Diagnostic Performance of a Fecal Immunochemical Test-Based Colorectal Cancer Screening Program According to Ambient Temperature and Humidity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Population
2.2. Screening Invitation Process
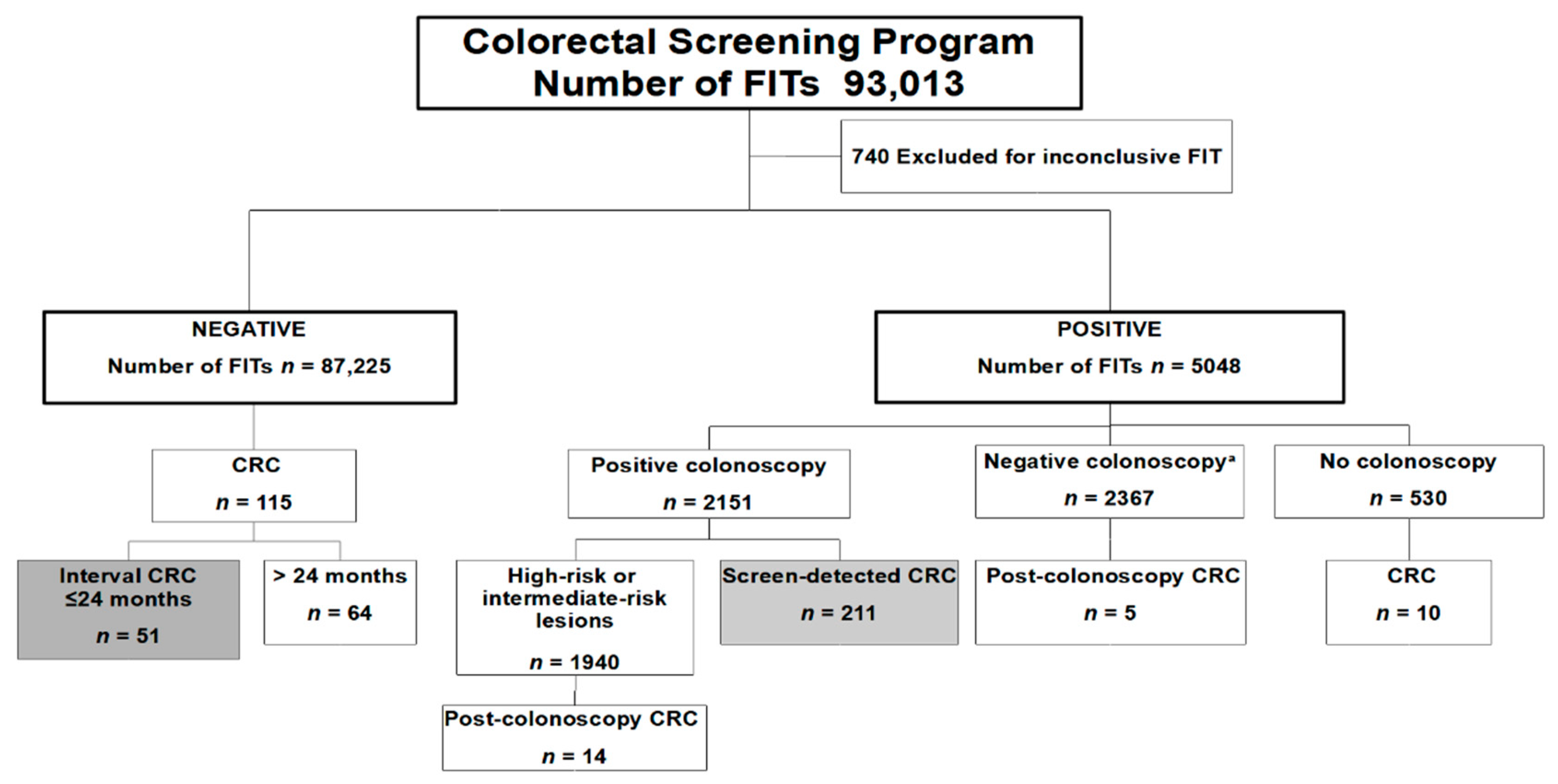
2.3. Study Population
2.4. Collection of Weather Information
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hewitson, P.; Glasziou, P.; Watson, E.; Towler, B.; Irwig, L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): An update. Am. J. Gastroenterol. 2008, 103, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Ponti, A.; Anttila, A.; Ronco, G.; Senore, C. Report on the Implementation of the Council Recommendation on Cancer Screening; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Alhomoud, S.; Blom, J.; Bretthauer, M.; Bulliard, J.L.; Corley, D.; Garcia Martinez, M.; Hoffmeister, M.; Hultcrantz, R.; Lansdorp-Vogelaar, I.; Nagtegaal, I.; et al. Colorectal Cancer Screening; IARC Working Group on the Evaluation of Cancer-Preventive Interventions: Lyon, France, 2019; Volume 17. [Google Scholar]
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 171. [Google Scholar] [CrossRef] [PubMed]
- Meklin, J.; SyrjAnen, K.; Eskelinen, M. Fecal Occult Blood Tests in Colorectal Cancer Screening: Systematic Review and Meta-analysis of Traditional and New-generation Fecal Immunochemical Tests. Anticancer Res. 2020, 40, 3591–3604. [Google Scholar] [CrossRef]
- Catomeris, P.; Baxter, N.N.; Boss, S.C.; Paszat, L.F.; Rabeneck, L.; Randell, E.; Serenity, M.L.; Sutradhar, R.; Tinmouth, J. Effect of Temperature and Time on Fecal Hemoglobin Stability in 5 Fecal Immunochemical Test Methods and One Guaiac Method. Arch. Pathol. Lab. Med. 2018, 142, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gies, A.; Cuk, K.; Schrotz-King, P.; Brenner, H. Direct comparison of ten quantitative fecal immunochemical tests for hemoglobin stability in colorectal cancer screening. Clin. Transl. Gastroenterol. 2018, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Vilkin, A.; Rozen, P.; Levi, Z.; Waked, A.; Maoz, E.; Birkenfeld, S.; Niv, Y. Performance characteristics and evaluation of an automated-developed and quantitative, immunochemical, fecal occult blood screening test. Am. J. Gastroenterol. 2005, 100, 2519–2525. [Google Scholar] [CrossRef]
- Symonds, E.L.; Osborne, J.M.; Cole, S.R.; Bampton, P.A.; Fraser, R.J.; Young, G.P. Factors affecting faecal immunochemical test positive rates: Demographic, pathological, behavioural and environmental variables. J. Med. Screen. 2015, 22, 187–193. [Google Scholar] [CrossRef]
- van Rossum, L.G.; van Rijn, A.F.; van Oijen, M.G.; Fockens, P.; Laheij, R.J.; Verbeek, A.L.; Jansen, J.B.; Dekker, E. False-negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int. J. Cancer 2009, 125, 746–750. [Google Scholar] [CrossRef]
