Differences in the Molecular Profile between Primary Breast Carcinomas and Their Cutaneous Metastases
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Histology
2.2. Immunohistochemistry
2.3. Massive Parallel Sequencing
2.4. Fluorescent In-Situ Hybridization on Tissue Microarrays
2.5. Statistical Methods
3. Results
3.1. Case Selection
3.2. Clinicopathological Features
3.3. Immunohistochemistry and HER2 FISH
3.3.1. Surrogate Molecular Types
3.3.2. Androgen Receptor in Triple-Negative Cases
3.4. Molecular Characterization
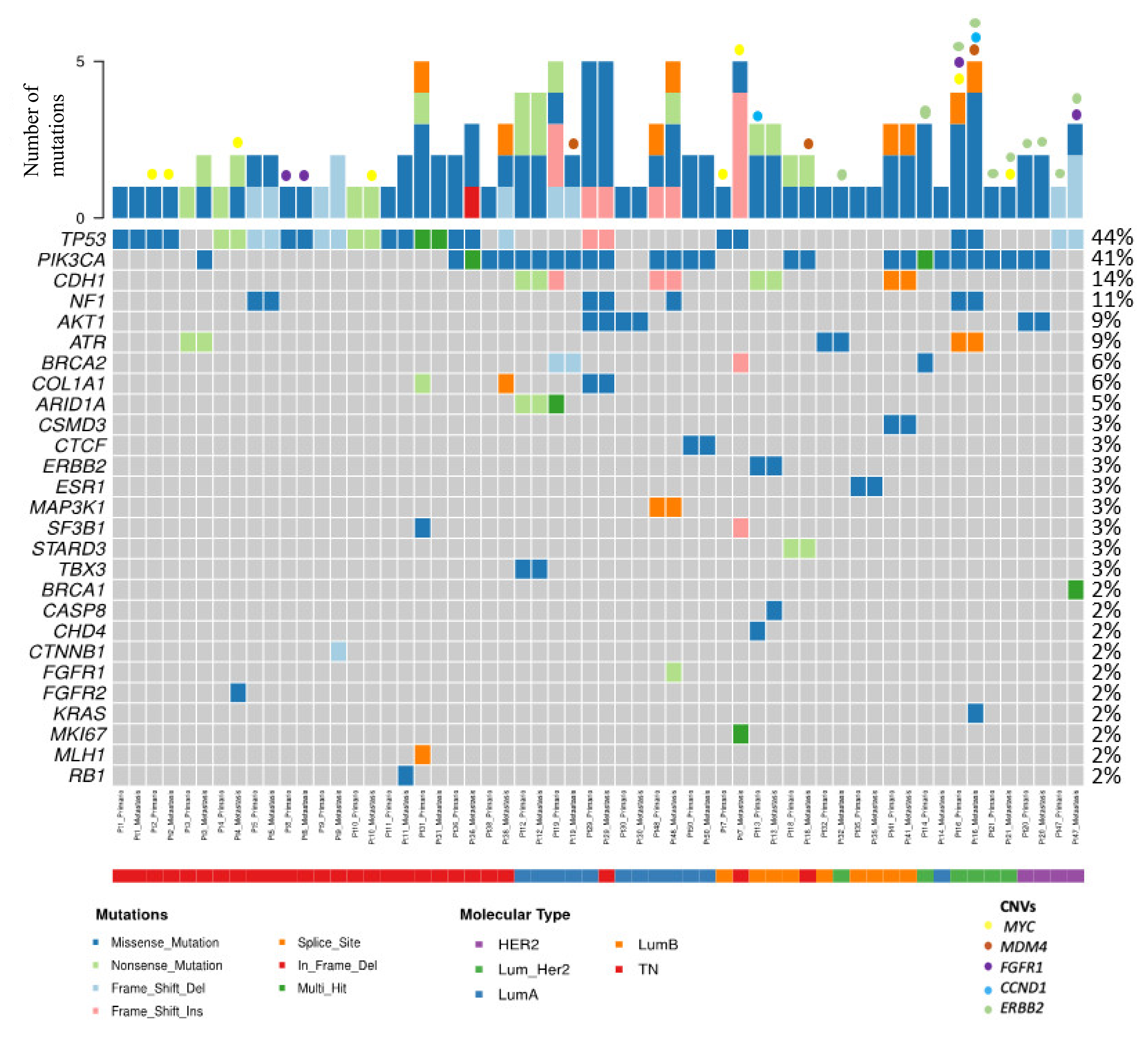
3.4.1. Most Frequently Altered Genes in Primary Tumors and Cutaneous Metastases
Enriched Molecular Alterations in Cutaneous Metastases
3.5. Survival Analysis
4. Discussion
4.1. Clinicopathologic Features of Breast Carcinomas That Develop Cutaneous Relapses
4.2. Molecular Alterations Involved in the Development of Cutaneous Metastasis in Breast Cancer
4.3. Therapeutic Implications
4.4. Prognosis
4.5. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol. Biomark. Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35. [Google Scholar] [CrossRef]
- Yam, C.; Mani, S.A.; Moulder, S.L. Targeting the Molecular Subtypes of Triple Negative Breast Cancer: Understanding the Diversity to Progress the Field. Oncologist 2017, 22, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Classification of Tumours Editorial Board; International Agency for Research on Cancer; World Health Organization. WHO Classification of Tumours. Breast Tumours; International Agency for Research on Cancer: Lyon, France, 2019; ISBN 978-92-832-4500-1. [Google Scholar]
- Scully, O.J.; Bay, B.-H.; Yip, G.; Yu, Y. Breast cancer metastasis. Cancer Genom. Proteom. 2012, 9, 311–320. [Google Scholar]
- Chiang, A.C.; Massagué, J. Molecular Basis of Metastasis. N. Engl. J. Med. 2008, 359, 2814–2823. [Google Scholar] [CrossRef]
- Liotta, L.A. Cancer invasion and metastases. JAMA J. Am. Med. Assoc. 1990, 263, 1123–1126. [Google Scholar] [CrossRef]
- Arozullah, A.M.; Calhoun, E.A.; Wolf, M.; Finley, D.K.; Fitzner, K.A.; Heckinger, E.A.; Gorby, N.S.; Schumock, G.T.; Bennett, C.L. The financial burden of cancer: Estimates from a study of insured women with breast cancer. J. Support. Oncol. 2004, 2, 271–278. [Google Scholar] [PubMed]
- Guanziroli, E.; Coggi, A.; Venegoni, L.; Fanoni, D.; Ercoli, G.; Boggio, F.; Veraldi, S.; Berti, E.; Gianotti, R.; Ferrero, S.; et al. Cutaneous metastases of internal malignancies: An experience from a single institution. Eur. J. Dermatol. 2017, 27, 609–614. [Google Scholar] [CrossRef]
