Proton Therapy for Prostate Cancer: Challenges and Opportunities
Abstract
Simple Summary
Abstract
1. Introduction
2. Potential Benefits of PT and Reported Clinical Outcomes of Prostate Treatments
3. Opportunities for Improvement with New Technologies and Innovative Techniques
- The large uncertainties in CT HU values, and CT conversion to mass density or SPR [45,50], can now be overcome by modern CT, which can acquire mass density maps or SPR directly [59] from CT image reconstruction data. Mass density and SPR are less sensitive to patient scan conditions than HU [60], and thus have less uncertainty. With this approach, the range uncertainty could be decreased from 3.5% to 2–2.5%. There is also an increasing interest in dual energy CT (DECT) as an alternative imaging modality for PT treatment planning because of its ability to discriminate between changes in patient density and chemical composition [61]. SPR calculation accuracy was found to be superior, on average, for DECT relative to single energy CT (SECT). Maximum errors of 12.8% and 2.2% were found in SPR data derived from SECT imaging and DECT imaging, respectively [61]. Quantitatively, the maximum dose calculation error in the SECT plan was 7.8%, compared to a value of 1.4% in the DECT plan [62]. Additionally, a novel spectral CT imaging technique based on a dual-layer detector-based approach has been used to demonstrate improvement in SPR prediction for particle therapy treatment planning, and would minimize the beam range uncertainty, allowing for the use of reduced safety margins in patient plans [63].
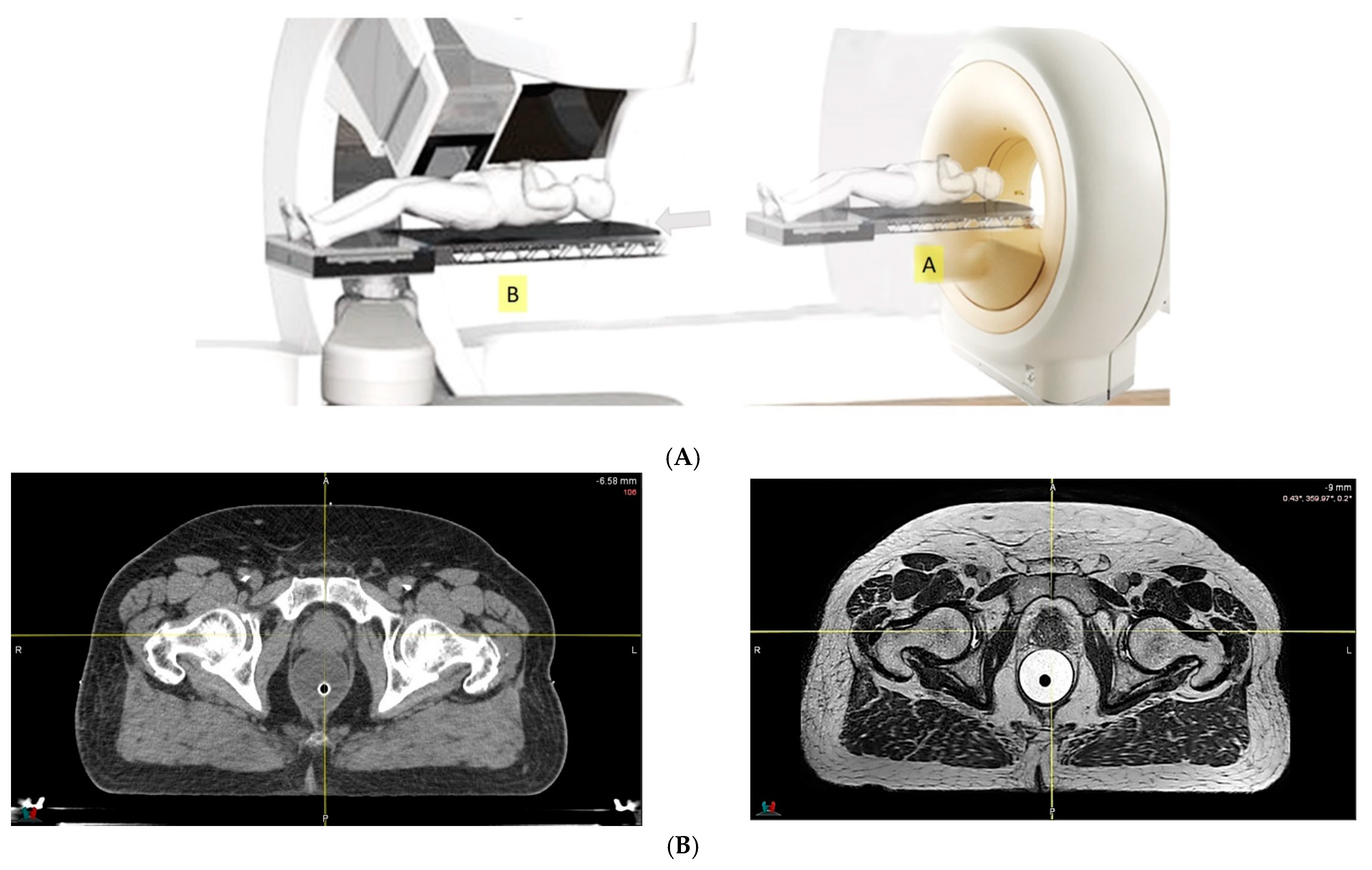
- Because of the inferior soft tissue contrast, orthogonal X-ray imaging systems rely on bony structures for verification of treatment position during patient setup. This type of setup technique can result in large positioning errors due to daily movement of the target and organs at risk (OARs) relative to the bony structures in the former technique. With fiducial markers implanted inside the prostate, many studies concluded that image registration by fiducial markers would reduce matching error. However, some patients may not accept marker implantation. Migration of markers with time may introduce registration errors. Such problems can now be minimized using on-board cone beam CT (CBCT). The better image quality of CBCT can provide 3D images and more information on the anatomic relationships between organs [64,65], which can be used to improve the accuracy of patient setup. Besides patient positioning, CBCT images can also provide information about inter-fractional changes in patient anatomy. In a recent study, an image-based correction method to generate pseudo-CT images from CBCT images was investigated for possible application in proton dose calculations [66] in adaptive PT. MRI, which has the ability to offer fast real-time imaging with high soft tissue contrast in the absence of ionizing radiation exposure [67], is being investigated for use in patient setup in RT. Our study using an external MRI setup room [68] and studies by others [69] indicated that patient positioning accuracy on the order of 1 mm is feasible, and is a significant reduction from that of conventional setup systems.