- Grazzini, G.; Ventura, L.; Zappa, M.; Ciatto, S.; Confortini, M.; Rapi, S.; Rubeca, T.; Visioli, C.B.; Halloran, S.P. Influence of seasonal variations in ambient temperatures on performance of immunochemical faecal occult blood test for colorectal cancer screening: Observational study from the Florence district. Gut 2010, 59, 1511–1515. [Google Scholar] [CrossRef]
- van Roon, A.H.; Hol, L.; van Vuuren, A.J.; Francke, J.; Ouwendijk, M.; Heijens, A.; Nagtzaam, N.; Reijerink, J.C.; van der Togt, A.C.; van Ballegooijen, M.; et al. Are fecal immunochemical test characteristics influenced by sample return time? A population-based colorectal cancer screening trial. Am. J. Gastroenterol. 2012, 107, 99–107. [Google Scholar] [CrossRef]
- Cha, J.M.; Suh, M.; Kwak, M.S.; Sung, N.Y.; Choi, K.S.; Park, B.; Jun, J.K.; Hwang, S.H.; Lee, D.H.; Kim, B.C.; et al. Risk of Interval Cancer in Fecal Immunochemical Test Screening Significantly Higher During the Summer Months: Results from the National Cancer Screening Program in Korea. Am. J. Gastroenterol. 2018, 113, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Doubeni, C.A.; Jensen, C.D.; Fedewa, S.A.; Quinn, V.P.; Zauber, A.G.; Schottinger, J.E.; Corley, D.A.; Levin, T.R. Fecal Immunochemical Test (FIT) for Colon Cancer Screening: Variable Performance with Ambient Temperature. J. Am. Board Fam. Med. 2016, 29, 672–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedermaier, T.; Weigl, K.; Gies, A.; Hoffmeister, M.; Brenner, H. Accuracy of a fecal immunochemical test according to outside temperature and travel time. Clin. Epidemiol. 2018, 10, 1203–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chausserie, S.; Levillain, R.; Puvinel, J.; Ferrand, O.; Ruiz, A.; Raginel, T.; Lantieri, O.; Launoy, G.; Guittet, L. Seasonal variations do not affect the superiority of fecal immunochemical tests over guaiac tests for colorectal cancer screening. Int. J. Cancer 2015, 136, 1827–1834. [Google Scholar] [CrossRef]
- Park, C.H.; Jung, Y.S.; Kim, N.H.; Lee, M.Y.; Park, J.H.; Park, D.I.; Sohn, C.I. Impact of temperature and humidity on performance of the fecal immunochemical test for advanced colorectal neoplasia. Sci. Rep. 2019, 9, 9824. [Google Scholar] [CrossRef] [Green Version]
- Dancourt, V.; Hamza, S.; Manfredi, S.; Drouillard, A.; Bidan, J.M.; Faivre, J.; Lepage, C. Influence of sample return time and ambient temperature on the performance of an immunochemical faecal occult blood test with a new buffer for colorectal cancer screening. Eur. J. Cancer Prev. 2016, 25, 109–114. [Google Scholar] [CrossRef]
