Overall Survival of Patients with Myxofibrosarcomas: An Epidemiological Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data
2.2. Statistical Analysis
3. Results
3.1. Patient and Clinical Characteristics
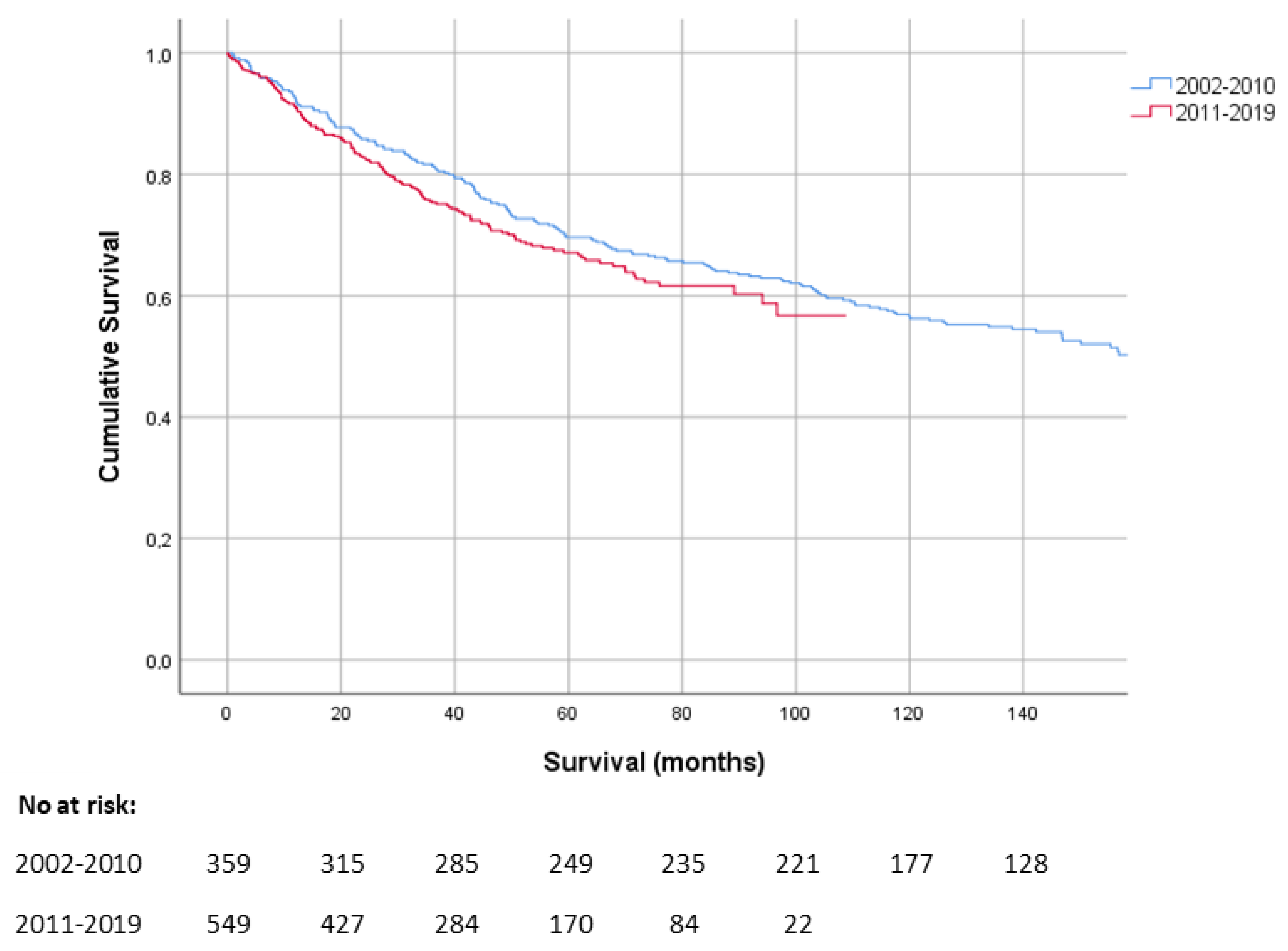
3.2. Overall Survival
3.3. Prognostic Factors
3.4. Recurrence and Distant Metastases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mentzel, T.; Calonje, E.; Wadden, C.; Camplejohn, R.S.; Beham, A.; Smith, M.A.; Fletcher, C.D. Myxofibrosarcoma. Clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am. J. Surg. Pathol. 1996, 20, 391–405. [Google Scholar] [CrossRef]
- Brennan, M.F.; Antonescu, C.R.; Alektiar, K.M.; Maki, R.G. Management of Soft Tissue Sarcoma, 2nd ed.; Springer: New York, NY, USA, 2016; pp. 143–152. [Google Scholar]
- Incidentie Bot- en Wekedelenkanker. Available online: https://iknl.nl/kankersoorten/bot-en-wekedelenkanker/registratie/incidentie (accessed on 10 August 2021).