- de Bittencourt, M.J.S.; Carvalho, A.H.; do Nascimento, B.A.M.; Freitas, L.K.M.; de Parijós, A.M. Cutaneous metastasis of a breast cancer diagnosed 13 years before. An. Bras. Dermatol. 2015, 90, 134–137. [Google Scholar] [CrossRef]
- De Giorgi, V.; Grazzini, M.; Alfaioli, B.; Savarese, I.; Corciova, S.A.; Guerriero, G.; Lotti, T. Cutaneous manifestations of breast carcinoma: A clinical guide. Dermatol. Ther. 2010, 23, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, I.; Cerroni, L.; Rütten, A.; Kutzner, H.; Requena, L. Cutaneous Metastases From Internal Malignancies: A Clinicopathologic and Immunohistochemical Review. Am. J. Dermatopathol. 2012, 34, 347–393. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.R. Cutaneous manifestations of breast cancer. Semin. Oncol. 2016, 43, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.C.-S.; Chen, G.-S.; Wu, C.-S.; Chai, C.-Y.; Chen, W.-T.; Lan, C.-C.E. Rates of cutaneous metastases from different internal malignancies: Experience from a Taiwanese medical center. J. Am. Acad. Dermatol. 2009, 60, 379–387. [Google Scholar] [CrossRef]
- Kong, J.H.; Park, Y.H.; Kim, J.A.; Kim, J.H.; Yun, J.; Sun, J.M.; Won, Y.W.; Lee, S.; Kim, S.T.; Cho, E.Y.; et al. Patterns of Skin and Soft Tissue Metastases from Breast Cancer according to Subtypes: Relationship between EGFR Overexpression and Skin Manifestations. Oncology 2011, 81, 55–62. [Google Scholar] [CrossRef]
- Krathen, R.A.; Orengo, I.F.; Rosen, T. Cutaneous Metastasis: A Meta-Analysis of Data. South Med. J. 2003, 96, 164–167. [Google Scholar] [CrossRef]
- Brownstein, M.H.; Helwig, E.B. Metastatic tumors of the skin. Cancer 1972, 29, 1298–1307. [Google Scholar] [CrossRef]
- Moore, S. Cutaneous Metastatic Breast Cancer. Clin. J. Oncol. Nurs. 2002, 6, 255–260. [Google Scholar] [CrossRef]
- Marcoval, J.; Gallego, M.I.; Moreno, A. Inflammatory Cutaneous Metastasis as a First Sign of Recurrence of Squamous Cell Carcinoma of the Lung. Actas Dermo-Sifiliográficas Engl. Ed. 2008, 99, 157–159. [Google Scholar] [CrossRef]
- Lin, L.; Sirohi, D.; Coleman, J.F.; Gulbahce, H.E. American Society of Clinical Oncology/College of American Pathologists 2018 Focused Update of Breast Cancer HER2 FISH Testing GuidelinesResults From a National Reference Laboratory. Am. J. Clin. Pathol. 2019, 152, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Koboldt, D.C.; Zhang, Q.; Larson, D.E.; Shen, D.; McLellan, M.D.; Lin, L.; Miller, C.A.; Mardis, E.R.; Ding, L.; Wilson, R.K. VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012, 22, 568–576. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Reis-Filho, J.S. Tackling the Diversity of Triple-Negative Breast Cancer. Clin. Cancer Res. 2013, 19, 6380–6388. [Google Scholar] [CrossRef]