- Chen, H.; Werner, S.; Brenner, H. Fresh vs Frozen Samples and Ambient Temperature Have Little Effect on Detection of Colorectal Cancer or Adenomas by a Fecal Immunochemical Test in a Colorectal Cancer Screening Cohort in Germany. Clin. Gastroenterol. Hepatol. 2017, 15, 1547–1556. [Google Scholar] [CrossRef]
- Adam, B.W.; Chafin, D.L.; De Jesus, V.R. Stabilities of hemoglobins A and S in dried blood spots stored under controlled conditions. Clin. Biochem. 2013, 46, 1089–1092. [Google Scholar] [CrossRef] [Green Version]
- Bretagne, J.F.; Carlo, A.; Piette, C.; Rousseau, C.; Cosson, M.; Lievre, A. Significant decrease in interval colorectal cancer incidence after implementing immunochemical testing in a multiple-round guaiac-based screening program. Br. J. Cancer 2021, 125, 1494–1502. [Google Scholar] [CrossRef]
- Robertson, D.J.; Lee, J.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Lieberman, D.; Levin, T.R.; Rex, D.K. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 152, 1217–1237. [Google Scholar] [CrossRef] [Green Version]
- Peris, M.; Espinas, J.A.; Munoz, L.; Navarro, M.; Binefa, G.; Borras, J.M.; Catalan Colorectal Cancer Screening Pilot Programme Group. Lessons learnt from a population-based pilot program for colorectal cancer screening in Catalonia (Spain). J. Med. Screen. 2007, 14, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Binefa, G.; Garcia, M.; Mila, N.; Fernandez, E.; Rodriguez-Moranta, F.; Gonzalo, N.; Benito, L.; Clopes, A.; Guardiola, J.; Moreno, V. Colorectal Cancer Screening Programme in Spain: Results of Key Performance Indicators After Five Rounds (2000–2012). Sci. Rep. 2016, 6, 19532. [Google Scholar] [CrossRef] [PubMed]
- Atkin, W.S.; Valori, R.; Kuipers, E.J.; Hoff, G.; Senore, C.; Segnan, N.; Jover, R.; Schmiegel, W.; Lambert, R.; Pox, C.; et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition—Colonoscopic surveillance following adenoma removal. Endoscopy 2012, 44 (Suppl. 3), SE151–SE163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanduleanu, S.; le Clercq, C.M.; Dekker, E.; Meijer, G.A.; Rabeneck, L.; Rutter, M.D.; Valori, R.; Young, G.P.; Schoen, R.E.; Expert Working Group on Right-sided lesions and interval cancers; et al. Definition and taxonomy of interval colorectal cancers: A proposal for standardising nomenclature. Gut 2015, 64, 1257–1267. [Google Scholar] [CrossRef]
- Rutter, M.D.; Beintaris, I.; Valori, R.; Chiu, H.M.; Corley, D.A.; Cuatrecasas, M.; Dekker, E.; Forsberg, A.; Gore-Booth, J.; Haug, U.; et al. World Endoscopy Organization Consensus Statements on Post-Colonoscopy and Post-Imaging Colorectal Cancer. Gastroenterology 2018, 155, 909–925. [Google Scholar] [CrossRef] [Green Version]
- Colls, C.; Mias, M.; Garcia-Altes, A. A deprivation index to reform the financing model of primary care in Catalonia (Spain). Gac. Sanit. 2020, 34, 44–50. [Google Scholar] [CrossRef]
