Characteristics and Outcome of Children with Wilms Tumor Requiring Intensive Care Admission in First Line Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Characteristics of Patients Requiring PICU Admission
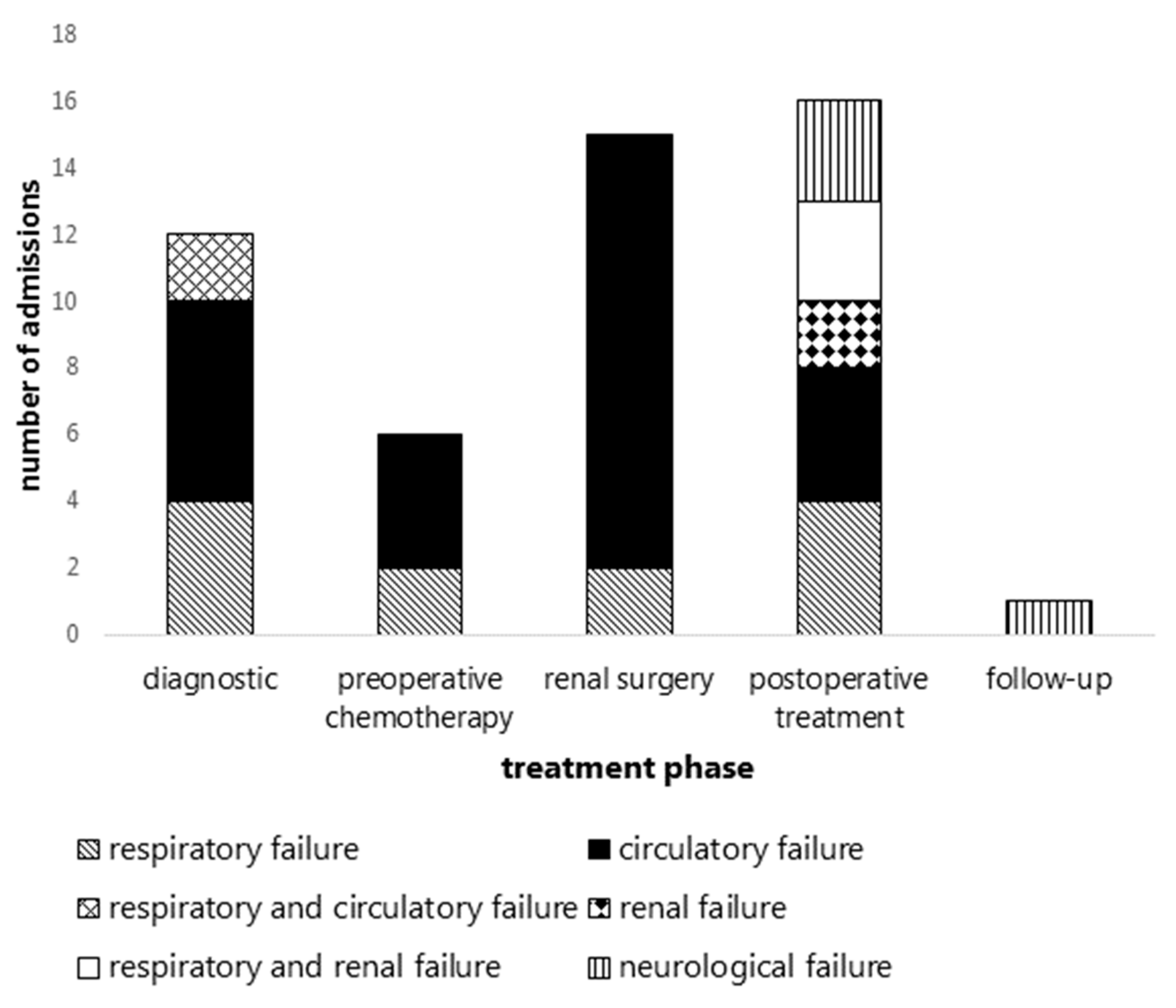
3.3. Indications for Unplanned PICU Admissions per Treatment Phase
3.4. PICU Stay Characteristics
3.5. Outcome of WT Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pastore, G.; Znaor, A.; Spreafico, F.; Graf, N.; Pritchard-Jones, K.; Steliarova-Foucher, E. Malignant renal tumours incidence and survival in European children (1978–1997): Report from the Automated Childhood Cancer Information System project. Eur. J. Cancer 2006, 42, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- De Kraker, J.; Graf, N.; van Tinteren, H.; Pein, F.; Sandstedt, B.; Godzinski, J.; Tournade, M.F. Reduction of postoperative chemotherapy in children with stage I intermediate-risk and anaplastic Wilms’ tumour (SIOP 93-01 trial): A randomised controlled trial. Lancet 2004, 364, 1229–1235. [Google Scholar] [CrossRef]
- Pritchard-Jones, K.; Bergeron, C.; de Camargo, B.; van den Heuvel-Eibrink, M.M.; Acha, T.; Godzinski, J.; Oldenburger, F.; Boccon-Gibod, L.; Leuschner, I.; Vujanic, G.; et al. Omission of doxorubicin from the treatment of stage II-III, intermediate-risk Wilms’ tumour (SIOP WT 2001): An open-label, non-inferiority, randomised controlled trial. Lancet 2015, 386, 1156–1164. [Google Scholar] [CrossRef] [Green Version]
- Van den Heuvel-Eibrink, M.M.; van Tinteren, H.; Bergeron, C.; Coulomb-L’Hermine, A.; de Camargo, B.; Leuschner, I.; Sandstedt, B.; Acha, T.; Godzinski, J.; Oldenburger, F.; et al. Outcome of localised blastemal-type Wilms tumour patients treated according to intensified treatment in the SIOP WT 2001 protocol, a report of the SIOP Renal Tumour Study Group (SIOP-RTSG). Eur. J. Cancer 2015, 51, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Dome, J.S.; Graf, N.; Geller, J.I.; Fernandez, C.V.; Mullen, E.A.; Spreafico, F.; Van Den Heuvel-Eibrink, M.; Pritchard-Jones, K. Advances in Wilms Tumor Treatment and Biology: Progress Through International Collaboration. J. Clin. Oncol. 2015, 33, 2999–3007. [Google Scholar] [CrossRef]
- Van den Heuvel-Eibrink, M.M.; Hol, J.A.; Pritchard-Jones, K.; van Tintern, H.; Furtwangler, R.; Verschuur, A.C.; Vujanic, G.M.; Leuschner, I.; Brok, J.; Rube, C.; et al. Position paper: Rationale for the treatment of Wilms tumour in the UMBRELLA SIOP-RTSG 2016 protocol. Nat. Rev. Urol. 2017, 14, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Wösten-van Asperen, R.M.; van Gestel, J.P.J.; van Grotel, M.; Tschiedel, E.; Dohna-Schwanke, C.; Valla, F.V.; Willems, J.; Angaard Nielsem, J.S.; Krause, M.F.; Potratz, J.; et al. PICU mortality of children with cancer admitted to pediatric intensive care unit a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2019, 142, 153–163. [Google Scholar] [CrossRef]