4. Potential Clinical Benefits and Cost Effectiveness of PT in Treatment of Prostate Cancer
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Endo, M.; Robert, R.W. (1914–2000): The first scientist to propose particle therapy—Use of particle beam for cancer treatment. Radiol. Phys. Technol. 2018, 11, 1–6. [Google Scholar] [CrossRef]
- Chera, B.S.; Vargas, C.; Morris, C.G.; Louis, D.; Flampouri, S.; Yeung, D.; Duvvuri, S.; Li, Z.; Mendenhall, N.P. Dosimetric study of pelvic proton radiotherapy for high-risk prostate cancer. Int. J. Radiat. Oncol. 2009, 75, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Rogers, K.; Lee, T.; Reed, D.; Biggs, C. Dosimetric impact of Acuros XB dose calculation algorithm in prostate cancer treatment using RapidArc. J. Cancer Res. Ther. 2013, 9, 430. [Google Scholar] [CrossRef]
- Lawrence, J.H. Proton irradiation of the pituitary. Cancer 1957, 10, 795–798. [Google Scholar] [CrossRef]
- Smith, A.R. Proton therapy. Phys. Med. Biol. 2006, 51, R491–R504. [Google Scholar] [CrossRef]
- The National Association for Proton Therapy. Proton Therapy Fact Sheet. Available online: https://www.proton-therapy.org/patient-resources/fact-sheet/ (accessed on 24 November 2021).
- Particle Therapy Co-Operative Group. Available online: https://www.ptcog.ch/ (accessed on 31 December 2020).
- Pan, H.Y.; Jiang, J.; Hoffman, K.E.; Tang, C.; Choi, S.L.; Nguyen, Q.-N.; Frank, S.J.; Anscher, M.S.; Shih, Y.-C.T.; Smith, B.D. Comparative toxicities and cost of intensity-modulated radiotherapy, proton radiation, and stereotactic body radiotherapy among younger men with prostate cancer. J. Clin. Oncol. 2018, 36, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Grosshans, D. Proton therapy—Present and future. Adv. Drug Deliv. Rev. 2017, 109, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ertner, D.; Tsujii, H. Particle radiation therapy using proton and heavier ion beams. J. Clin. Oncol. 2007, 25, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT Trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose escalated external beam boost for high- and intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Mohan, R.; Park, P.; Liu, Z.; Li, H.; Li, X.; Li, Y.; Wu, R.; Sahoo, N.; Dong, L.; et al. Dosimetric benefits of robust treatment planning for intensity modulated proton therapy for base-of-skull cancers. Pract. Radiat. Oncol. 2014, 4, 384–391. [Google Scholar] [CrossRef]
- Vargas, C.; Fryer, A.; Mahajan, C.; Indelicato, D.; Horne, D.; Chellini, A.; McKenzie, C.; Lawlor, P.; Henderson, R.; Li, Z.; et al. Dose–volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int. J. Radiat. Oncol. 2008, 70, 744–751. [Google Scholar] [CrossRef]
- Mock, D.D.U.; Bogner, J.; Georg, D.; Auberger, T.; Pötter, R. Comparative treatment planning on localized prostate carcinoma. Strahlenther. Onkol. 2005, 181, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Cella, L.; Lomax, A.; Miralbell, R. Potential role of intensity modulated proton beams in prostate cancer radiotherapy. Int. J. Radiat. Oncol. 2001, 49, 217–223. [Google Scholar] [CrossRef]
- Moteabbed, M.; Trofimov, A.; Sharp, G.C.; Wang, Y.; Zietman, A.L.; Efstathiou, J.A.; Lu, H.-M. Proton therapy of prostate cancer by anterior-oblique beams: Implications of setup and anatomy variations. Phys. Med. Biol. 2017, 62, 1644–1660. [Google Scholar] [CrossRef] [PubMed]
- Cuaron, J.J.; Harris, A.A.; Chon, B.; Tsai, H.; Larson, G.; Hartsell, W.F.; Hug, E.; Cahlon, O. Anterior-oriented proton beams for prostate cancer: A multi-institutional experience. Acta Oncol. 2015, 54, 868–874. [Google Scholar] [CrossRef]
- Polf, J.C.; Chuong, M.; Zhang, B.; Mehta, M. Anteriorly oriented beam arrangements with daily in vivo range verification for proton therapy of prostate cancer: Rectal toxicity rates. Int. J. Part. Ther. 2016, 2, 509–517. [Google Scholar] [CrossRef]
- Murray, L.; Henry, A.; Hoskin, P.; Siebert, F.-A.; Venselaar, J. Second primary cancers after radiation for prostate cancer: A systematic review of the clinical data and impact of treatment technique. Radiother. Oncol. 2014, 110, 213–228. [Google Scholar] [CrossRef]
- Davis, E.J.; Beebe-Dimmer, J.L.; Ms, C.L.Y.; Cooney, K.A. Risk of second primary tumors in men diagnosed with prostate cancer: A population-based cohort study. Cancer 2014, 120, 2735–2741. [Google Scholar] [CrossRef]
- Slater, J.D.; Rossi, C.J.; Yonemoto, L.T.; Bush, D.; Jabola, B.; Levy, R.P.; Grove, R.I.; Preston, W.; Slater, J.M. Proton therapy for prostate cancer: The initial Loma Linda University experience. Int. J. Radiat. Oncol. 2004, 59, 348–352. [Google Scholar] [CrossRef]