- Johnson, C. Cutaneous Manifestation as Initial Presentation of Metastatic Breast Cancer: A Systematic Review. Cutis 2021, 107, E29–E36. [Google Scholar] [CrossRef] [PubMed]
- Lookingbill, D.P.; Spangler, N.; Sexton, F.M. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J. Am. Acad. Dermatol. 1990, 22, 19–26. [Google Scholar] [CrossRef]
- Atış, G.; Tükenmez Demirci, G.; Kıvanç Atunay, İ.; Sakız, D. Derinin metastatik tümörlerinin primer deri tümörleri arasındaki sıklığı ve klinik özellikleri. Turkderm 2013, 47, 166–169. [Google Scholar] [CrossRef]
- Bansal, R.; Patel, T.; Sarin, J.; Parikh, B.; Ohri, A.; Trivedi, P. Cutaneous and subcutaneous metastases from internal malignancies: An analysis of cases diagnosed by fine needle aspiration. Diagn. Cytopathol. 2011, 39, 882–887. [Google Scholar] [CrossRef]
- Benmously, R.; Souissi, A.; Badri, T.; Ben Jannet, S.; Marrak, H.; Mokhtar, I.; Fenniche, S. Cutaneous metastases from internal cancers. Acta Dermatovenerol. Alp. Pannonica Adriat. 2008, 17, 167–170. [Google Scholar] [PubMed]
- Chopra, R.; Chhabra, S.; Samra, S.G.; Thami, G.P.; Punia, R.P.S.; Mohan, H. Cutaneous metastases of internal malignancies: A clinicopathologic study. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 125–131. [Google Scholar] [CrossRef]
- El Khoury, J.; Khalifeh, I.; Kibbi, A.-G.; Abbas, O. Cutaneous metastasis: Clinicopathological study of 72 patients from a tertiary care center in Lebanon. Int. J. Dermatol. 2014, 53, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Flores, A. Cutaneous metastases: A study of 78 biopsies from 69 patients. Am. J. Dermatopathol. 2010, 32, 222–239. [Google Scholar] [CrossRef]
- Gómez Sánchez, M.E.; Martinez Martinez, M.L.; Martín De Hijas, M.C.; López Villaescusa, M.T.; Faura Berruga, C.; Rodríguez Vázquez, M.; Pérez García, L.J. Metástasis cutáneas de tumores sólidos. Estudio descriptivo retrospectivo. Piel 2014, 29, 207–212. [Google Scholar] [CrossRef]
