Benign adenoma | 0 | 4 (21.1) | 8 (42.1) | 7 (36.8) | 19 (100) | |
| Borderline tumor | 2 (20.0) | 5 (50.0) | 2 (20.0) | 1 (10.0) | 10 (100) | |
| Invasive carcinoma | 46 (56.1) | 16 (19.5) | 6 (7.3) | 14 (17.1) | 82 (100) | |
| Mucinous | 10 | 0 | 1 | 2 | 13 | |
| Serous | 26 | 11 | 4 | 1 | 42 | |
| Endometrioid | 3 | 2 | 0 | 0 | 5 | |
| Transitional cell | 0 | 1 | 0 | 11 | 12 | |
| Clear cell | 1 | 1 | 1 | 0 | 3 | |
| Undifferentiated | 6 | 1 | 0 | 0 | 7 | |
| Clinicopathological Parameters | N (%) | p-Value | |
|---|---|---|---|
| Univariate | Multivariate | ||
| Age | |||
| Mean (range), year | 53.2 (24–77) | ||
| CA125 | |||
| ≤35 U/mL | 3 (7.1) | ||
| >35 U/mL | 39 (92.9) | ||
| FIGO stage | 0.015 | * NS | |
| I–II | 14 (33.3) | ||
| III–IV | 28 (66.7) | ||
| Type | NS | ||
| I | 12 (28.6) | ||
| II | 30 (71.4) | ||
| DKK3 protein expression | 0.042 | NS | |
| Negative | 26 (61.9) | ||
| Positive | 16 (38.1) | ||
| Debulking surgery | 0.031 | NS | |
| Optimal | 18 (42.9) | ||
| Suboptimal | 24 (57.1) | ||
| Chemo-response | <0.001 | <0.01 | |
| Sensitive | 20 (47.6) | ||
| Resistant | 19 (45.2) | ||
| Unknown | 3 (7.2) | ||
| Clinicopathological Parameters | DKK3 Expression | ||
|---|---|---|---|
| Negative (n = 26) | Positive (n = 16) | p-Value | |
| Age | * NS | ||
| Mean (±SD), year | 52.9 (±11.8) | 54.3 (±12.5) | |
| CA125 | NS | ||
| ≤35 U/mL | 1 | 2 | |
| >35 U/mL | 25 | 14 | |
| FIGO stage | NS | ||
| I–II | 7 | 7 | |
| III–IV | 19 | 9 | |
| Type | NS | ||
| I | 7 | 5 | |
| II | 19 | 11 | |
| Debulking operation | NS | ||
| Optimal | 10 | 8 | |
| Suboptimal | 16 | 8 | |
| Chemo-response | 0.029 | ||
| Sensitive | 9 | 11 | |
| Resistant | 15 | 4 | |
| Unknown | 2 | 1 | |
| Recurrence | NS | ||
| No | 13 | 12 | |
| Yes | 13 | 4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, Q.T.T.; Park, H.S.; Lee, T.J.; Choi, K.-M.; Park, J.Y.; Kim, D.; Kim, J.H.; Park, J.; Lee, E.-J. DKK3, Downregulated in Invasive Epithelial Ovarian Cancer, Is Associated with Chemoresistance and Enhanced Paclitaxel Susceptibility via Inhibition of the β-Catenin-P-Glycoprotein Signaling Pathway. Cancers 2022, 14, 924. https://doi.org/10.3390/cancers14040924
Nguyen QTT, Park HS, Lee TJ, Choi K-M, Park JY, Kim D, Kim JH, Park J, Lee E-J. DKK3, Downregulated in Invasive Epithelial Ovarian Cancer, Is Associated with Chemoresistance and Enhanced Paclitaxel Susceptibility via Inhibition of the β-Catenin-P-Glycoprotein Signaling Pathway. Cancers. 2022; 14(4):924. https://doi.org/10.3390/cancers14040924
Chicago/Turabian StyleNguyen, Que Thanh Thanh, Hwang Shin Park, Tae Jin Lee, Kyung-Mi Choi, Joong Yull Park, Daehan Kim, Jae Hyung Kim, Junsoo Park, and Eun-Ju Lee. 2022. "DKK3, Downregulated in Invasive Epithelial Ovarian Cancer, Is Associated with Chemoresistance and Enhanced Paclitaxel Susceptibility via Inhibition of the β-Catenin-P-Glycoprotein Signaling Pathway" Cancers 14, no. 4: 924. https://doi.org/10.3390/cancers14040924