- Vassil, A.D.; Murphy, E.S.; Reddy, C.A.; Angermeier, K.W.; Altman, A.; Chehade, N.; Ulchaker, J.; Klein, E.A.; Ciezki, J.P. Five year biochemical recurrence free survival for intermediate risk prostate cancer after radical prostatectomy, external beam ra-diation therapy or permanent seed implantation. Urology 2010, 76, 1251–1257. [Google Scholar] [CrossRef]
- Coen, J.J.; Zietman, A.L.; Rossi, C.J.; Grocela, J.A.; Efstathiou, J.A.; Yan, Y.; Shipley, W.U. Comparison of high-dose proton radiotherapy and brachytherapy in localized prostate cancer: A case-matched analysis. Int. J. Radiat. Oncol. 2012, 82, e25–e31. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Demizu, Y.; Fujii, O.; Terashima, K.; Niwa, Y.; Daimon, T.; Tokumaru, S.; Fuwa, N.; Hareyama, M.; Okimoto, T. Proton therapy for localized prostate cancer: Long-term results from a single-center experience. Int. J. Radiat. Oncol. 2020, 109, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.J.; Bs, J.J.P.; Yeap, B.Y.; Sanda, M.G.; Sandler, H.M.; Michalski, J.M.; Talcott, J.A.; Coen, J.J.; Hamstra, D.A.; Shipley, W.U.; et al. Patient-reported outcomes after 3-dimensional conformal, intensity-modulated, or proton beam radiotherapy for localized prostate cancer. Cancer 2013, 119, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.S.; Michalski, J.M.; Mendenhall, N.; Ms, C.G.M.; Henderson, R.; Nichols, R.C.; Mendenhall, W.M.; Williams, C.R.; Regan, M.M.; Ms, J.J.C.; et al. Comparative effectiveness study of patient-reported outcomes after proton therapy or intensity-modulated radiotherapy for prostate cancer. Cancer 2013, 120, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Ms, R.M.; Deville, C.; Both, S.; Bekelman, J.E.; Christodouleas, J.P.; Guzzo, T.J.; Tochner, Z.; Hahn, S.M.; Vapiwala, N. A case-matched study of toxicity outcomes after proton therapy and intensity-modulated radiation therapy for prostate cancer. Cancer 2014, 121, 1118–1127. [Google Scholar] [CrossRef]
- Kim, S.; Shen, S.; Moore, D.F.; Shih, W.; Lin, Y.; Li, H.; Dolan, M.; Shao, Y.-H.; Lu-Yao, G.L. Late gastrointestinal toxicities following radiation therapy for prostate cancer. Eur. Urol. 2011, 60, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.B.; Soulos, P.R.; Herrin, J.; Cramer, L.D.; Potosky, A.L.; Roberts, K.B.; Gross, C.P. Proton versus intensity-modulated radiotherapy for prostate cancer: Patterns of care and early toxicity. JNCI J. Natl. Cancer Inst. 2013, 105, 25–32. [Google Scholar] [CrossRef]
- Royce, T.J.; Lee, D.H.; Keum, N.; Permpalung, N.; Chiew, C.J.; Epstein, S.; Pluchino, K.M.; D’Amico, A.V. Conventional versus hypofractionated radiation therapy for localized prostate cancer: A meta-analysis of randomized noninferiority trials. Eur. Urol. Focus 2017, 5, 577–584. [Google Scholar] [CrossRef]
- Al-Mamgani, A.; van Putten, W.L.; Heemsbergen, W.D.; van Leenders, G.J.; Slot, A.; Dielwart, M.F.; Incrocci, L.; Lebesque, J.V. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int. J. Radiat. Oncol. 2008, 72, 980–988. [Google Scholar] [CrossRef]
- Dearnaley, D.P.; Jovic, G.; Syndikus, I.; Khoo, V.; Cowan, R.; Graham, J.; Aird, E.G.; Bottomley, D.; Huddart, R.A.; Jose, C.C.; et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: Long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014, 15, 464–473. [Google Scholar] [CrossRef]
- Kuban, D.A.; Tucker, S.L.; Dong, L.; Starkschall, G.; Huang, E.H.; Cheung, M.R.; Lee, A.K.; Pollack, A. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Beckendorf, V.; Guerif, S.; Le Prisé, E.; Cosset, J.-M.; Bougnoux, A.; Chauvet, B.; Salem, N.; Chapet, O.; Bourdain, S.; Bachaud, J.-M.; et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int. J. Radiat. Oncol. Biol. Physics. 2011, 80, 1056–1063. [Google Scholar] [CrossRef]
- Zietman, A.L.; Bae, K.; Slater, J.D.; Shipley, W.U.; Efstathiou, J.A.; Coen, J.J.; Bush, D.A.; Lunt, M.; Spiegel, D.Y.; Skowronski, R.; et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: Long-term results from Proton Radiation Oncology Group/American College of Radiology 95–09. J. Clin. Oncol. 2010, 28, 1106–1111. [Google Scholar] [CrossRef]
- Sheets, N.C.; Goldin, G.H.; Meyer, A.-M.; Wu, Y.; Chang, Y.; Stürmer, T.; Holmes, J.A.; Reeve, B.B.; Godley, P.A.; Carpenter, W.R.; et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 2012, 307, 1611–1620. [Google Scholar] [PubMed]
- Rodda, S.; Tyldesley, S.; Morris, W.J.; Keyes, M.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; et al. ASCENDE-RT: An analysis of treatment-related morbidity for a randomized trial comparing a low-dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int. J. Radiat. Oncol. 2017, 98, 286–295. [Google Scholar] [CrossRef]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar] [CrossRef] [PubMed]