- Fletcher, C.D.; Gustafson, P.; Rydholm, A.; Willén, H.; Akerman, M. Clinicopathologic re-evaluation of 100 malignant fibrous histiocytomas: Prognostic relevance of subclassification. J. Clin. Oncol. 2001, 19, 3045–3050. [Google Scholar] [CrossRef]
- Dewan, V.; Darbyshire, A.; Sumathi, V.; Jeys, L.; Grimer, R. Prognostic and survival factors in myxofibrosarcomas. Sarcoma 2012, 2012, 830879. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Miceli, R.; Grosso, F.; Fiore, M.; Puma, E.; Pennacchioli, E.; Barisella, M.; Sangalli, C.; Mariani, L.; Casali, P.G.; et al. Myxofibrosarcoma: Prognostic factors and survival in a series of patients treated at a single institution. Ann. Surg. Oncol. 2011, 18, 720–725. [Google Scholar] [CrossRef]
- Mühlhofer, H.M.L.; Lenze, U.; Gersing, A.; Lallinger, V.; Burgkart, R.; Obermeier, A.; von Eisenhart-Rothe, R.; Knebel, C. Prognostic Factors and Outcomes for Patients With Myxofibrosarcoma: A 13-Year Retrospective Evaluation. Anticancer Res. 2019, 39, 2985–2992. [Google Scholar] [CrossRef]
- Hong, N.J.L.; Hornicek, F.J.; Raskin, K.A.; Yoon, S.S.; Szymonifka, J.; Yeap, B.; Chen, Y.L.; DeLaney, T.F.; Nielsen, G.P.; Mullen, J.T. Prognostic factors and outcomes of patients with myxofibrosarcoma. Ann. Surg. Oncol. 2013, 20, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Teurneau, H.; Engellau, J.; Ghanei, I.; von Steyern, F.V.; Styring, E. High Recurrence Rate of Myxofibrosarcoma: The Effect of Radiotherapy Is Not Clear. Sarcoma 2019, 2019, 8517371. [Google Scholar] [CrossRef]
- Kaya, M.; Wada, T.; Nagoya, S.; Yamashita, T. Bone and/or joint attachment is a risk factor for local recurrence of myxofibrosarcoma. J. Orthop. Sci. 2011, 16, 413–417. [Google Scholar] [CrossRef]
- Weiss, S.W.; Enzinger, F.M. Myxoid variant of malignant fibrous histiocytoma. Cancer 1977, 39, 1672–1685. [Google Scholar] [CrossRef]
- Gronchi, A.; Vullo, S.L.; Colombo, C.; Collini, P.; Stacchiotti, S.; Mariani, L.; Fiore, M.; Casali, P.G. Extremity soft tissue sarcoma in a series of patients treated at a single institution: Local control directly impacts survival. Ann. Surg. 2010, 251, 506–511. [Google Scholar] [CrossRef]
- Angervall, L.; Kindblom, L.G.; Merck, C. Myxofibrosarcoma. A study of 30 cases. Acta Pathol. Microbiol. Scand. A 1977, 85a, 127–140. [Google Scholar]
- Sambri, A.; Bianchi, G.; Righi, A.; Ferrari, C.; Donati, D. Surgical margins do not affect prognosis in high grade myxofibrosarcoma. Eur. J. Surg. Oncol. 2016, 42, 1042–1048. [Google Scholar] [CrossRef]
- Huang, H.Y.; Lal, P.; Qin, J.; Brennan, M.F.; Antonescu, C.R. Low-grade myxofibrosarcoma: A clinicopathologic analysis of 49 cases treated at a single institution with simultaneous assessment of the efficacy of 3-tier and 4-tier grading systems. Hum. Pathol. 2004, 35, 612–621. [Google Scholar] [CrossRef]
