The Role of Neuroaxis Irradiation in the Treatment of Intraspinal Ewing Sarcoma: A Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Data Extraction and Study Quality Assessment
2.3. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Patients
3.3. Treatment Modalities
3.4. Analysis of Efficacy
| Author | Sex/ Age at Diagnosis | Localization | Multifocal Tumor/ Leptomeningeal Spread | Radiotherapy | Surgery | Chemotherapy | Clinical Response | Radiological Response | Survival/Months | |
|---|---|---|---|---|---|---|---|---|---|---|
| CSI Cumulative/ Fraction Dose (Gy) | Boost * Cumulative/ Fraction Dose (Gy) | |||||||||
| Own case | F/27 | intradural extramedullary | No/No | 36/1.5 | 18/1.8 | n.p. | MTX/ VCR_IFO_ETO/ HD-TREO_MEL | CR | CR | 38/alive |
| Huguenard et al., 2021 [14] | M/34 | intradural extramedullary | Yes/Yes | 30.6/1.8 | 12.6/2.5 | STR spinal lesion | CPM_CyT_VCR/ HD-IFO/ETP | PD | PD | 6 |
| Weil et al., 2001 [21] | M/21 | intramedullary intracranially | Yes/No | 37.8/1.8 | 7.2/1.8 | STR cranial & spinal lesion | VCR_DXR_CPM/ ETP_ IFO | CR | CR | 30/alive |
| Tan et al., 2019 [22] | F/34 | intradural extramedullary | Yes/No | 36/2 | n.p. | STR | n.p. | PR, then PD | PR, then PD | 11 |
| Izubuchi et al., 2020 [15] | F/35 | intradural extramedullary, | Yes/Yes | 45/1.8 | n.p. | STR | VCR_DXR_CPM/ IFO_ETP | PR, then PD | PR, then PD | 16 |
| Bostelmann et al., 2016 [23] | M/29 | extradural | No/No | 36/n.a. | 14.4/1.8 | GTR | VCR_IFO_DXR/ ETP_TOPO_CPM | PR | CR | 18/alive |
| Chihak et al., 2016 [24] | M/25 | intradural extramedullary | No/No | 39.6/n.a. | 14.4/n.a. | STR | IFO_ETP_VCR/ DXR_CPM | CR | CR | 20/alive |
| Chihak et al. 2016 [24] | M/34 | intradural extramedullary | Yes/No | 30/n.a. | 29.4/n.a. | STR | VCR_DXR_CPM/ IFO_ETP | CR | CR | 3/alive |
| Khwaja et al. 2019 [25] | F/44 | intramedullary | No/No | 30.6/1.8 | 12.6/1.8 | STR | CIS_CCNU_IFO/ CPM_ETP | CR | CR | 96/alive |
| Isotalo et al., 2000 [26] | M/52 | intradural extramedullary | No/No | 38.5/1.75 | 17.5/1.75 | STR | n.a. | PR | CR | 12/alive |
| Johnson et al., 2020 [27] | M/42 | intradural extramedullary, | No/Yes | 37.8/1.8 | 16.2/1.8 | STR | VCR_DXR_CPM/IFO_VCR/ TMZ_IRT | PR | PR | 1/alive |
| Lu et al., 2019 [12] | M/25 | intradural extramedullary | No/No | 36/1.8 | 14.4/1.8 | STR | IFO_VCR/ CPM_CyT | CR | CR | 62/alive |
| Benesch et al., 2011 [28] | F/14 | intramedullary | No/No | 40.5/1.5 | 14.4/1.8 | STR | CPM_VCR_ETO_CPM HD-MTX/ intrathecal MTX | CR | CR | 44/alive |
| Benesch et al., 2011 [28] | M/2 | intramedullary | Yes/Yes | 35.2/1.6 | 9.6/1.6 | STR | CAR_ETP/ CIS_ETP_VCR/ HD-MTX | PR | CR | 40/alive |
| Takahashi et al., 2017 [29] | F/50 | intramedullary | No/No | 39.6/1.8 | 14.4/1.8 | STR | CAR_ETO | PR | PR | n/a |
| Albrecht et al., 2003 [30] | F/29 | intramedullary | Yes/No | 35.2/1.6 | 18/1.8 | n.p. | ADR_ETO_CPM | PR | PR | 17 |