- Soukup, M.; Alber, M. Influence of dose engine accuracy on the optimum dose distribution in intensity-modulated proton therapy treatment plans. Phys. Med. Biol. 2007, 52, 725–740. [Google Scholar] [CrossRef]
- Szymanowski, H.; Oelfke, U. Two-dimensional pencil beam scaling: An improved proton dose algorithm for heterogeneous media. Phys. Med. Biol. 2002, 47, 3313–3330. [Google Scholar] [CrossRef] [PubMed]
- Titt, U.; Sahoo, N.; Ding, X.; Zheng, Y.; Newhauser, W.D.; Zhu, X.R.; Polf, J.C.; Gillin, M.T.; Mohan, R. Assessment of the accuracy of an MCNPX-based Monte Carlo simulation model for predicting three-dimensional absorbed dose distributions. Phys. Med. Biol. 2008, 53, 4455–4470. [Google Scholar] [CrossRef]
- Petti, P.L. Evaluation of a pencil-beam dose calculation technique for charged particle radiotherapy. Int. J. Radiat. Oncol. 1996, 35, 1049–1057. [Google Scholar] [CrossRef]
- Schaffner, B.; Pedroni, E.; Lomax, A. Dose calculation models for proton treatment planning using a dynamic beam delivery system: An attempt to include density heterogeneity effects in the analytical dose calculation. Phys. Med. Biol. 1999, 44, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Holloway, S.M.; Holloway, M.D.; Thomas, S. A method for acquiring random range uncertainty probability distributions in proton therapy. Phys. Med. Biol. 2017, 63, 01NT02. [Google Scholar] [CrossRef] [PubMed]
- Chvetsov, A.V.; Paige, S.L. The influence of CT image noise on proton range calculation in radiotherapy planning. Phys. Med. Biol. 2010, 55, N141–N149. [Google Scholar] [CrossRef] [PubMed]
- Newhauser, W.D.; Giebeler, A.; Langen, K.M.; Mirkovic, D.; Mohan, R. Can megavoltage computed tomography reduce proton range uncertainties in treatment plans for patients with large metal implants? Phys. Med. Biol. 2008, 53, 2327–2344. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, O.; Reiss, P. The influence of metal artefacts on the range of ion beams. Phys. Med. Biol. 2007, 52, 635–644. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, X.R.; Park, P.C.; Titt, U.; Mohan, R.; Virshup, G.; Clayton, J.E.; Dong, L. Comprehensive analysis of proton range uncertainties related to patient stopping-power-ratio estimation using the stoichiometric calibration. Phys. Med. Biol. 2012, 57, 4095–4115. [Google Scholar] [CrossRef]
- Wohlfahrt, P.; Richter, C. Status and innovations in pre-treatment CT imaging for proton therapy. Br. J. Radiol. 2020, 93. [Google Scholar] [CrossRef]
- Paganetti, H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys. Med. Biol. 2012, 57, R99–R117. [Google Scholar] [CrossRef]
- Moyers, M.F. Comparison of x ray computed tomography number to proton relative linear stopping power conversion functions using a standard phantom. Med. Phys. 2014, 41, 061705. [Google Scholar] [CrossRef][Green Version]
- Park, P.C.; Cheung, J.P.; Zhu, X.R.; Lee, A.K.; Sahoo, N.; Tucker, S.L.; Liu, W.; Li, H.; Mohan, R.; Court, L.; et al. Statistical assessment of proton treatment plans under setup and range uncertainties. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 1007–1013. [Google Scholar] [CrossRef]
- Wouters, B.G.; Skarsgard, L.D.; Gerweck, L.E.; Cárabe-Fernández, A.; Wong, M.; Durand, R.E.; Nielson, D.; Bussière, M.R.; Wagner, M.; Biggs, P.; et al. Radiobiological intercomparison of the 160 MeV and 230 MeV proton therapy beams at the Harvard Cyclotron Laboratory and at Massachusetts General Hospital. Radiat. Res. 2015, 183, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Yasui, H.; Matsuura, T.; Yamamori, T.; Suzuki, M.; Nagane, M.; Nam, J.-M.; Inanami, O.; Shirato, H. Evaluation of the relative biological effectiveness of spot-scanning proton irradiation in vitro. J. Radiat. Res. 2016, 57, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Mairani, A.; Dokic, I.; Magro, G.; Tessonnier, T.; Bauer, J.; Böhlen, T.T.; Ciocca, M.; Ferrari, A.; Sala, P.; Jäkel, O.; et al. A phenomenological relative biological effectiveness approach for proton therapy based on an improved description of the mixed radiation field. Phys. Med. Biol. 2017, 62, 1378–1395. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J. Margins for treatment planning of proton therapy. Phys. Med. Biol. 2006, 51, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Royce, T.J.; Efstathiou, J.A. Proton therapy for prostate cancer: A review of the rationale, evidence, and current state. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Tattenberg, S.; Madden, T.M.; Gorissen, B.L.; Bortfeld, T.; Parodi, K.; Verburg, J. Proton range uncertainty reduction benefits for skull base tumors in terms of normal tissue complication probability (NTCP) and healthy tissue doses. Med. Phys. 2021, 48, 5356–5366. [Google Scholar] [CrossRef] [PubMed]