- Handa, U.; Kundu, R.; Dimri, K. Cutaneous Metastasis: A Study of 138 Cases Diagnosed by Fine-Needle Aspiration Cytology. Acta Cytol. 2017, 61, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Itin, P.; Tomaschett, S. Hautmetastasen bei viszeralen Malignomen: Eine epidemiologische Studie. Internist 2009, 50, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Razavi, P.; Chang, M.T.; Xu, G.; Bandlamudi, C.; Ross, D.S.; Vasan, N.; Cai, Y.; Bielski, C.M.; Donoghue, M.T.A.; Jonsson, P.; et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell 2018, 34, 427–438.e6. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, B.; Guo, J.; Shao, H.; Del Priore, I.S.; Chang, Q.; Kudo, R.; Li, Z.; Razavi, P.; Liu, B.; et al. INK4 tumor suppressor proteins mediate resistance to CDK4/6 kinase inhibitors. Cancer Discov. 2021, 12, 356–371. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, S.; Pizarro, D.; Pérez-Mies, B.; Caniego-Casas, T.; Curigliano, G.; Cortés, J.; Palacios, J. Clinical, Pathological, and Molecular Features of Breast Carcinoma Cutaneous Metastasis. Cancers 2021, 13, 5416. [Google Scholar] [CrossRef]
- Luna, A.; Rabassa, M.E.; Isla Larrain, M.; Cabaleiro, P.; Zwenger, A.; Canzoneri, R.; Segal-Eiras, A.; Abba, M.C.; Croce, M.V. Breast cancer cutaneous metastases are associated to uMUC1 and sialyl Lewis x and to highly malignant primary tumors. Pathol.-Res. Pract. 2020, 216, 152859. [Google Scholar] [CrossRef]
- Yates, L.R.; Knappskog, S.; Wedge, D.; Farmery, J.H.R.; Gonzalez, S.; Martincorena, I.; Alexandrov, L.B.; Van Loo, P.; Haugland, H.K.; Lilleng, P.K.; et al. Genomic Evolution of Breast Cancer Metastasis and Relapse. Cancer Cell 2017, 32, 169–184.e7. [Google Scholar] [CrossRef]
- Lefebvre, C.; Bachelot, T.; Filleron, T.; Pedrero, M.; Campone, M.; Soria, J.-C.; Massard, C.; Lévy, C.; Arnedos, M.; Lacroix-Triki, M.; et al. Mutational Profile of Metastatic Breast Cancers: A Retrospective Analysis. PLoS Med. 2016, 13, e1002201. [Google Scholar] [CrossRef]
- Schrijver, W.A.; Selenica, P.; Lee, J.Y.; Ng, C.K.Y.; Burke, K.; Piscuoglio, S.; Berman, S.H.; Reis-Filho, J.S.; Weigelt, B.; Van Diest, P.J.; et al. Mutation profiling of key cancer genes in primary breast cancers and their distant metastases. Cancer Res. 2018, 78, 3112–3121. [Google Scholar] [CrossRef]
- Paul, M.R.; Pant, D.K.; Shih, N.N.C.; Chen, Y.; Harvey, K.L.; Solomon, A.; Lieberman, D.; Morrissette, J.J.D.; Soucier-Ernst, D.; Goodman, N.G.; et al. Genomic landscape of metastatic breast cancer identifies preferentially dysregulated pathways and targets. J. Clin. Investig. 2020, 130, 4252–4265. [Google Scholar] [CrossRef]