- Gronchi, A.; Casali, P.G.; Mariani, L.; Miceli, R.; Fiore, M.; Vullo, S.L.; Bertulli, R.; Collini, P.; Lozza, L.; Olmi, P.; et al. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: A series of patients treated at a single institution. J. Clin. Oncol. 2005, 23, 96–104. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Leung, D.H.; Hoos, A.; Jaques, D.P.; Lewis, J.J.; Brennan, M.F. Analysis of the prognostic significance of microscopic margins in 2084 localized primary adult soft tissue sarcomas. Ann. Surg. 2002, 235, 424–434. [Google Scholar] [CrossRef]
- Wada, T.; Hasegawa, T.; Nagoya, S.; Kawaguchi, S.; Kaya, M.; Ishii, S. Myxofibrosarcoma with an infiltrative growth pattern: A case report. Jpn. J. Clin. Oncol. 2000, 30, 458–462. [Google Scholar] [CrossRef] [Green Version]
- Roland, C.L.; Wang, W.L.; Lazar, A.J.; Torres, K.E. Myxofibrosarcoma. Surg. Oncol. Clin. N. Am. 2016, 25, 775–788. [Google Scholar] [CrossRef]
- Haglund, K.E.; Raut, C.P.; Nascimento, A.F.; Wang, Q.; George, S.; Baldini, E.H. Recurrence patterns and survival for patients with intermediate- and high-grade myxofibrosarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 361–367. [Google Scholar] [CrossRef]
- Mutter, R.W.; Singer, S.; Zhang, Z.; Brennan, M.F.; Alektiar, K.M. The enigma of myxofibrosarcoma of the extremity. Cancer 2012, 118, 518–527. [Google Scholar] [CrossRef]
- Odei, B.; Rwigema, J.C.; Eilber, F.R.; Eilber, F.C.; Selch, M.; Singh, A.; Chmielowski, B.; Nelson, S.D.; Wang, P.C.; Steinberg, M.; et al. Predictors of Local Recurrence in Patients With Myxofibrosarcoma. Am. J. Clin. Oncol. 2018, 41, 827–831. [Google Scholar] [CrossRef]
- Boers-Sonderen, M.J.; Desar, I.M.; Koopman, M.; Punt, C.J.; van Herpen, C.M. Capecitabine, irinotecan (CAPIRI) and sunitinib in metastatic colorectal cancer. Acta Oncol. 2013, 52, 1778. [Google Scholar] [CrossRef] [Green Version]
- Micheli, A.; Ciampichini, R.; Oberaigner, W.; Ciccolallo, L.; de Vries, E.; Izarzugaza, I.; Zambon, P.; Gatta, G.; de Angelis, R. The advantage of women in cancer survival: An analysis of EUROCARE-4 data. Eur. J. Cancer 2009, 45, 1017–1027. [Google Scholar] [CrossRef]
- Lin, C.N.; Chou, S.C.; Li, C.F.; Tsai, K.B.; Chen, W.C.; Hsiung, C.Y.; Yen, C.F.; Huang, H.Y. Prognostic factors of myxofibrosarcomas: Implications of margin status, tumor necrosis, and mitotic rate on survival. J. Surg. Oncol. 2006, 93, 294–303. [Google Scholar] [CrossRef]
- Kikuta, K.; Kubota, D.; Yoshida, A.; Suzuki, Y.; Morioka, H.; Toyama, Y.; Kobayashi, E.; Nakatani, F.; Chuuman, H.; Kawai, A. An analysis of factors related to recurrence of myxofibrosarcoma. Jpn. J. Clin. Oncol. 2013, 43, 1093–1104. [Google Scholar] [CrossRef] [Green Version]
- Sarcomenzorg in Nederland. Available online: https://www.iknl.nl/getmedia/b2538482-8e79-40d5-a4ff-90e4b1e54838/Rapport-Sarcomenzorg-in-Nederland_2020_IKNL_NKR.pdf (accessed on 10 August 2021).