- van der Heyden, B.; Öllers, M.; Ritter, A.; Verhaegen, F.; van Elmpt, W. Clinical evaluation of a novel CT image reconstruction algorithm for direct dose calculations. Phys. Imaging Radiat. Oncol. 2017, 2, 11–16. [Google Scholar] [CrossRef]
- Zhao, T.; Mistry, N.; Ritter, A.; Sun, B.; Li, H.; Mutic, S. Dosimetric evaluation of direct electron density computed tomography images for simplification of treatment planning workflow. Int. J. Radiat. Oncol. 2016, 96, E674–E675. [Google Scholar] [CrossRef]
- Bär, E.; Lalonde, A.; Royle, G.; Lu, H.-M.; Bouchard, H. The potential of dual-energy CT to reduce proton beam range uncertainties. Med. Phys. 2017, 44, 2332–2344. [Google Scholar] [CrossRef]
- Zhu, J.; Penfold, S.N. Dosimetric comparison of stopping power calibration with dual-energy CT and single-energy CT in proton therapy treatment planning. Med. Phys. 2016, 43, 2845–2854. [Google Scholar] [CrossRef]
- Faller, F.K.; Mein, S.; Ackermann, B.; Debus, J.; Stiller, W.; Mairani, A. Pre-clinical evaluation of dual-layer spectral computed tomography-based stopping power prediction for particle therapy planning at the Heidelberg Ion Beam Therapy Center. Phys. Med. Biol. 2020, 65, 095007. [Google Scholar] [CrossRef] [PubMed]
- Landry, G.; Hua, C. Current state and future applications of radiological image guidance for particle therapy. Med. Phys. 2018, 45, e1086–e1095. [Google Scholar] [CrossRef] [PubMed]
- Posiewnik, M.; Piotrowski, T. A review of cone-beam CT applications for adaptive radiotherapy of prostate cancer. Phys. Med. 2019, 59, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Thummerer, A.; Zaffino, P.; Meijers, A.; Marmitt, G.G.; Seco, J.; Steenbakkers, R.J.H.M.; Langendijk, J.A.; Both, S.; Spadea, M.F.; Knopf, A.-C. Comparison of CBCT based synthetic CT methods suitable for proton dose calculations in adaptive proton therapy. Phys. Med. Biol. 2020, 65, 095002. [Google Scholar] [CrossRef]
- Wyatt, J.J.; Brooks, R.L.; Ainslie, D.; Wilkins, E.; Raven, E.; Pilling, K.; Pearson, R.A.; McCallum, H.M. The accuracy of Magnetic Resonance—Cone Beam Computed Tomography soft-tissue matching for prostate radiotherapy. Phys. Imaging Radiat. Oncol. 2019, 12, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Businesswire. Hong Kong Sanatorium Radiotherapy Department and MedLever to Streamline Patient Setup & Imaging Process. Available online: https://www.businesswire.com/news/home/20170913005133/en/Hong-Kong-Sanatorium-Radiotherapy-Department-and-MedLever-To-Streamline-Patient-Setup-Imaging-Process (accessed on 24 November 2021).
- Nyholm, T.; Nyberg, M.; Karlsson, M.G.; Karlsson, M. Systematisation of spatial uncertainties for comparison between a MR and a CT-based radiotherapy workflow for prostate treatments. Radiat. Oncol. 2009, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Oborn, B.; Moteabbed, M.; Yan, S.; Bortfeld, T.; Knopf, A.; Fuchs, H.; Georg, D.; Seco, J.; Spadea, M.F.; et al. MR-guided proton therapy: A review and a preview. Radiat. Oncol. 2020, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Oborn, B.; Dowdell, S.; Metcalfe, P.; Crozier, S.; Mohan, R.; Keall, P. Future of medical physics: Real-time MRI guided proton therapy. Med. Phys. 2017, 44, e77–e90. [Google Scholar] [CrossRef]
- Depauw, N.; Keyriläinen, J.; Suilamo, S.; Warner, L.; Bzdusek, K.; Olsen, C.; Kooy, H. MRI-based IMPT planning for prostate cancer. Radiother. Oncol. 2020, 144, 79–85. [Google Scholar] [CrossRef]
- Pepin, M.; Tryggestad, E.; Tseung, H.; Johnson, J.; Herman, M.; Beltran, C. A Monte-Carlo-based and GPU-accelerated 4D-dose calculator for a pencil-beam scanning proton therapy system. Med. Phys. 2018, 45, 5293–5304. [Google Scholar] [CrossRef]
- Parodi, K.; Polf, J.C. In vivo range verification in particle therapy. Med. Phys. 2018, 45, e1036–e1050. [Google Scholar] [CrossRef]
- Wrońska, A. Prompt gamma imaging in proton therapy—Status, challenges and developments. J. Phys. Conf. Ser. 2020, 1561, 012021. [Google Scholar] [CrossRef]
- Zhu, X.; El Fakhri, G. Proton therapy verification with PET imaging. Theranostics 2013, 3, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T. Online MR imaging verifies proton beam range. Physicsworld. Available online: https://physicsworld.com/a/online-mr-imaging-verifies-proton-beam-range/ (accessed on 6 December 2021).