- Kajal, K.; Munirathinam, G.; Mandal, B.; Gandhi, K.; Singh, H.; Kanojia, R. Wilm’s tumor with intracardiac extension causing dynamic tricuspid valve obstruction: An anesthetic challenge. Saudi J. Anaesth. 2018, 12, 321–323. [Google Scholar] [CrossRef]
- Chalavon, E.; Lampin, M.E.; Lervat, C.; Leroy, X.; Bonnevalle, M.; Recher, M.; Sudour-Bonnange, H. Dilated Cardiomyopathy Caused by Wilms Tumor. Pediatr. Emerg. Care 2017, 33, 41–42. [Google Scholar] [CrossRef]
- Abraham, M.; Samuel, M.; Mathew, M. Wilms tumour with intracardiac extension–multimodal approach to a challenging case. Indian J. Anaesth. 2016, 60, 216–218. [Google Scholar] [CrossRef]
- Dhar, M.; Prakash, S.; Pandey, V.; Pai, V. Intraoperative tumor lysis syndrome in a child with Wilms’ tumor. Anesth. Essays Res. 2016, 10, 145–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genc, D.B.; Vural, S.; Telhan, L. Wilms Tumor Presenting with Fulminant Hepatic Failure and Succesful Initial Treatment with Cyclophosphamide. Pediatr. Blood Cancer 2016, 63, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Light, J.; Marchick, M.; Hoelle, R. Dyspnea, Tachycardia, and New Onset Seizure as a Presentation of Wilms Tumor: A Case Report. Case Rep. Emerg. Med. 2014, 2014, 562672. [Google Scholar] [CrossRef] [PubMed]
- Martín-Lázaro, J.F.; Palanca, D.; Garcia-Iñiguez, J.P.; Madurga, P.; Carboné, A. Hepatopathy-thrombocytopenia syndrome after actinomycin-D therapy: Treatment with defibrotide. Pediatr. Hematol. Oncol. 2013, 30, 25–27. [Google Scholar] [CrossRef]
- Cooper, L.; Moore, C.; Branchford, B.; Greffe, B.; Capocelli, K.; Kuder, A.; Mehta, I.; Mourani, P.M. Succesful Pulmonary Artery Embolectomy in a Patient with a Saddle Wilms Tumor Embolus. Pediatr. Blood Cancer 2012, 58, 806–809. [Google Scholar] [CrossRef]
- Dressler, A.M.; Finck, C.M.; Carroll, C.L.; Bonanni, C.C.; Spinella, P.C. Use of a massive transfusion protocol with hemostatic resuscitation for severe intraoperative bleeding in a child. J. Pediatr. Surg. 2010, 45, 1530–1533. [Google Scholar] [CrossRef]
- Nagappa, M.; Bhat, R.R.; Sudeep, K.; Mishra, S.K.; Badhe, A.S.; Hemavathi, B. Vincristine-induced acute life-threatening hyponatremia resulting in seizure and coma. Indian J. Crit. Care Med. 2009, 13, 167–168. [Google Scholar]
- Van Toorn, R.; Wessels, G.; Stefan, C. Catastrophic intracerebral hemorrhage in a young infant with Wilms tumor. J. Pediatr. Hematol. Oncol. 2007, 29, 298–300. [Google Scholar] [CrossRef]
- Vujanić, G.M.; Sandstedt, B.; Harms, D.; Leuschner, I.; Kelsey, A.; de Kraker, J. Revised International Society of Paediatric Oncology (SIOP) working classification of renal tumors of childhood. Med. Pediatr. Oncol. 2002, 38, 79–82. [Google Scholar] [CrossRef]