| Yavuz et al., 2002 [31] | F/18 | intradural extramedullary | No/No | 34/n.a. | 20/n.a. | STR | VCR_CPM_DXR_IFO_ ETO | CR | CR | 25/alive |
| Izycka–Swieszewska et al., 2001 [32] | F/13 | epidural | Yes/No | 33/n.a. | n.p. | n.p. | CAR_EPI_VPS_VCR_IFO_ACT/ TRO_IDA_VPS | CR | CR | 18/alive |
| Kim et al., 2004 [33] | M/17 | intramedullary | No/No | 50.4/1.8 | n.p. | STR | n.p. | n.a. | n.a. | 4/alive |
| Nutman et al., 2007 [34] | F/19 | intradural extramedullary | No/No | 36/1.5 | 9/1.8 | GTR | CPM_VCR/ CAR_THI_ETO | CR | CR | 24/alive |
| Papadatos et al., 1998 [20] | F/23 | intradural extramedullary | No/No | 36/1.5 | 9/1.8 | STR | CPM_CIS_ETO | PR | CR | 12/alive |
| Weber et al., 2004 [35] | M/26 | epidural | No/No | 36/1.8 | 21/1.6 | GTR | VCR_ACT_CPM | CR | CR | 9/alive |
| Gollard et al., 2011 [36] | F/21 | intramedullary | No/No | 36/n.a. | 18/n.a. | n.p. | VCR_CIS_CPM | CR | CR | 120/alive |
| Alexander et al., 2010 [37] | M/45 | intradural extramedullary | No/No | 36/1.8 | 18/1.8 | STR | n.p. | PR | n.a. | 13/alive |
3.5. Comparison with Focal Irradiation
| Characteristic | Focal Radiotherapy 55 Patients | Craniospinal Irradiation 24 Patients | p |
|---|---|---|---|
| Age, years, median (range) | 22 (1–70) | 25.5 (2–52) | 0.09 * |
| Sex | 0.33 # | ||
| Male, n (%) | 34 (61.8) | 12 (50.0) | |
| Female, n (%) | 21 (38.2) | 12 (50.0) | |
| Tumor localization | <0.001 # | ||
| Intradural, n (%) | 21 (38.2) | 21 (87.5) | |
| Epidural/extradural, n (%) | 34 (61.8) | 3 (12.5) | |
| Multiple lesions or leptomeningeal spread at time of diagnosis, n (%) | 3 (5.5) | 8 (33.3) | 0.001 # |
| Localization of the tumor(s) at time of diagnosis | 0.21 # | ||
| Cervical spine | 10 (18.2) | 4 (16.7) | |
| Thoracic spine | 9 (16.4) | 4 (16.7) | |
| Lumbosacral spine | 18 (32.7) | 3 (12.5) | |
| Multiple segments involved | 18 (32.7) | 13 (54.2) | |
| Follow-up, months, median (range) | 13 (2–120) | 17.5 (0–120) | 0.72 * |
| Death during follow-up, n (%) | 12 (21.8) | 4 (16.7) | 0.60 # |
| Development of neuroaxis metastases after treatment (only patients with singular tumor at time of diagnosis considered), n (%) | 10 (19.2) | 1 (6.3) | 0.22 # |
| Radiation dose to the tumor region (Gy) | 50.0 (30.0–65.0) | 51.8 (33.0–59.4) | 0.08 # |
| Dose per fraction (Gy) x | 2 (1.75–3) | 1.8 (1.5–2) | <0.001 * |
3.6. Prognostic Parameters in the CSI Group
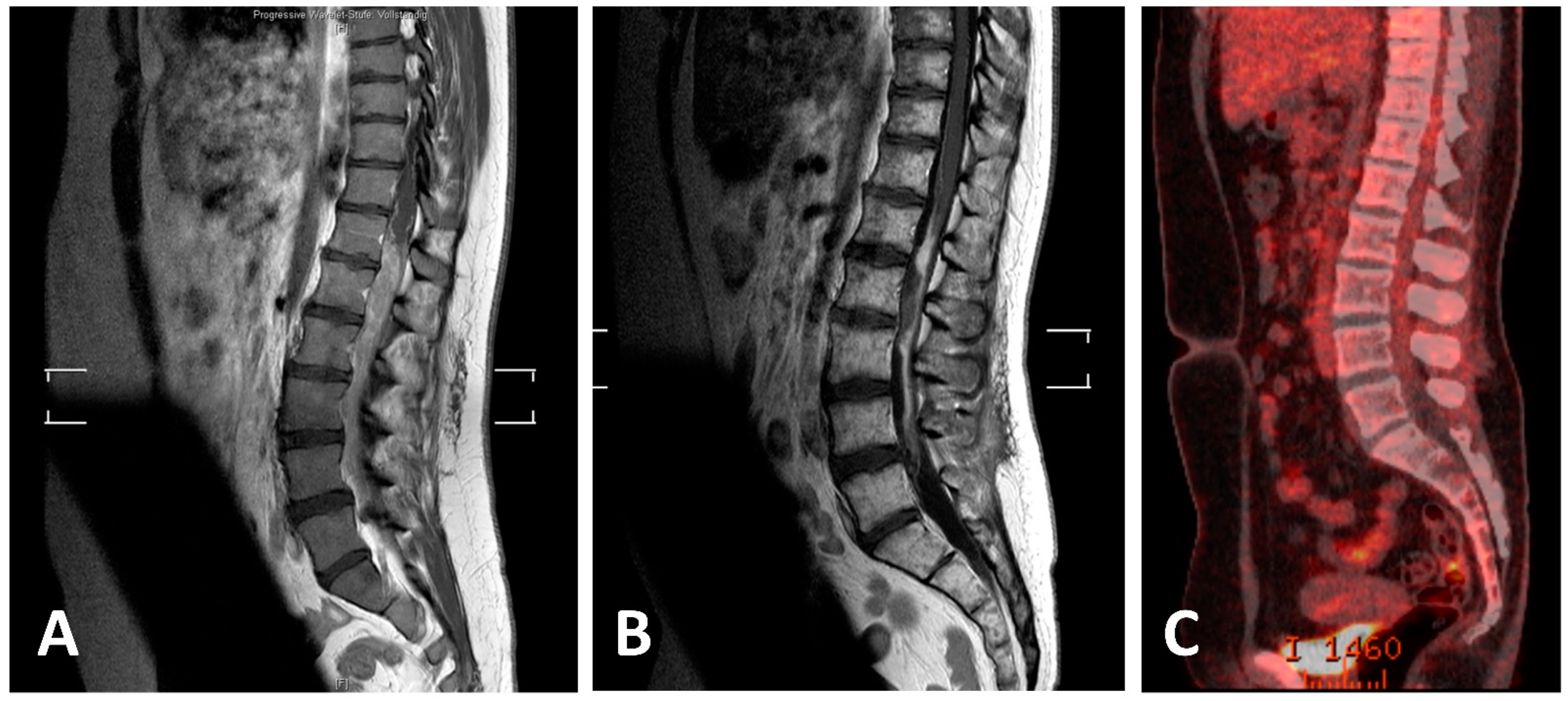
3.7. Own Case
4. Discussion
4.1. Therapeutic Regimens
4.2. CSI for Local and Distant Control
4.3. CSI for Survival
4.4. Compatibility of CSI with Systemic Therapy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludwig, J.A. Ewing sarcoma: Historical perspectives, current state-of-the-art, and opportunities for targeted therapy in the future. Curr. Opin. Oncol. 2008, 20, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Tefft, M.; Vawter, G.F.; Mitus, A. Paravertebral “round cell” tumors in children. Radiology 1969, 92, 1501–1509. [Google Scholar] [CrossRef]