- Muller, K.E.; Marotti, J.D.; de Abreu, F.B.; Peterson, J.D.; Miller, T.W.; Chamberlin, M.D.; Tsongalis, G.J.; Tafe, L.J. Targeted next-generation sequencing detects a high frequency of potentially actionable mutations in metastatic breast cancers. Exp. Mol. Pathol. 2016, 100, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.J.; Naidu, S.; Topham, A.K.; Guiles, F.; Xu, Y.; McCue, P.; Schwartz, G.F.; Park, P.K.; Rosenberg, A.L.; Brill, K.; et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: A single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer 2007, 110, 876–884. [Google Scholar] [CrossRef]
- Kurozumi, S.; Alsaleem, M.; Monteiro, C.J.; Bhardwaj, K.; Joosten, S.E.P.; Fujii, T.; Shirabe, K.; Green, A.R.; Ellis, I.O.; Rakha, E.A.; et al. Targetable ERBB2 mutation status is an independent marker of adverse prognosis in estrogen receptor positive, ERBB2 non-amplified primary lobular breast carcinoma: A retrospective in silico analysis of public datasets. Breast Cancer Res. 2020, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Rosa, J.; Caniego-Casas, T.; Leskela, S.; Cristobal, E.; González-Martínez, S.; Moreno-Moreno, E.; López-Miranda, E.; Holgado, E.; Pérez-Mies, B.; Garrido, P.; et al. High Frequency of ERBB2 Activating Mutations in Invasive Lobular Breast Carcinoma with Pleomorphic Features. Cancers 2019, 11, 74. [Google Scholar] [CrossRef]
- Lookingbill, D.P.; Spangler, N.; Helm, K.F. Cutaneous metastases in patients with metastatic carcinoma: A retrospective study of 4020 patients. J. Am. Acad. Dermatol. 1993, 29, 228–236. [Google Scholar] [CrossRef]



| Clinicopathological Features | Categories | n (%) | |
|---|---|---|---|
| Sex | Female | 33 (100) | |
| Age | <60 | 14 (42.4) | |
| >60 | 19 (57.6) | ||
| Cutaneous metastases location | Local * | 15 (46.9) | |
| Distance | 17 (53.1) | ||
| NA | 1 | ||
| Menopausal status at diagnosis | Yes | 27 (90) | |
| No | 3 (10) | ||
| NA | 3 | ||
| pT | 1 | 5 (18.5) | |
| 2 | 14 (51.9) | ||
| 3 | 5 (18.5) | ||
| 4 | 3 (11.1) | ||
| NA | 6 ** | ||
| pN | 0 | 8 (30.8) | |
| 1 | 10 (38.5) | ||
| 2 | 2 (7.7) | ||
| 3 | 6 (23) | ||
| NA | 7 ** | ||
| Clinical stage | I | 4 (12.5) | |
| II | 5 (15.6) | ||
| III | 14 (43.7) | ||
| IV | 9 (28.1) | ||
| NA | 1 | ||
| Histological Grade | 1 | 1 (3) | |
| 2 | 13 (39.4) | ||
| 3 | 19 (57.6) | ||
| LIV | Yes | 11 (33.3) | |
| No | 22 (66.7) | ||
| Immunohistochemical markers | ER+ | 19 (57.6) | |
| PR+ | 11 (33.3) | ||
| HER2+ | 5 (15.2) | ||
| Ki67 | ≤15% | 9 (27.3) | |
| 16–29% | 5 (15.2) | ||
| ≥30% | 19 (57.6) | ||