- Bernal-Delgado, E.E.; Martos, C.; Martinez, N.; Chirlaque, M.D.; Marquez, M.; Navarro, C.; Hernando, L.; Palomar, J.; Izarzugaza, I.; Larranaga, N.; et al. Is hospital discharge administrative data an appropriate source of information for cancer registries purposes? Some insights from four Spanish registries. BMC Health Serv. Res. 2010, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.M.; Lee, J.I.; Joo, K.R.; Shin, H.P.; Park, J.J.; Jeun, J.W.; Lim, J.U.; Hwang, S.H. Performance of the fecal immunochemical test is not decreased by high ambient temperature in the rapid return system. Dig. Dis. Sci. 2012, 57, 2178–2183. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.P.; Zhai, A.; Pirani, S.L.; Connors, C.; Péan, S.; Berger, N.; Caud, Y.; Chen, L.; Goldfarb, M.I.; Gomis, M.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021; Available online: https://www.ipcc.ch/sr15/chapter/spm/ (accessed on 1 December 2021).
- Ibanez-Sanz, G.; Sanz-Pamplona, R.; Garcia, M.; MSIC-SC Research Group. Future Prospects of Colorectal Cancer Screening: Characterizing Interval Cancers. Cancers 2021, 13, 1328. [Google Scholar] [CrossRef]


| Variation | Negative | Positive |
|---|---|---|
| N = 87,225 | N = 5048 | |
| Sex | ||
| Female | 48,789 (55.9%) | 2185 (43.3%) |
| Male | 38,436 (44.1%) | 2863 (56.7%) |
| Age (years) | ||
| 50–59 | 42,485 (48.7%) | 2095 (41.5%) |
| 60–69 | 44,740 (51.3%) | 2953 (58.5%) |
| Screening episode | ||
| Initial | 50,554 (58.0%) | 3306 (65.5%) |
| Successive | 36,671 (42.0%) | 1742 (34.5%) |
| Socioeconomic Score | ||
| 0–39 (least deprived) | 6671 (7.65%) | 378 (7.49%) |
| 39–51 | 33,665 (38.6%) | 1913 (37.9%) |
| 52–100 (most deprived) | 46,889 (53.8%) | 2757 (54.6%) |
| Quarter year of FIT performance | ||
| 1st quarter (January–March) | 27,278 (31.3%) | 1675 (33.2%) |
| 2nd quarter (April–June) | 18,091 (20.7%) | 1007 (19.9%) |
| 3rd quarter (July–September) | 8376 (9.60%) | 449 (8.89%) |
| 4th quarter (October–December) | 33,480 (38.4%) | 1917 (38.0%) |
| Maximum ambient temperature | ||
| ≤24 °C | 60,375 (69.2%) | 3589 (71.1%) |
| >24 °C | 26,850 (30.8%) | 1459 (28.9%) |
| Maximum ambient humidity | ||
| ≤89% | 76,933 (88.2%) | 4446 (88.1%) |
| >89% | 10,292 (11.8%) | 602 (11.9%) |
| Quarter Year of FIT Performance | Mean (95% CI) Hb | Number of FIT Results | Min Hb | Max Hb | p25 | p50 | p75 |
|---|---|---|---|---|---|---|---|
| 1st quarter (January–March) | 30.5 | 28,953 | 0 | 1000 | 0 | 0 | 10 |
| 2nd quarter (April–June) | 28.9 | 19,098 | 0 | 1000 | 0 | 0 | 5 |
| 3rd quarter (July–September) | 27.2 | 8825 | 0 | 1000 | 0 | 0 | 4 |
| 4th quarter (October–December) | 28.8 | 35,397 | 0 | 1000 | 0 | 0 | 7 |
| Variation | Number of FITs | OR 2 | 95% CI | p-Value |
|---|---|---|---|---|
| N = 5048 | ||||
| Sex | ||||
| Female | 2185 (43.3%) | 1.00 | <0.001 | |