- Slater, A.; Shann, F.; Pearson, G. PIM2: A revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003, 29, 278–285. [Google Scholar] [CrossRef]
- Alexander, S.; Pole, J.D.; Gibson, P.; Lee, M.; Hesser, T.; Chi, S.N.; Dvorak, C.; Fisher, B.; Hasle, H.; Kanerva, J.; et al. Classification of treatment-related mortality in children with cancer: A systematic assessment. Lancet Oncol. 2015, 16, e604–e610. [Google Scholar] [CrossRef]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; De Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.M.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Roy, P.; van Peer, S.E.; de Witte, M.M.; Tytgat, G.A.M.; Karim-Kos, H.E.; van Grotel, M.; van de Ven, C.P.; Mavinkurve-Groothius, A.M.C.; Merks, J.H.M.; Kuiper, R.P.; et al. Characteristics and outcome of children with renal tumors in the Netherlands: The first five-year’s experience of national centralization. PLoS ONE 2022, 17, e0261729. [Google Scholar] [CrossRef] [PubMed]
- Voûte, P.A.; van der Meer, J.; Staugaard-Kloosterziel, W. Plasmin Renin Activity in Wilms Tumor. Acta Endocrinol. 1971, 67, 197–202. [Google Scholar] [CrossRef]
- Maas, M.H.; Cransberg, K.; van Grotel, M.; Pieters, R.; van den Heuvel-Eibrink, M.M. Renin-Induced Hypertension in Wilms Tumor Patients. Pediatr. Blood Cancer 2007, 48, 500–503. [Google Scholar] [CrossRef]
- Charlton, G.A.; Sedgwick, J.; Sutton, D.N. Anaesthetic Management of Renin Secreting Nephroblastoma. Br. J. Anaesth. 1992, 69, 206–209. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Akingbola, O.; Yosypiv, I.; El-Dahr, S. Emergency Management of Hypertension in Children. Int. J. Nephrol. 2012, 2012, 420247. [Google Scholar] [CrossRef] [Green Version]
- Steinbrecher, H.A.; Malone, P.S.J. Wilms’ tumour and hypertension: Incidence and outcome. Br. J. Urol. 1995, 76, 241–243. [Google Scholar] [CrossRef]
- Hallahan, A.R.; Shaw, P.J.; Rowell, G.; O’Connell, A.; Schell, D.; Gillis, J. Improved outcomes of children with malignancy admitted to a pediatric intensive care unit. Crit. Care Med. 2000, 28, 3718–3721. [Google Scholar] [CrossRef]
- Abraham, R.B.; Toren, A.; Ono, N.; Weinbroum, A.A.; Vardi, A.; Barzilay, Z.; Paret, G. Predictors of outcome in the pediatric intensive care units of children with malignancies. J. Pediatr. Hematol. Oncol. 2012, 24, 23–26. [Google Scholar] [CrossRef]
- Hol, J.A.; Lopez-Yurda, M.I.; Van Tinteren, H.; Van Grotel, M.; Godzinski, J.; Vujanic, G.; Oldenburger, F.; De Camargo, B.; Ramirez-Villar, G.L.; Bergeron, C.; et al. Prognostic significance of age in 5631 patients with Wilms tumour prospectively registered in International Society of Paediatric oncology (SIOP) 93-01 and 2001. PLoS ONE 2019, 14, e0221373. [Google Scholar] [CrossRef] [Green Version]