- Uesaka, T.; Amano, T.; Inamura, T.; Ikezaki, K.; Inoha, S.; Takamatsu, M.; Iwaki, T.; Fukui, M. Intradural, extramedullary spinal Ewing’s sarcoma in childhood. J. Clin. Neurosci. 2003, 10, 122–125. [Google Scholar] [CrossRef]
- Bouffet, E.; Marec-Berard, P.; Thiesse, P.; Carrie, C.; Risk, T.; Jouvet, A.; Brunat-Mentigny, M.; Mottolese, C. Spinal cord compression by secondary epi- and intradural metastases in childhood. Childs. Nerv. Syst. 1997, 13, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Karikari, I.O.; Mehta, A.I.; Nimjee, S.; Hodges, T.R.; Tibaleka, J.; Montgomery, C.; Simpson, L.; Cummings, T.J.; Bagley, C.A. Primary intradural extraosseous ewing sarcoma of the spine: Case report and literature review. Neurosurgery 2011, 69, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Pancucci, G.; Simal-Julian, J.A.; Plaza-Ramirez, E.; García-Marcos, R.; Mayordomo-Aranda, E.; Botella-Asunción, C. Primary extraosseous intradural spinal Ewing’s sarcoma: Report of two cases. Acta Neurochir. 2013, 155, 1229–1234. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Chiang, Y.H.; Tsai, W.C.; Sheu, L.F.; Liu, M.Y. Primary spinal epidural Ewing sarcoma: A case report and review of the literature. Turk. J. Pediatr. 2008, 50, 282–286. [Google Scholar]
- Saeedinia, S.; Nouri, M.; Alimohammadi, M.; Moradi, H.; Amirjamshidi, A. Primary spinal extradural Ewing’s sarcoma (primitive neuroectodermal tumor): Report of a case and meta-analysis of the reported cases in the literature. Surg. Neurol. Int. 2012, 3, 55. [Google Scholar]
- Kogawa, M.; Asazuma, T.; Iso, K.; Koike, Y.; Domoto, H.; Aida, S.; Fujikawa, K. Primary cervical spinal epidural Extra-osseous Ewing’s sarcoma. Acta Neurochir. 2004, 146, 1051–1053. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Keole, S.R.; Shahlaee, A.H.; Morris, C.G.; Gibbs, C.P.; Scarborough, M.T.; Pincus, D.W.; Marcus, R.B. Spinal and Paraspinal Ewing Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1463–1471. [Google Scholar] [CrossRef]
- Harimaya, K.; Oda, Y.; Matsuda, S.; Tanaka, K.; Chuman, H.; Iwamoto, Y. Primitive neuroectodermal tumor and extraskeletal Ewing sarcoma arising primarily around the spinal column: Report of four cases and a review of the literature. Spine 2003, 28, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.M.; Goyal, A.; Alvi, M.A.; Kerezoudis, P.; Haddock, M.G.; Bydon, M. Primary intradural Ewing’s sarcoma of the spine: A systematic review of the literature. Clin. Neurol. Neurosurg. 2019, 177, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xu, T.; Chen, J.; Hu, G.; Lu, Y. Intraspinal Ewing’s sarcoma/primitive neuroectodermal tumors. J. Clin. Neurosci. 