- Parodi, K.; Paganetti, H.; Cascio, E.; Flanz, J.B.; Bonab, A.A.; Alpert, N.M.; Lohmann, K.; Bortfeld, T. PET/CT imaging for treatment verification after proton therapy: A study with plastic phantoms and metallic implants. Med. Phys. 2007, 34, 419–435. [Google Scholar] [CrossRef]
- Guan, F.; Bronk, L.; Titt, U.; Lin, S.H.; Mirkovic, D.; Kerr, M.D.; Zhu, X.R.; Dinh, J.; Sobieski, M.; Stephan, C.; et al. Spatial mapping of the biologic effectiveness of scanned particle beams: Towards biologically optimized particle therapy. Sci. Rep. 2015, 5, 9850. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Marshall, T.I.; Perozziello, F.M.; Manti, L.; Currell, F.J.; Hanton, F.; McMahon, S.J.; Kavanagh, J.N.; Cirrone, G.A.P.; Romano, F.; et al. Relative biological effectiveness variation along monoenergetic and modulated Bragg peaks of a 62-MeV therapeutic proton beam: A preclinical assessment. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 27–35. [Google Scholar] [CrossRef]
- Rørvik, E.; Thörnqvist, S.; Stokkevåg, C.; Dahle, T.; Fjæra, L.; Ytre-Hauge, K. A phenomenological biological dose model for proton therapy based on linear energy transfer spectra. Med. Phys. 2017, 44, 2586–2594. [Google Scholar] [CrossRef]
- Grün, R.; Friedrich, T.; Krämer, M.; Scholz, M. Systematics of relative biological effectiveness measurements for proton radiation along the spread out Bragg peak: Experimental validation of the local effect model. Phys. Med. Biol. 2017, 62, 890–908. [Google Scholar] [CrossRef]
- McNamara, A.; Schuemann, J.; Paganetti, H. SU-F-BRD-13: A phenomenological relative biological effectiveness (RBE) model for proton therapy based on all published in vitro cell survival data. Med. Phys. 2015, 42, 3528. [Google Scholar] [CrossRef]
- Ma, D.; Bronk, L.; Kerr, M.; Sobieski, M.; Chen, M.; Geng, C.; Yiu, J.; Wang, X.; Sahoo, N.; Cao, W.; et al. Exploring the advantages of intensity-modulated proton therapy: Experimental validation of biological effects using two different beam intensity-modulation patterns. Sci. Rep. 2020, 10, 3199. [Google Scholar] [CrossRef]
- Michaelidesová, A.; Vachelová, J.; Puchalska, M.; Brabcová, K.P.; Vondráček, V.; Sihver, L.; Davídková, M. Relative bio-logical effectiveness in a proton spread-out Bragg peak formed by pencil beam scanning mode. Australas Phys. Eng. Sci. Med. 2017, 40, 359–368. [Google Scholar] [CrossRef]
- Underwood, T.; Paganetti, H. Variable proton relative biological effectiveness: How do we move forward? Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 56–58. [Google Scholar] [CrossRef]
- Wedenberg, M.; Beltran, C.; Mairani, A.; Alber, M. Advanced Treatment Planning. Med. Phys. 2018, 45, e1011–e1023. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.D.; Carlson, D.J.; Butkus, M.P.; Hawkins, R.; Friedrich, T.; Scholz, M. A comparison of mechanism-inspired models for particle relative biological effectiveness (RBE). Med. Phys. 2018, 45, e925–e952. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Patel, S.A.; Jani, A.B.; Gillespie, T.W.; Patel, P.R.; Godette, K.D.; Hershatter, B.W.; Shelton, J.W.; McDonald, M.W. Overall survival after treatment of localized prostate cancer with proton beam therapy, external-beam photon therapy, or brachy-therapy. Clin. Genitourin. Cancer 2020, 19, 255–266. [Google Scholar] [CrossRef]