- Breslow, N.E.; Palmer, N.F.; Hill, L.R.; Buring, J.; D’Angio, G.J. Wilm’s tumor: Prognostic factors for patients without metastases at diagnosis: Result of the National Wilms’ Tumor Study. Cancer 1978, 41, 1577–1589. [Google Scholar] [CrossRef]
- Jones, B.; Breslow, N.E.; Takashima, J. Toxic deaths in the second National Wilms’ Tumor Study. J. Clin. Oncol. 1984, 2, 1028–1033. [Google Scholar] [CrossRef]
- Coppes, M.J.; Tournade, M.F.; Lemerle, J.; Weitzman, S.; Rey, A.; Burger, D.; Carli, M.; Voûte, P.A. Preoperative Care of Infants with Nephroblastoma: The International Society of Pediatric Oncology 6 Experience. Cancer 1992, 69, 2721–2725. [Google Scholar] [CrossRef]
- Ritchey, M.L.; Shamberger, R.C.; Haase, G.; Horwitz, J.; Bergemann, T.; Breslow, N.E. Surgical complications after primary nephrectomy for Wilms’ tumor: Report from the National Wilms’ Tumor Study Group. J. Am. Coll. Surg. 2001, 192, 63–68. [Google Scholar] [CrossRef]
- Charlton, J.; Irtan, S.; Bergeron, C.; Pritchard-Jones, K. Bilateral Wilms tumour: A review of clinical and molecular features. Expert Rev. Mol. Med. 2017, 19, e8. [Google Scholar] [CrossRef] [PubMed]
- Neu, M.A.; Russo, A.; Wingerter, A.; Alt, F.; Theruvath, J.; El Malki, K.; Kron, B.; Dittrich, M.; Lotz, J.; Stein, R.; et al. Prospective analysis of long-term renal function in survivors of childhood Wilms tumor. Pediatr. Nephrol. 2017, 32, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Mavinkurve-Groothuis, A.M.; van de Kracht, F.; Westland, R.; van Wijk, J.A.; Loonen, J.J.; Schreuder, M.F. Long-term follow-up of blood pressure and glomerular filtration rate in patients with a solitary functioning kidney: A comparison between Wilms tumor survivors and nephrectomy for other reasons. Pediatr. Nephrol. 2016, 31, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Dekkers, I.A.; Blijdorp, K.; Cransberg, K.; Pluijm, S.M.; Pieters, R.; Neggers, S.J.; van den Heuvel-Eibrink, M.M. Long-Term nephrotoxicity in adult survivors of childhood cancer. Clin. J. Am. Soc. Nephrol. 2013, 8, 922–929. [Google Scholar] [CrossRef] [Green Version]
- Kooijmans, E.C.; Bökenkamp, A.; Tjahjadi, N.S.; Tettero, J.M.; van Dulmen-den Broeder, E.; van der Pal, H.J.H.; Veening, M.A. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syt. Rev. 2019, 3, CD008944. [Google Scholar] [CrossRef] [PubMed]

| Total Population n = 175 Patients | Control Group (No PICU Admission or Planned PICU Admission Only) n = 142 Patients | Unplanned PICU Admission n = 33 Patients | |
|---|---|---|---|
| Gender, male, n (%) | 82 (46.9) | 70 (49.3) | 12 (36.4) |
| Age in months, median (IQR) | 38.5 (22.0–57.0) | 42.2 (27.4–61.1) | 22.0 (13.8–38.8) † |
| Disease stage, n (%) | |||
| I | 60 (34.3) | 53 (37.3) | 7 (21.2) |
| II | 35 (20.0) | 32 (22.5) | 3 (9.1) |
| III | 38 (21.7) | 27 (19.0) | 11 (33.3) |
| IV * | 24 (13.7) | 18 (12.7) | 6 (18.2) |
| V | 18 (10.2) | 12 (8.5) | 6 (18.2) |
