[68Ga]DOTATOC PET/CT Radiomics to Predict the Response in GEP-NETs Undergoing [177Lu]DOTATOC PRRT: The “Theragnomics” Concept
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
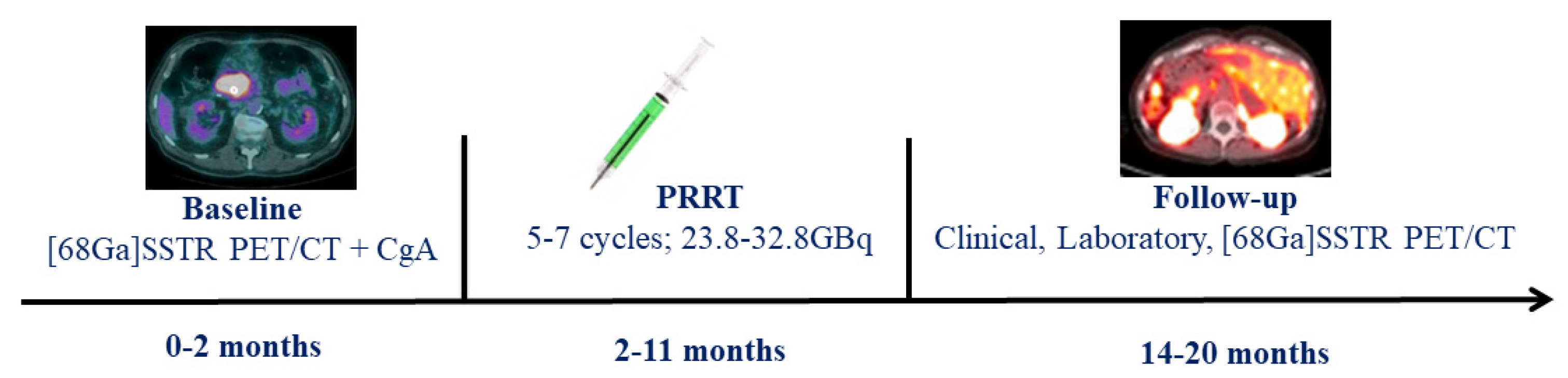
2.1. Patients
2.2. [68Ga]DOTATOC PET/CT
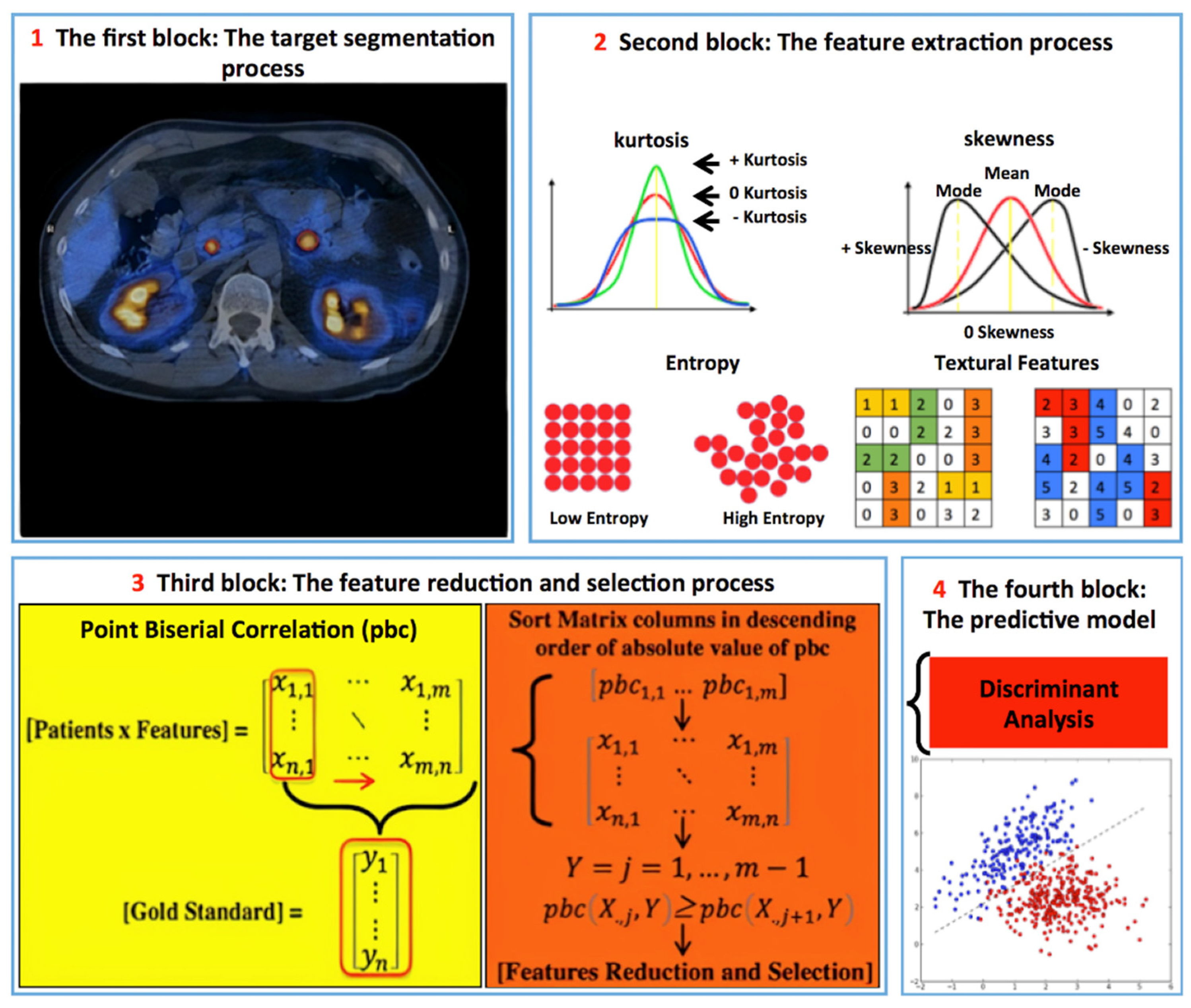
2.3. Image Analysis
2.4. PRRT
2.5. Radiomics [68Ga]DOTATOC PET/CT Analysis
2.6. Statistical Analysis
3. Results
3.1. [68Ga]DOTATOC PET/CT Findings
3.2. Radiomics Analysis
3.3. Lesions’ Per-Site Sub-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Baratto, L.; Laudicella, R.; Stracuzzi, F.; Baldari, S.; Iagaru, A. Molecular imaging of pancreatic neoplasms. Clin. Transl. Imaging 2021, 9, 141–151. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; the WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauckneht, M.; Albano, D.; Annunziata, S.; Santo, G.; Guglielmo, P.; Frantellizzi, V.; Branca, A.; Ferrari, C.; Vento, A.; Mirabile, A.; et al. Somatostatin Receptor PET/CT Imaging for the Detection and Staging of Pancreatic NET: A Systematic Review and Meta-Analysis. Diagnostics 2020, 10, 598. [Google Scholar] [CrossRef]
- Minutoli, F.; Laudicella, R.; Burger, I.A.; Baldari, S. Combined use of peptide receptor radionuclide therapy and metronomic chemotherapy in neuroendocrine tumors: A possible choice driven by nuclear medicine molecular imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3041–3042. [Google Scholar] [CrossRef]
- Hennrich, U.; Kopka, K. Lutathera((R)): The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef] [Green Version]
- Bauckneht, M.; Albano, D.; Annunziata, S.; Santo, G.; Guglielmo, P.; Frantellizzi, V.; Branca, A.; Ferrari, C.; Vento, A.; Mirabile, A.; et al. A Delphic consensus assessment: Imaging and biomarkers in gastroenteropancreatic neuroendocrine tumor disease management. Endocr. Connect 2016, 5, 174–187. [Google Scholar]
- Liberini, V.; Huellner, M.; Grimaldi, S.; Finessi, M.; Thuillier, P.; Muni, A.; Pellerito, R.; Papotti, M.; Piovesan, A.; Arvat, E.; et al. The Challenge of Evaluating Response to Peptide Receptor Radionuclide Therapy in Gastroenteropancreatic Neuroendocrine Tumors: The Present and the Future. Diagnostics 2020, 10, 1083. [Google Scholar] [CrossRef]
- Knigge, U.; Capdevila, J.; Bartsch, D.K.; Baudin, E.; Falkerby, J.; Kianmanesh, R.; Kos-Kudła, B.; Niederle, B.; van Dijkum, E.N.; O’Toole, D.; et al. ENETS Consensus Recommendations for the Standards of Care in Neuroendocrine Neoplasms: Follow-Up and Documentation. Neuroendocrinology 2017, 105, 310–319. [Google Scholar] [CrossRef] [Green Version]