- Tang, Q.; Zhao, F.; Yu, X.; Wu, L.; Lu, Z.; Yan, S. The role of radioprotective spacers in clinical practice: A review. Quant. Imaging Med. Surg. 2018, 8, 514–524. [Google Scholar] [CrossRef]
- Chung, H.; Polf, J.; Badiyan, S.; Biagioli, M.; Fernandez, D.; Latifi, K.; Wilder, R.; Mehta, M.; Chuong, M. Rectal dose to prostate cancer patients treated with proton therapy with or without rectal spacer. J. Appl. Clin. Med. Phys. 2016, 18, 32–39. [Google Scholar] [CrossRef]
- Polamraju, P.; Bagley, A.F.; Williamson, T.; Zhu, X.R.; Frank, S.J. Hydrogel spacer reduces rectal dose during proton therapy for prostate cancer: A dosimetric analysis. Int. J. Part. Ther. 2019, 5, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.-K.T.; Lee, H.J.; Macomber, M.W.; Apisarnthanarax, S.; Zeng, J.; Laramore, G.E.; Rengan, R.; Russell, K.J.; Chen, J.J.; Ellis, W.J.; et al. Rectal hydrogel spacer improves late gastrointestinal toxicity compared to rectal balloon immobilization after proton beam radiation therapy for localized prostate cancer: A retrospective observational study. Int. J. Radiat. Oncol. 2020, 108, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.; Henry, A.; Hoskin, P.; Siebert, F.-A.; Venselaar, J. Second primary cancers after radiation for prostate cancer: A review of data from planning studies. Radiat. Oncol. 2013, 8, 172. [Google Scholar] [CrossRef]
- Yoon, M.; Ahn, S.H.; Kim, J.; Shin, D.H.; Park, S.Y.; Lee, S.B.; Shin, K.H.; Cho, K.H. Radiation-induced cancers from modern radiotherapy techniques: Intensity-modulated radiotherapy versus proton therapy. Int. J. Radiat. Oncol. 2010, 77, 1477–1485. [Google Scholar] [CrossRef]
- Weber, D.C.; Wang, H.; Cozzi, L.; Dipasquale, G.; Khan, H.G.; Ratib, O.; Rouzaud, M.; Vees, H.; Zaidi, H.; Miralbell, R. RapidArc, intensity modulated photon and proton techniques for recurrent prostate cancer in previously irradiated patients: A treatment planning comparison study. Radiat. Oncol. 2009, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Peeters, A.; Grutters, J.; Pijls-Johannesma, M.; Reimoser, S.; De Ruysscher, D.; Severens, J.L.; Joore, M.; Lambin, P. How costly is particle therapy? Cost analysis of external beam radiotherapy with carbon-ions, protons and photons. Radiother. Oncol. 2010, 95, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Mishra, M.V.; Mehta, M.P. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer 2016, 122, 1483–1501. [Google Scholar] [CrossRef] [PubMed]
- Schippers, J.M.; Lomax, A.J. Emerging technologies in proton therapy. Acta Oncol. 2011, 50, 838–850. [Google Scholar] [CrossRef]
- Schippers, M. New developments in cyclotrons and gantries for proton therapy. Verh. Der Dtsch. Phys. Ges. 2019, 50, 1. [Google Scholar]