| * abdominal stage | I (n = 3), II (n = 3), III (n = 11), V (n = 1) | I (n = 1), II (n= 0), III (n = 3), V (n = 2) | |
| Metastases, n (%) | |||
| Lung | 17 (9.6) | 13 (9.2) | 4 (11.4) |
| Liver | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other site | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Multiple sites | 7 (4.1) | 5 (3.5) | 2 (5.8) |
| Histology, n (%) | |||
| Low risk | 9 (5.1) | 6 (4.2) | 3 (9.1) |
| Intermediate risk | 147 (84.0) | 120 (84.5) | 27 (81.8) |
| High risk | 19 (10.8) | 16 (11.3) | 3 (9.1) |
| -Diffuse anaplasia | 7 (4.0) | 6 (4.2) | 1 (3.0) |
| -Blastemal predominant | 10 (5.7) | 8 (5.6) | 2 (6.1) |
| -Diffuse anaplasia and Blasternal predominant | 2 (1.1) | 2 (1.4) | 0 (0.0) |
| Documented genetic anomaly or congenital malformation, n (%) ** | |||
| Beckwith-Wiedeman syndrome | 5 (2.9) | 5 (3.5) | 0 (0.0) |
| Hemihypertrophy NOS | 9 (5.1) | 7 (4.9) | 2 (6.1) |
| WT1 gene variant | 8 (4.6) | 4 (2.8) | 4 (12.1) |
| Genito-urinary malformation NOS | 3 (1.7) | 3 (2.1) | 0 (0.0) |
| Other | 17 (9.7) | 11 (7.7) | 6 (18.2) |
| Preoperative treatment, n (%) | |||
| None | 8 (4.6) | 6 (4.2) | 2 (6.1) |
| AV | 138 (78.8) | 116 (82.0) | 22 (66.7) |
| AVD | 24 (13.7) | 18 (13.4) | 6 (18.2) |
| >3 drugs | 5 (2.9) | 2 (1.4) | 3 (9.1) † |
| Renal surgery, n (%) | |||
| Nilateral | 156 (89.1) | 131 (92.2) | 25 (75.7) † |
| Bilateral | 19 (10.8) | 11 (7.7) | 8 (24.2) † |
| Thrombectomy, n (%) | 10 (5.8) | 7 (4.9) | 3 (9.1) |
| Metastectomy, n (%) | 6 (3.4) | 4 (2.8) | 2 (6.1) |
| Postoperative treatment, n (%) | |||
| None | 5 (2.9) | 4 (2.8) | 1 (3.0) |
| VCR | 3 (1.7) | 2 (1.4) | 1 (3.0) |
| AV-1 | 41 (23.4) | 38 (26.8) | 3 (9.1) † |
| AV-2 | 57 (32.6) | 43 (30.3) | 14 (42.4) |
| AVD | 38 (21.7) | 33 (23.2) | 5 (15.2) † |
| >3 drugs | 31 (17.7) | 22 (15.5) | 9 (27.3) † |
| Radiotherapy, n (%) | |||
| Abdominal | 53 (30.3) | 41 (28.9) | 12 (36.4) |
| Pulmonary | 2 (1.1) | 2 (1.4) | 0 (0) |
| Combined | 6 (3.4) | 3 (2.1) | 3 (9.1) |
| Clinical Characteristics (Gender, Age, Stage, Histology, History) | PICU Admission Indication | Causes Underlying PICU Admission Indication |
|---|---|---|
| Diagnostic phase | ||
| F, 6 mo, I, IR | circulatory failure | hypertension |
| M, 1.6 y, I, IR | circulatory failure | intratumoral hemorrhage |
| F, 6 mo, I, IR | circulatory failure | hypertension |
| F, 1.3 y, II, IR, Fanconi anemia | respiratory and circulatory failure | ARDS (suspected infection), abdominal tumor mass, hypertension |
| F, 1.6 y, II, IR | circulatory failure | hypertension |
| M, 6 mo, III, LR * | circulatory failure | hypertension |
| F, 1.8 y, III, IR * | circulatory failure | intratumoral hemorrhage |
| F, 0 mo, III, IR, antenatal WT * | respiratory and circulatory failure | antenatal abdominal tumor mass |