- Spada, F.; Campana, D.; Lamberti, G.; Laudicella, R.; Dellamano, R.; Dellamano, L.; Leeuwenkamp, O.; Baldari, S. [(177)Lu]Lu-DOTA-TATE versus standard of care in adult patients with gastro-enteropancreatic neuroendocrine tumours (GEP-NETs): A cost-consequence analysis from an Italian hospital perspective. Eur. J. Nucl. Med. Mol. Imaging 2021, 1–12. [Google Scholar] [CrossRef]
- Laudicella, R.; Comelli, A.; Stefano, A.; Szostek, M.; Crocè, L.; Vento, A.; Spataro, A.; Comis, A.D.; La Torre, F.; Gaeta, M.; et al. Artificial Neural Networks in Cardiovascular Diseases and its Potential for Clinical Application in Molecular Imaging. Curr. Radiopharm. 2021, 14, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Alongi, P.; Laudicella, R.; Stefano, A.; Caobelli, F.; Comelli, A.; Vento, A.; Sardina, D.; Ganduscio, G.; Toia, P.; Ceci, F.; et al. Choline PET/CT features to predict survival outcome in high risk prostate cancer restaging: A preliminary machine-learning radiomics study. Q. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef] [PubMed]
- Alongi, P.; Stefano, A.; Comelli, A.; Laudicella, R.; Scalisi, S.; Arnone, G.; Barone, S.; Spada, M.; Purpura, P.; Bartolotta, T.V.; et al. Radiomics analysis of 18F-Choline PET/CT in the prediction of disease outcome in high-risk prostate cancer: An explorative study on machine learning feature classification in 94 patients. Eur. Radiol. 2021, 31, 4595–4605. [Google Scholar] [CrossRef]
- Liberini, V.; Laudicella, R.; Capozza, M.; Huellner, M.; Burger, I.; Baldari, S.; Terreno, E.; Deandreis, D. The Future of Cancer Diagnosis, Treatment and Surveillance: A Systemic Review on Immunotherapy and Immuno-PET Radiotracers. Molecules 2021, 26, 2201. [Google Scholar] [CrossRef]
- Laudicella, R.; Iagaru, A.; Minutoli, F.; Gaeta, M.; Baldari, S.; Bisdas, S. PET/MR in neuro-oncology: Is it ready for prime-time? Clin. Transl. Imaging 2020, 8, 233–235. [Google Scholar] [CrossRef]
- Liberini, V.; Rampado, O.; Gallio, E.; De Santi, B.; Ceci, F.; Dionisi, B.; Thuillier, P.; Ciuffreda, L.; Piovesan, A.; Fioroni, F.; et al. (68)Ga-DOTATOC PET/CT-Based Radiomic Analysis and PRRT Outcome: A Preliminary Evaluation Based on an Exploratory Radiomic Analysis on Two Patients. Front. Med. 2020, 7, 601853. [Google Scholar] [CrossRef]