| TN | 12 (36.4) | ||
| Surrogated molecular type | Luminal HER2- | 16 (48.5) | |
| Luminal HER2+ | 3 (9) | ||
| HER2+ (non-luminal) | 2 (6) | ||
| Tumor | Surrogated Molecular Type |
|---|---|
| Pt7_Primary | Luminal HER2- |
| Pt7_Metastasis | TN |
| Pt18_Primary | Luminal HER2- |
| Pt18_Metastasis | TN |
| Pt29_Primary | Luminal HER2- |
| Pt29_Metastasis | TN |
| Pt14_Primary | Luminal HER2+ |
| Pt14_Metastasis | Luminal HER2- |
| Pt32_Primary | Luminal HER2- |
| Pt32_Metastasis | Luminal HER2+ |
| Surrogate Molecular Types | Gene | Primary Tumors n (%) | Cutaneous Metastases n (%) | |
|---|---|---|---|---|
| TN n = 12 | Mutations | TP53 | 10 (83) | 11 (92) |
| PIK3CA | 2 (17) | 4 (33) | ||
| NF1 | 1 (8.3) | 1 (8.3) | ||
| TN n = 7 | CNVs | MYC | 1 (14.3) | 3 (42.9) |
| MDM4 | 0 | 1 (14.3) | ||
| FGFR1 | 1 (14.3) | 1 (14.3) | ||
| Luminal HER2- n = 16 | Mutations | TP53 | 1 (6) | 1 (6) |
| PIK3CA | 7 (39) | 7 (39) | ||
| NF1 | 1 (6.2) | 2 (12.5) | ||
| AKT1 | 2 (12.5) | 2 (12.5) | ||
| Luminal HER2- n = 9 | CNVs | MYC | 1 (11.1) | 1 (11.1) |
| MDM4 | 0 | 1 (11.1) | ||
| CCND1 | 1 (11.1) | 0 | ||
| HER2+ n = 5 | Mutations | TP53 | 2 (40) | 2 (40) |
| PIK3CA | 4 (80) | 4 (80) | ||
| NF1 | 1 (20) | 1 (20) | ||
| AKT1 | 1 (20) | 1 (20) | ||
| HER2+ n = 4 | CNVs | MYC | 1 (25) | 1 (25) |
| MDM4 | 0 | 1 (25) | ||
| FGFR1 | 1 (25) | 1 (25) | ||
| CCND1 | 0 | 1 (25) |
| Location | Surrogated Molecular Type | Gene | Cases with Additional Mutation in Cutaneous Metastasis n |
|---|---|---|---|
| Distant cutaneous metastasis n = 17 | TN | PIK3CA | 1 |
| RB1 | 1 | ||
| FGFR2 + MYC (amplification) | 1 | ||
| CTNNB1 | 1 | ||
| MDM4 (amplification) | 1 | ||
| Luminal HER2- | FGFR1 + NF1 | 1 | |
| BRCA2 + MKI67 + SF3B1 | 1 | ||
| CASP8 + ERBB2 | 1 | ||
| MDM4 (amplification) | 1 | ||
| HER2+ | KRAS + CCND1 (amplification) + MDM4 (amplification) | 1 | |
| BRCA1 + FGFR1 (polysomy) | 1 | ||
| Local cutaneous metastasis n = 15 | TN | TP53 + PIK3CA + COL1A1 | 1 |
| PIK3CA | 1 | ||
| MYC (amplification) | 1 | ||
| Luminal HER2- | ERBB2 (polysomy) | 1 | |
| HER2+ | MYC (amplification) | 1 |
| Authors | n | Luminal HER2− n (%) | HER2+ n (%) | TN n (%) | Unknown n (%) |
|---|---|---|---|---|---|
| Yates y col. [39] | 19 | 9 (47) | 2 (10) | 5 (26) | 3 (16) |
| Kong y col. [15] | 125 | 53 (42.4) | 43 (34) | 29 (23) | |
| Luna y col. [38] | 26 | 7 (27) | 7 (27) | 10 (39) | 2 (7) |
| González-Martínez y col. [37] | 58 | 29 (50) | 8 (14) | 15 (26) | 6 (10) |