| (2 PICU admissions) | respiratory failure | subglottic stenosis (post-extubation), laryngomalacia, omphalitis |
| F, 3.3 y, IV (lung, bone), IR * | respiratory failure | pulmonary metastases, suspected pneumonia |
| F, 1.5 y, IV (lung), IR * | respiratory failure | abdominal tumor mass, pulmonary metastases |
| F, 2.3 y, IV (lung), bilateral, IR | respiratory failure | abdominal tumor mass, pulmonary metastases, pleural effusion |
| Preoperative chemotherapy phase | ||
| F, 2 mo, I, IR | respiratory failure | abdominal mass with chyloperitoneum |
| M, 6 mo, III, LR * | circulatory failure | hypertension |
| F, 1.8 y, III, IR * | circulatory failure | abdominal tumor mass causing bowel obstruction |
| F, 2.5 y, III, IR | circulatory failure | hypertension |
| F, 3.7 y, III, IR * | circulatory failure | hypertension |
| M, 9 mo, V, IR, WT1 mutation | respiratory failure | abdominal tumor mass |
| Renal tumor surgery | ||
| F, 1.5 y, I, LR | circulatory failure | left TN: transection SMA |
| F, 1.7 y, I, IR, hemihypertrophy ** | circulatory failure | left NSS: intraoperative hemorrhage |
| (2 PICU admissions) | circulatory failure | left TN: hypotension |
| M, 4.3 y, II, IR, prematurity GA 26 wk | respiratory failure | left TN: inability to wean postoperatively, BPD |
| M, 2.8 y, III, IR | circulatory failure | right TN, thrombectomy VCI / RA: hypotension |
| M, 1.5 y, III, IR, WT1 mutation | circulatory failure | right TN: intraoperative hemorrhage |
| F, 3.7 y, III, IR * | circulatory failure | right TN, partial hepatectomy, hemicolectomy: hypotension |
| M, 2.9 y, III, IR * | circulatory failure | right TN: intraoperative hemorrhage |
| F, 3.0 y, III, IR ** | respiratory failure | right TN, partial hepatectomy: post-extubation subglottic stenosis |
| M, 2.9 y, III, IR, chr16 q deletion | circulatory failure | left TN: transection SMA |
| F, 7.1 y, III, HR (BP) * | circulatory failure | left TN, thrombectomy VCI: intraoperative hemorrhage |
| F, 3.2 y, IV (lung), IR, skeletal dysplasia * | circulatory failure | left TN: hypotension |
| M, 3.8 y, V, IR | circulatory failure | right TN and left NSS, day + 2: intra-abdominal urine leakage |
| F, 11 mo, V, IR, WT1 mutation | circulatory failure | left TN, right tumor biopsy: hypotension |
| F, 5.1 y, V, IR, TSC1 mutation | circulatory failure | left NSS: intraoperative hemorrhage |
| Postoperative chemotherapy phase | ||
| F, 1.8 y, I, LR | circulatory failure | urosepsis (Citrobacter spp.) |
| F, 1.7 y, I, IR, hemihypertrophy ** | respiratory failure, kidney injury | kidney injury (acute on chronic) with fluid overload, ascites, suspected infection |
| M, 2.9 y, III, IR * | respiratory failure | pleural empyema |
| F, 3.0 y, III, IR ** | respiratory failure | subglottic stenosis (recurrence post-extubation stenosis) |
| (2 PICU admissions) | respiratory failure | subglottic stenosis (recurrence post-extubation stenosis) |