| Male | 2863 (56.7%) | 1.67 | 1.58–1.77 | |
| Age 1 (years) | 60.7 (5.8) | 1.03 | 1.03–1.04 | <0.001 |
| Screening episode | ||||
| Initial | 3306 (65.5%) | 1.00 | <0.001 | |
| Successive | 1742 (34.5%) | 0.68 | 0.64–0.72 | |
| Socioeconomic score 1 | 53.5 (10.9) | 1.01 | 1.00–1.01 | <0.001 |
| Maximum ambient temperature | ||||
| ≤24 °C | 3589 (71.1%) | 1.00 | <0.001 | |
| >24 | 1459 (28.9%) | 0.88 | 0.83–0.94 | |
| Maximum ambient humidity | ||||
| ≤89% | 4446 (88.1%) | 1.00 | 0.06 | |
| >89% | 602 (11.9%) | 1.09 | 1.00–1.19 |
| Variation | No Advanced Neoplasia | Advanced Neoplasia | OR 2 | 95% CI | p-Value |
|---|---|---|---|---|---|
| N = 2367 | N = 2151 | ||||
| Sex | |||||
| Female | 1278 (54.0%) | 678 (31.5%) | 1.00 | ||
| Male | 1089 (46.0%) | 1473 (68.5%) | 2.53 | 2.24–2.86 | <0.001 |
| Age 1 (mean (SD)) (years) | 60.6 (5.8) | 60.8 (5.8) | 1.01 | 1.00–1.02 | 0.01 |
| Screening episode | |||||
| Initial | 1422 (60.1%) | 1515 (70.4%) | 1.00 | ||
| Successive | 945 (39.9%) | 636 (29.6%) | 0.63 | 0.56–0.72 | <0.001 |
| Socioeconomic score 1 (mean (SD)) | 53.3 (10.8) | 53.5 (11.1) | 1.00 | 1.00–1.01 | 0.54 |
| Maximum ambient temperature | |||||
| ≤24 °C | 1706 (72.1%) | 1513 (70.3%) | 1.00 | ||
| >24 °C | 661 (27.9%) | 638 (29.7%) | 1.08 | 0.94–1.25 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez-Sanz, G.; Milà, N.; Vives, N.; Vidal, C.; Binefa, G.; Rocamora, J.; Atencia, C.; Moreno, V.; Sanz-Pamplona, R.; Garcia, M.; et al. Diagnostic Performance of a Fecal Immunochemical Test-Based Colorectal Cancer Screening Program According to Ambient Temperature and Humidity. Cancers 2022, 14, 1153. https://doi.org/10.3390/cancers14051153
Ibáñez-Sanz G, Milà N, Vives N, Vidal C, Binefa G, Rocamora J, Atencia C, Moreno V, Sanz-Pamplona R, Garcia M, et al. Diagnostic Performance of a Fecal Immunochemical Test-Based Colorectal Cancer Screening Program According to Ambient Temperature and Humidity. Cancers. 2022; 14(5):1153. https://doi.org/10.3390/cancers14051153
Chicago/Turabian StyleIbáñez-Sanz, Gemma, Núria Milà, Núria Vives, Carmen Vidal, Gemma Binefa, Judith Rocamora, Carmen Atencia, Víctor Moreno, Rebeca Sanz-Pamplona, Montse Garcia, and et al. 2022. "Diagnostic Performance of a Fecal Immunochemical Test-Based Colorectal Cancer Screening Program According to Ambient Temperature and Humidity" Cancers 14, no. 5: 1153. https://doi.org/10.3390/cancers14051153
APA StyleIbáñez-Sanz, G., Milà, N., Vives, N., Vidal, C., Binefa, G., Rocamora, J., Atencia, C., Moreno, V., Sanz-Pamplona, R., Garcia, M., & on behalf of the MSIC-SC Research Group. (2022). Diagnostic Performance of a Fecal Immunochemical Test-Based Colorectal Cancer Screening Program According to Ambient Temperature and Humidity. Cancers, 14(5), 1153. https://doi.org/10.3390/cancers14051153