- Weber, M.; Kessler, L.; Schaarschmidt, B.M.; Fendler, W.P.; Lahner, H.; Antoch, G.; Umutlu, L.; Herrmann, K.; Rischpler, C. Treatment-related changes in neuroendocrine tumors as assessed by textural features derived from (68)Ga-DOTATOC PET/MRI with simultaneous acquisition of apparent diffusion coefficient. BMC Cancer 2020, 20, 326. [Google Scholar] [CrossRef] [Green Version]
- Werner, R.A.; Lapa, C.; Ilhan, H.; Higuchi, T.; Buck, A.K.; Lehner, S.; Bartenstein, P.; Bengel, F.; Schatka, I.; Muegge, D.O.; et al. Survival prediction in patients undergoing radionuclide therapy based on intratumoral somatostatin-receptor heterogeneity. Oncotarget 2017, 8, 7039–7049. [Google Scholar] [CrossRef] [Green Version]
- Werner, R.A.; Ilhan, H.; Lehner, S.; Papp, L.; Zsótér, N.; Schatka, I.; Muegge, D.O.; Javadi, M.S.; Higuchi, T.; Buck, A.K.; et al. Pre-therapy Somatostatin Receptor-Based Heterogeneity Predicts Overall Survival in Pancreatic Neuroendocrine Tumor Patients Undergoing Peptide Receptor Radionuclide Therapy. Mol. Imaging Biol. 2019, 21, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Onner, H.; Abdulrezzak, U.; Tutus, A. Could the skewness and kurtosis texture parameters of lesions obtained from pretreatment Ga-68 DOTA-TATE PET/CT images predict receptor radionuclide therapy response in patients with gastroenteropancreatic neuroendocrine tumors? Nucl. Med. Commun. 2020, 41, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with (68)Ga-DOTA-conjugated somatostatin receptor targeting peptides and (18)F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601. [Google Scholar] [CrossRef]
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Pavel, M.E.; Baum, R.P.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinol, M.; Paganelli, G. Radionuclide Peptide Cancer Therapy, 1st ed.; Taylor & Francis Group: Abingdon, UK, 2006. [Google Scholar]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Comelli, A.; Stefano, A.; Coronnello, C.; Russo, G.; Vernuccio, F.; Cannella, R.; Salvaggio, G.; Lagalla, R.; Barone, S. Radiomics: A New Biomedical Workflow to Create a Predictive Model; Springer International Publishing: Cham, Switzerland, 2020; pp. 280–293. [Google Scholar]
- Barone, S.; Cannella, R.; Comelli, A.; Pellegrino, A.; Salvaggio, G.; Stefano, A.; Vernuccio, F. Hybrid descriptive-inferential method for key feature selection in prostate cancer radiomics. Appl. Stoch. Model. Bus. Ind. 2021, 37, 961–972. [Google Scholar] [CrossRef]
- Comelli, A.; Coronnello, C.; Dahiya, N.; Benfante, V.; Palmucci, S.; Basile, A.; Vancheri, C.; Russo, G.; Yezzi, A.; Stefano, A. Lung Segmentation on High-Resolution Computerized Tomography Images Using Deep Learning: A Preliminary Step for Radiomics Studies. J. Imaging 2020, 6, 125. [Google Scholar] [CrossRef]