| F, 7.1 y, III, HR (BP) * | neurological failure | intracranial hemorrhage (thrombocytopenia) |
| F, 3.3 y, IV (lung, bone), IR * | neurological failure | raised ICP leptomeningeal secondary malignancy |
| F, 1.5 y, IV (lung), IR * | respiratory failure | influenza A and post-irradiation ARDS |
| F, 4.9 y, IV (lung, liver), HR (DA) | circulatory failure | sepsis, chylothorax, chyloperitoneum |
| M, 1.8 y, IV (lung), bilateral, IR ** | circulatory failure | cardiomyopathy |
| (3 PICU admissions) | kidney injury | prerenal kidney injury (vomiting, gastric ulcer) in setting of CKD |
| kidney injury | prerenal kidney injury (vomiting) in setting of CKD | |
| F, 7 mo, V, IR, WT1 mutation | respiratory failure, kidney injury | suspected viral airway infection, ESDR with fluid overload |
| M, 4.3 y, V, HR (BP), hemihypertrophy ** | respiratory failure, kidney injury | ESKD with fluid overload, neutropenic sepsis |
| (3 PICU admissions) | neurological failure | seizures secondary to malignant hypertension and hypokalemia |
| circulatory failure | massive bowel ischemia in setting of IRIS following invasive candidiasis | |
| Follow-up phase | ||
| F, 3.2 y, IV (lung), IR, skeletal dysplasia * | neurological failure | scoliosis correction: loss of neuromonitoring |
| Planned PICU Admissions (n = 70 Admissions) | Unplanned PICU Admissions (n = 50 Admissions) | |
|---|---|---|
| Length of stay | ||
| LOS in days, median (IQR) | 1.0 (1.0–1.0) | 3.0 (1.0–6.0) |
| LOS in days, range | <1–6 | <1–100 |
| PIM2 score * | ||
| PIM2 score, mortality probability %, median (IQR) | 0.25 (0.2–0.57) | 1.0 (0.5–1.38) |
| PIM2 score, mortality probability %, range | 0.1–2.4 | 0.1–18.9 |
| Invasive ventilation, n (%) | 23 (32.9) | 25 (50.0) |
| Days on invasive ventilation, median (IQR) | 0.1 (0.1–0.2) | 4.0 (1.0–1.5) |
| Days on invasive ventilation, range | 0.1–2 | <1–91 |
| Vasopressor/inotropic support, n (%) | 1 (1.4) | 14 (28.0) |
| Days on vasopressor/inotropic support, median (IQR) | (-) | 1.0 (0.3–1.0) |
| Days on vasopressor/inotropic support, range | <1 | <1–4 |
| ECMO, n (%) | 0 | 0 |
| iNO, n (%) | 0 | 1 (2.0) |
| Renal replacement therapy, n (%) | 2 (2.8) | 4 (8.0) |
| Days on renal replacement therapy, range | 2 | 2–47 |
| Renal replacement therapy at discharge, n (%) | 2 (2.8) | 3 (9.1) |
| PICU mortality, n (%) | 0 | 2 (4.0) |
| Total Cohort (n = 175 Patients) | Control Group (No or Planned PICU Admissions Only) (n= 142 Patients) | Unplanned PICU Admission (n = 33 Patients) | |
|---|---|---|---|
| Follow-up time in months, median (IQR) | 52 (30–115) | 52 (31–124) | 50 (21–85) |
| Hypertension, n (%) At end of treatment At last follow-up | 13 (7.4) 12 (6.9) | 7 (4.9) 6 (4.2) | 6 (18.2) † 6 (18.2) † |