- Vos, M.; Blaauwgeers, H.G.T.; Ho, V.K.Y.; van Houdt, W.J.; van der Hage, J.A.; Been, L.B.; Bonenkamp, J.J.; Bemelmans, M.H.A.; van Dalen, T.; Haas, R.L.; et al. Increased survival of non low-grade and deep-seated soft tissue sarcoma after surgical management in high-volume hospitals: A nationwide study from the Netherlands. Eur. J. Cancer 2019, 110, 98–106. [Google Scholar] [CrossRef]
- Blay, J.Y.; Soibinet, P.; Penel, N.; Bompas, E.; Duffaud, F.; Stoeckle, E.; Mir, O.; Adam, J.; Chevreau, C.; Bonvalot, S.; et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann. Oncol. 2017, 28, 2852–2859. [Google Scholar] [CrossRef]
- Lombardi, R.; Jovine, E.; Zanini, N.; Salone, M.C.; Gambarotti, M.; Righi, A.; Balladelli, A.; Colangeli, M.; Rocca, M. A case of lung metastasis in myxoinflammatory fibroblastic sarcoma: Analytical review of one hundred and thirty eight cases. Int. Orthop. 2013, 37, 2429–2436. [Google Scholar] [CrossRef] [Green Version]
- Maretty-Nielsen, K.; Baerentzen, S.; Keller, J.; Dyrop, H.B.; Safwat, A. Low-Grade Fibromyxoid Sarcoma: Incidence, Treatment Strategy of Metastases, and Clinical Significance of the FUS Gene. Sarcoma 2013, 2013, 256280. [Google Scholar] [CrossRef] [Green Version]

| Variable | Overall Survival | p-Value 2 |
|---|---|---|
| Hazard Ratio (95% CI) 1 | ||
| Age | ||
| <65 years | Ref. | |
| ≥65 years | 2.7 (2.0–3.6) | <0.01 3 |
| Sex | ||
| Male | Ref. | |
| Female | 0.7 (0.6–0.9) | <0.01 3 |
| Cancer in medical history | ||
| No | Ref. | |
| Yes | 1.5 (1.2–2.1) | <0.01 3 |
| Tumour size | ||
| ≤5 cm | Ref. | |
| >5 cm | 2.0 (1.5–2.7) | <0.01 3 |
| Tumour depth | ||
| Superficial | Ref. | |
| Deep | 1.3 (0.9–1.8) | 0.10 |
| Histological grade | ||
| I | Ref. | |
| II | 2.3 (1.5–3.8) | <0.01 3 |
| III | 3.1 (1.8–5.2) | <0.01 3 |
| Distant metastases at diagnosis | ||
| No | Ref. | |
| Yes | 2.5 (1.4–4.7) | <0.01 3 |
| Surgery | ||
| No | Ref. | |
| Yes | 0.2 (0.1–0.4) | <0.01 3 |
| Residual disease | ||
| R0 | Ref. | |
| R1/R2 | 1.7 (1.2–2.4) | <0.01 3 |
| Radiotherapy | ||
| No | Ref. | |
| Neoadjuvant | 0.8 (0.6–1.1) | 0.20 |
| Adjuvant | 0.6 (0.4–0.8) | <0.01 3 |
| Neoadjuvant + adjuvant | 0.1 (0.02–0.9) | 0.04 3 |
| Primary | 0.4 (0.2–0.8) | 0.02 3 |
| Surgery | ||
| Sarcoma centre | Ref | |
| Other | 1.3 (1.0–1.6) | 0.087 |
| Recurrence or Metastases | No (%) | Median Time to Recurrence (Range) | Median OS (Months. 95% CI) 1 | p-Value 2 |
|---|---|---|---|---|
| Local recurrence | <0.01 3 | |||
| Yes | 69 (39) | 20.0 (1.7–88.5) | 64.0 (38.5–89.5) | |
| No | 108 (61) | 194.1 (60.3–327.8) | ||
| Regional recurrence | <0.015 3 | |||
| Yes | 6 (3) | 17.4 (3.8–82.4) | 23.8 (13.4–34.2) | |
| No | 171 (97) | 103.5 (51.9–155.2) | ||
| Distant metastases | <0.01 3 | |||
| Yes | 50 (28) | 15.3 (3.8–155.0) | 34.3(28.8–39.8) | |
| No | 127 (72) | 194.1 (88.4–299.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Horst, C.A.J.; Bongers, S.L.M.; Versleijen-Jonkers, Y.M.H.; Ho, V.K.Y.; Braam, P.M.; Flucke, U.E.; de Wilt, J.H.W.; Desar, I.M.E. Overall Survival of Patients with Myxofibrosarcomas: An Epidemiological Study. Cancers 2022, 14, 1102. https://doi.org/10.3390/cancers14051102
van der Horst CAJ, Bongers SLM, Versleijen-Jonkers YMH, Ho VKY, Braam PM, Flucke UE, de Wilt JHW, Desar IME. Overall Survival of Patients with Myxofibrosarcomas: An Epidemiological Study. Cancers. 2022; 14(5):1102. https://doi.org/10.3390/cancers14051102
Chicago/Turabian Stylevan der Horst, Chiel A. J., Sabien L. M. Bongers, Yvonne M. H. Versleijen-Jonkers, Vincent K. Y. Ho, Pètra M. Braam, Uta E. Flucke, Johannes H. W. de Wilt, and Ingrid M. E. Desar. 2022. "Overall Survival of Patients with Myxofibrosarcomas: An Epidemiological Study" Cancers 14, no. 5: 1102. https://doi.org/10.3390/cancers14051102
APA Stylevan der Horst, C. A. J., Bongers, S. L. M., Versleijen-Jonkers, Y. M. H., Ho, V. K. Y., Braam, P. M., Flucke, U. E., de Wilt, J. H. W., & Desar, I. M. E. (2022). Overall Survival of Patients with Myxofibrosarcomas: An Epidemiological Study. Cancers, 14(5), 1102. https://doi.org/10.3390/cancers14051102