- Couvelard, A.; Deschamps, L.; Ravaud, P.; Baron, G.; Sauvanet, A.; Hentic, O.; Colnot, N.; Paradis, V.; Belghiti, J.; Bedossa, P.; et al. Heterogeneity of tumor prognostic markers: A reproducibility study applied to liver metastases of pancreatic endocrine tumors. Mod. Pathol. 2009, 22, 273–281. [Google Scholar] [CrossRef]
- Wetz, C.; Apostolova, I.; Steffen, I.G.; Hofheinz, F.; Furth, C.; Kupitz, D.; Ruf, J.; Venerito, M.; Klose, S.; Amthauer, H. Predictive Value of Asphericity in Pretherapeutic [(111)In]DTPA-Octreotide SPECT/CT for Response to Peptide Receptor Radionuclide Therapy with [(177)Lu]DOTATATE. Mol. Imaging Biol. 2017, 19, 437–445. [Google Scholar] [CrossRef]
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef]

| Patients’ Number (female–male) | 38 (15 F—23 M) |
| Mean/median age (Range) | 59.4 ± 10.3 y/58 y (35–79) |
| Mean/median administred activity (range) | 29 ± 1.5 GBq/29 GBq (23.9–32.8) |
| Mean/median PRRT cycles (range) | 5.3 ± 0.5/5 (5–7) |
| GEP NET origin | |
| Pancreas | 17/38 (45%) |
| Ileum | 14/38 (37%) |
| Colon | 3/38 (8%) |
| Stomach | 2/38 (5%) |
| Jejunum | 2/38 (5%) |
| Grading (n) | |
| G1 | 9/38 (23.7%) |
| G2 | 27/38 (71%) |
| G3 | 2/38 (5.3%) |
| Lesions’ distribution | |
| Bone Lesions | 42/324 (12.9%) |
| Lymph nodal Lesions | 91/324 (28.1%) |
| Liver Lesions | 169/324 (52.2%) |
| Parenchimal Lesions (no liver) | 22/324 (6.8%) |
| Singular lesion response to PRRT | |
| PD | 133/324 (41%) |
| SD | 79/324 (24.4%) |
| PR | 92/324 (28.4%) |
| CR | 20/324 (6.2%) |
| Lesions’ distribution according to response (SD, PR, CR) and grading | |
| G1 | 28/82 (34.1%) |
| G2 | 157/232 (67.7%) |
| G3 | 6/10 (60%) |
| Scanner types | n patients—n lesions |
| GE Discovery 690 | 15/38—135/324 |
| Siemens biograph horizon | 14/38—133/324 |
| GE Discovery ST | 4/38—34/324 |
| Philips Gemini GXL 16 | 4/38—18/324 |
| GE Discovery 600 | 1/38—4/324 |
| District | Responders | Non-Responders | p |
|---|---|---|---|
| Lymph nodes (n = 91) | |||
| HISTO_Skewness | 2.01 ± 2.12 (−1.10–7.66) | 3.02 ± 1.44 (0.02–5.60) | 0.006 |
| HISTO_Kurtosis | 11.03 ± 11.79 (1.66–60.40) | 13.72 ± 8.85 (1.85–36.05) | 0.028 |
| SUVmax | 18.67 ± 12.14 (2.88–51.88) | 18.16 ± 13.86 (2.77–75.17) | 0.738 |
| Liver (n = 169) | |||
| HISTO_Skewness | 1.35 ± 2.25 (−4.47–7.66) | 3.63 ± 1.90 (−0.51–7.63) | 0.0001 |
| HISTO_Kurtosis | 9.04 ± 11.90 (1.81–60.40) | 19.34 ± 13.86 (1.75–60.09) | 0.0001 |
| SUVmax | 19.39 ± 10.17 (4.91–55.86) | 20.87–10.14 (9.12–55.26) | 0.326 |