| Impaired renal function, n (%) Any at end of treatment CKD stage ≥2 at end of treatment CKD stage ≥3 at end of treatment Any at last follow-up CKD stage ≥2 at last follow-up CKD stage ≥3 at last follow-up | 6 (3.4) 6 (3.4) 4 (2.3) 15 (8.6) 12 (8.9) 5 (2.8) | 1 (0.7) 1 (0.7) 0 (0) 10 (7.0) 7 (4.9) 0 (0) | 5 (15.2) † 5 (15.2) † 4 (12.1) † 5 (15.2) † 5 (15.2) † 5 (15.2) † |
| Dialysis, n (%) At end of treatment At last follow-up | 2 (1.1) 1 (0.6) | 0 (0) 0 (0) | 2 (6.1) 1 (3.0) |
| Kidney transplant, n (%) | 1 (0.6) | 0 (0) | 1 (3.0) |
| Cardiomyopathy, n (%) At end of treatment At last follow-up | 2 (1.1) 3 (1.7) | 1 (0.7) 2 (1.4) | 1 (3.0) 1 (3.0) |
| Relapse/Refractory WT, n (%) Relapsed WT Refractory WT Time to relapse/progression in months, median (IQR) | 11 (6.3) 4 (2.3) 16.0 (8–30.5) | 8 (5.6) 3 (2.1) 15.5 (6.5–27) | 3 (9.1) 1 (3.0) 30.5 (8.5–30.5) |
| Overall mortality, n (%) Time to death in months, median (IQR) Cause of death, n (%)
| 6 (3.4) 20.5 (8–26.5) 4 (2.3) 1 (0.6) 1 (0.6) | 3 (2.1) 26.5 (21.5–26.5) 3 (2.1) 0 (0) 0 (0) | 3 (9.1) † 9.5 (6.5–9.5) 1 (3.0) 1 (3.0) 1 (3.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steur, A.; Raymakers-Janssen, P.A.M.A.; Kneyber, M.C.J.; Dijkstra, S.; van Woensel, J.B.M.; van Waardenburg, D.A.; van de Ven, C.P.; van der Steeg, A.F.W.; Wijnen, M.; Lilien, M.R.; et al. Characteristics and Outcome of Children with Wilms Tumor Requiring Intensive Care Admission in First Line Therapy. Cancers 2022, 14, 943. https://doi.org/10.3390/cancers14040943
Steur A, Raymakers-Janssen PAMA, Kneyber MCJ, Dijkstra S, van Woensel JBM, van Waardenburg DA, van de Ven CP, van der Steeg AFW, Wijnen M, Lilien MR, et al. Characteristics and Outcome of Children with Wilms Tumor Requiring Intensive Care Admission in First Line Therapy. Cancers. 2022; 14(4):943. https://doi.org/10.3390/cancers14040943
Chicago/Turabian StyleSteur, Anouk, Paulien A. M. A. Raymakers-Janssen, Martin C. J. Kneyber, Sandra Dijkstra, Job B. M. van Woensel, Dick A. van Waardenburg, Cornelis P. van de Ven, Alida F. W. van der Steeg, Marc Wijnen, Marc R. Lilien, and et al. 2022. "Characteristics and Outcome of Children with Wilms Tumor Requiring Intensive Care Admission in First Line Therapy" Cancers 14, no. 4: 943. https://doi.org/10.3390/cancers14040943
APA StyleSteur, A., Raymakers-Janssen, P. A. M. A., Kneyber, M. C. J., Dijkstra, S., van Woensel, J. B. M., van Waardenburg, D. A., van de Ven, C. P., van der Steeg, A. F. W., Wijnen, M., Lilien, M. R., de Krijger, R. R., van Tinteren, H., Littooij, A. S., Janssens, G. O., Peek, A. M. L., Tytgat, G. A. M., Mavinkurve-Groothuis, A. M., van Grotel, M., van den Heuvel-Eibrink, M. M., & Asperen, R. M. W.-v. (2022). Characteristics and Outcome of Children with Wilms Tumor Requiring Intensive Care Admission in First Line Therapy. Cancers, 14(4), 943. https://doi.org/10.3390/cancers14040943