- Lee, W.R. Prostate cancer and the hypofractionation hypothesis. J. Clin. Oncol. 2013, 31, 3849–3851. [Google Scholar] [CrossRef]
- Tree, A.C.; Alexander, E.J.; Van As, N.J.; Dearnaley, D.P.; Khoo, V. Biological dose escalation and hypofractionation: What is there to be gained and how will it best be done? Clin. Oncol. 2013, 25, 483–498. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Cho, K.H.; Pyo, H.R.; Lee, K.H.; Moon, S.H.; Kim, T.H.; Shin, K.H.; Kim, J.-Y.; Lee, S.B.; Nam, B.H. A phase II study of hypofractionated proton therapy for prostate cancer. Acta Oncol. 2013, 52, 477–485. [Google Scholar] [CrossRef]
- Cohilis, P.; Jongen, Y. Some factors influencing the cost of a hospital based proton therapy centre. Strahlenther. Onkol. 1999, 175, 102–104. [Google Scholar] [CrossRef]
- Bolsi, A.; Lomax, A.J.; Pedroni, E.; Goitein, G.; Hug, E. Experiences at the Paul Scherrer Institute with a remote patient positioning procedure for high-throughput proton radiation therapy. Int. J. Radiat. Oncol. 2008, 71, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Fava, G.; Widesott, L.; Fellin, F.; Amichetti, M.; Viesi, V.; Lomax, A.J.; Lederer, L.; Hug, E.B.; Fiorino, C.; Salvadori, G.; et al. In-gantry or remote patient positioning? Monte Carlo simulations for proton therapy centers of different sizes. Radiother. Oncol. 2012, 103, 18–24. [Google Scholar] [CrossRef] [PubMed]

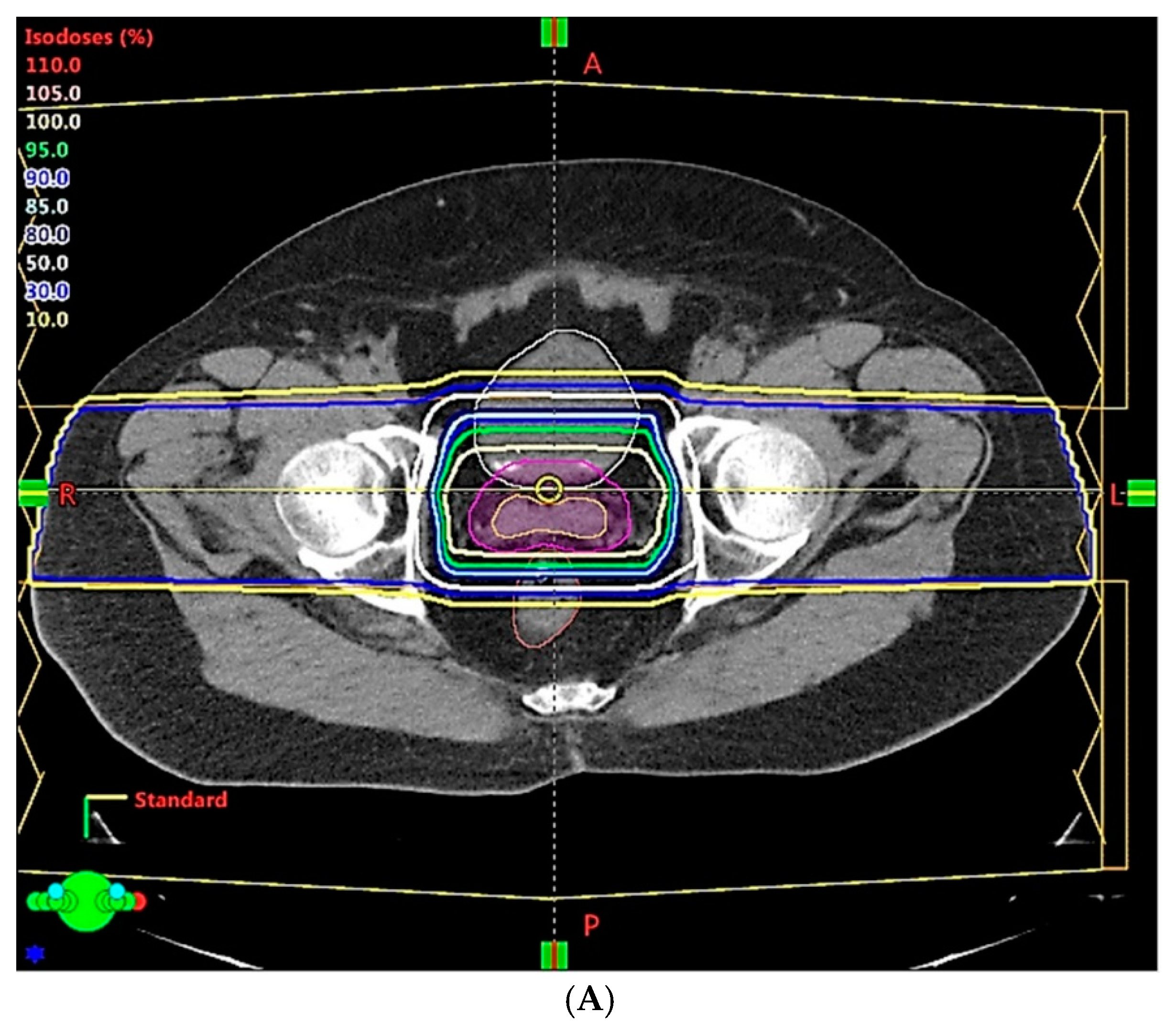
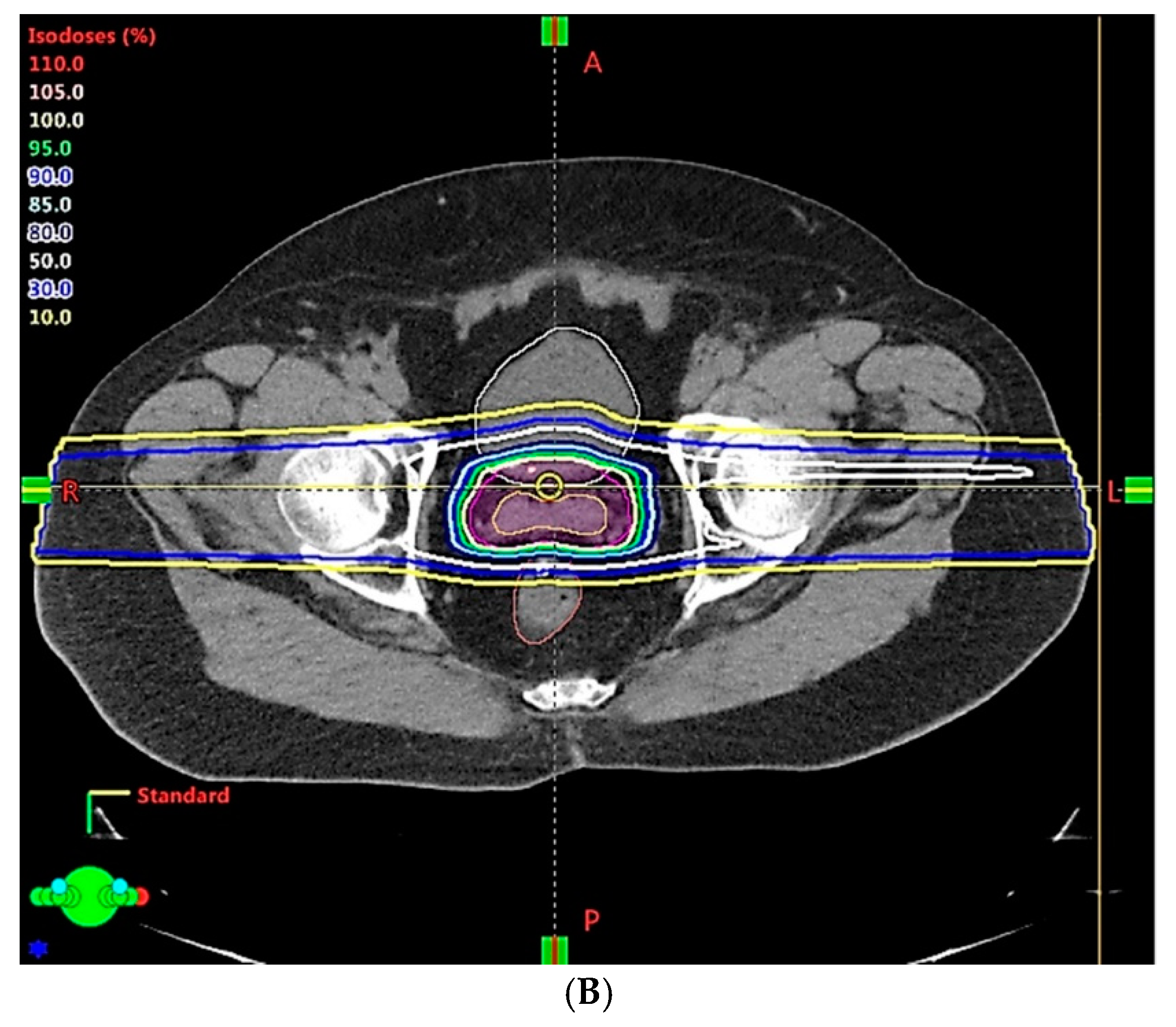
| Clinical Scenarios | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Range uncertainty | 3.5% | 3.0% | 2.5% | 2.0% | 1.5% | 1.0% | |||
| Setup error | 5 mm | 3 mm | 2 mm | 1 mm | 1 mm | ||||
| CTV, V78Gy | 99.9% | 99.6% | 99.1% | 98.4% | 98.8% | 98.5% | 98.8% | 99.3% | 99.6% |
| Rectum, V70Gy | 30.8% | 25.9% | 24.5% | 20.1% | 19.7% | 20.1% | 18.2% | 19.5% | 19.5% |
| Bladder, V70Gy | 35.2% | 30.6% | 29.1% | 25.3% | 24.7% | 24.7% | 24.5% | 23.6% | 23.5% |
| Rectum, Dmean (Gy) | 37.1 | 33.3 | 32.4 | 28.8 | 28.5 | 28.9 | 27.1 | 28.4 | 28.5 |
| Bladder, Dmean (Gy) | 40.4 | 37.0 | 36.1 | 32.8 | 32.3 | 32.5 | 32.2 | 31.8 | 31.7 |
| Non-target tissue, Dmean (Gy) | 14.6 | 13.6 | 13.3 | 12.3 | 12.1 | 12.1 | 11.7 | 11.8 | 11.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poon, D.M.C.; Wu, S.; Ho, L.; Cheung, K.Y.; Yu, B. Proton Therapy for Prostate Cancer: Challenges and Opportunities. Cancers 2022, 14, 925. https://doi.org/10.3390/cancers14040925
Poon DMC, Wu S, Ho L, Cheung KY, Yu B. Proton Therapy for Prostate Cancer: Challenges and Opportunities. Cancers. 2022; 14(4):925. https://doi.org/10.3390/cancers14040925
Chicago/Turabian StylePoon, Darren M. C., Stephen Wu, Leon Ho, Kin Yin Cheung, and Ben Yu. 2022. "Proton Therapy for Prostate Cancer: Challenges and Opportunities" Cancers 14, no. 4: 925. https://doi.org/10.3390/cancers14040925
APA StylePoon, D. M. C., Wu, S., Ho, L., Cheung, K. Y., & Yu, B. (2022). Proton Therapy for Prostate Cancer: Challenges and Opportunities. Cancers, 14(4), 925. https://doi.org/10.3390/cancers14040925