| Present series | 33 | 16 (48.5) | 5 (15.2) | 12 (36.4) |
| Authors | Paired Cases of Cutaneous Metastases n | Cases with Additional Mutation | Additional Molecular Alterations in Cutaneous Metastases | |
|---|---|---|---|---|
| Schrijver and col. [41] | 8 * | 6 | 33 mutations (ATR, BRCA1, SMAD4, CDH1, ARID1A, ERBB2, IDH1, PIK3R1, RB1, etc.) and FGF3 amplification | |
| Yates and col. [39] | Cohort 1: 2 | 2 | 4 molecular alterations (FGFR1 amplification/TP53 structural variant, RB1 indel/TERC amplification) | |
| Cohort 2: 4 | 4 | 8 mutations (JAK2, NF1, TP53, AKT1, ARID1A, ARID1A, RB1) and 2 amplifications (MYC and FGFR1) | ||
| Paul and col. [42] | 1 | 1 | 54 mutations (PIK3CA, TP53, etc.) | |
| Present series | 33 | 12 TN | 6 mutations (TP53 + PIK3CA + COL1A1, PIK3CA, RB1, FGFR2, CTNNB1) and 3 amplifications (2 in MYC and one in MDM4) | |
| 17 | 16 RH + HER2- | 7 mutations (FGFR1 + NF1, BRCA2 + MKI67 + SF3B1, CASP8+ ERBB2) and 2 amplifications or polysomy (ERBB2 and MDM4) | ||
| 5 HER2+ | 2 mutations (KRAS, BRCA1), 4 amplifications or polysomy (CCND1, MYC, FGFR1 and MDM4) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Martínez, S.; Pizarro, D.; Pérez-Mies, B.; Caniego-Casas, T.; Rodríguez-Peralto, J.L.; Curigliano, G.; Cortés, A.; Gión, M.; Cortés, J.; Palacios, J. Differences in the Molecular Profile between Primary Breast Carcinomas and Their Cutaneous Metastases. Cancers 2022, 14, 1151. https://doi.org/10.3390/cancers14051151
González-Martínez S, Pizarro D, Pérez-Mies B, Caniego-Casas T, Rodríguez-Peralto JL, Curigliano G, Cortés A, Gión M, Cortés J, Palacios J. Differences in the Molecular Profile between Primary Breast Carcinomas and Their Cutaneous Metastases. Cancers. 2022; 14(5):1151. https://doi.org/10.3390/cancers14051151
Chicago/Turabian StyleGonzález-Martínez, Silvia, David Pizarro, Belén Pérez-Mies, Tamara Caniego-Casas, José Luis Rodríguez-Peralto, Giuseppe Curigliano, Alfonso Cortés, María Gión, Javier Cortés, and José Palacios. 2022. "Differences in the Molecular Profile between Primary Breast Carcinomas and Their Cutaneous Metastases" Cancers 14, no. 5: 1151. https://doi.org/10.3390/cancers14051151
APA StyleGonzález-Martínez, S., Pizarro, D., Pérez-Mies, B., Caniego-Casas, T., Rodríguez-Peralto, J. L., Curigliano, G., Cortés, A., Gión, M., Cortés, J., & Palacios, J. (2022). Differences in the Molecular Profile between Primary Breast Carcinomas and Their Cutaneous Metastases. Cancers, 14(5), 1151. https://doi.org/10.3390/cancers14051151