| Bone (n = 42) | |||
| HISTO_Skewness | 2.40 ± 1.89 (0.51–6.67) | 4.03 ± 1.87 (0.49–7.74) | 0.014 |
| HISTO_Kurtosis | 11.57 ± 12.83 (2.35–48.00) | 23.13 ± 15.46 (2.17–61.34) | 0.015 |
| SUVmax | 10.31 ± 9.41 (2.06–36.07) | 28.42 ± 28.61 (1.67–93.50) | 0.047 |
| District | Responders | Non-Responders | p |
|---|---|---|---|
| Lymph node (n = 91) | |||
| ΔHISTO_Skewness | 21.18 ± 265.75% (−880.0–1533.3) | 176.83 ± 469.34% (−96.3–2550.0) | 0.886 |
| ΔHISTO_Kurtosis | 13.97 ± 83.08% (−82.9–340.5) | −4.48 ± 40.84% (−85.2–96.2) | 0.604 |
| Liver (n = 169) | |||
| ΔHISTO_Skewness | −17.72 ± 865.36% (−6300.0–4800.0) | 134.23 ± 324.32% (−180.00–1203.82) | 0.031 |
| ΔHISTO_Kurtosis | 9.76 ± 52.45% (−94.83–193.68) | 14.64 ± 60.64% (−94.07–175.68) | 0.906 |
| Bone (n = 42) | |||
| ΔHISTO_Skewness | 6.84 ± 70.95% (−125.0–134.53) | −24.54 ± 71.06% (−240.8–56.6) | 0.334 |
| ΔHISTO_Kurtosis | 66.15 ± 113.10% (−28.1–338.5) | −0.33 ± 41.43% (−55.7–103.7) | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laudicella, R.; Comelli, A.; Liberini, V.; Vento, A.; Stefano, A.; Spataro, A.; Crocè, L.; Baldari, S.; Bambaci, M.; Deandreis, D.; et al. [68Ga]DOTATOC PET/CT Radiomics to Predict the Response in GEP-NETs Undergoing [177Lu]DOTATOC PRRT: The “Theragnomics” Concept. Cancers 2022, 14, 984. https://doi.org/10.3390/cancers14040984
Laudicella R, Comelli A, Liberini V, Vento A, Stefano A, Spataro A, Crocè L, Baldari S, Bambaci M, Deandreis D, et al. [68Ga]DOTATOC PET/CT Radiomics to Predict the Response in GEP-NETs Undergoing [177Lu]DOTATOC PRRT: The “Theragnomics” Concept. Cancers. 2022; 14(4):984. https://doi.org/10.3390/cancers14040984
Chicago/Turabian StyleLaudicella, Riccardo, Albert Comelli, Virginia Liberini, Antonio Vento, Alessandro Stefano, Alessandro Spataro, Ludovica Crocè, Sara Baldari, Michelangelo Bambaci, Desiree Deandreis, and et al. 2022. "[68Ga]DOTATOC PET/CT Radiomics to Predict the Response in GEP-NETs Undergoing [177Lu]DOTATOC PRRT: The “Theragnomics” Concept" Cancers 14, no. 4: 984. https://doi.org/10.3390/cancers14040984
APA StyleLaudicella, R., Comelli, A., Liberini, V., Vento, A., Stefano, A., Spataro, A., Crocè, L., Baldari, S., Bambaci, M., Deandreis, D., Arico’, D., Ippolito, M., Gaeta, M., Alongi, P., Minutoli, F., Burger, I. A., & Baldari, S. (2022). [68Ga]DOTATOC PET/CT Radiomics to Predict the Response in GEP-NETs Undergoing [177Lu]DOTATOC PRRT: The “Theragnomics” Concept. Cancers, 14(4), 984. https://doi.org/10.3390/cancers14040984