2011, 18, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Huguenard, A.L.; Li, Y.D.; Sharifai, N.; Perkins, S.M.; Dahiya, S.; Chicoine, M.R. Multifocal primary central nervous system Ewing sarcoma presenting with intracranial hemorrhage and leptomeningeal dissemination: Illustrative case. J. Neurosurg. Case Lessons 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Izubuchi, Y.; Nakajima, H.; Honjoh, K.; Imamura, Y.; Nojima, T.; Matsumine, A. Primary intradural extramedullary Ewing Sarcoma: A case report and literature review. Oncol. Lett. 2020, 20, 2347–2355. [Google Scholar] [CrossRef]
- Chhabda, S.; Carney, O.; D’Arco, F.; Jacques, T.S.; Mankad, K. The 2016 World Health Organization classification of tumours of the Central Nervous System: What the paediatric neuroradiologist needs to know. Quant. Imaging Med. Surg. 2016, 6, 486–489. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Onwuegbuzie, A.J.; Frels, R. Resources to help you teach online Seven Steps to a Comprehensive Literature Review; SAGE Publications Ltd.: Thousands Oaks, CA, USA, 2021; ISBN 9781446248928. [Google Scholar]
- Williams, J. A Comprehensive Review of Seven Steps to a Comprehensive Literature Review. Qual. Rep. 2018, 23, 345–349. [Google Scholar] [CrossRef]
- Papadatos, D.; Albrecht, S.; Mohr, G.; Del Carpio-O’Donovan, R. Exophytic primitive neuroectodermal tumor of the spinal cord. Am. J. Neuroradiol. 1998, 19, 787–789. [Google Scholar]
- Weil, R.J.; Zhuang, Z.; Pack, S.; Kumar, S.; Helman, L.; Fuller, B.G.; Mackall, C.L.; Oldfield, E.H. Intramedullary Ewing sarcoma of the spinal cord: Consequences of molecular diagnostics. Case report. J. Neurosurg. 2001, 95, 270–275. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, D.; Phung, T.B.; Lai, L.T. Primary intradural extramedullary Ewing sarcoma of the cervical spine: A case report and review of the literature. J. Clin. Neurosci. 2019, 66, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Bostelmann, R.; Leimert, M.; Steiger, H.J.; Gierga, K.; Petridis, A.K. The Importance of Surgery as Part of Multimodal Therapy in Rapid Progressive Primary Extraosseous Ewing Sarcoma of the Cervical Intra- and Epidural Space. Clin. Pract. 2016, 6, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Chihak, M.A.; Ahmed, S.K.; Lachance, D.H.; Nageswara Rao, A.A.; Laack, N.N. Patterns of failure and optimal radiotherapy target volumes in primary intradural extramedullary Ewing sarcoma. Acta Oncol. 2016, 55, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, R.; Mantilla, E.; Fink, K.; Pan, E. Adult primary peripheral PNET/Ewing’s sarcoma of the cervical and thoracic spine. Anticancer Res. 2019, 39, 4463–4465. [Google Scholar] [CrossRef] [PubMed]
- Isotalo, P.A.; Agbi, C.; Davidson, B.; Girard, A.; Verma, S.; Robertson, S.J. Primary primitive neuroectodermal tumor of the cauda equina. Hum. Pathol. 2000, 31, 999–1001. [Google Scholar] [CrossRef]
- Johnson, M.D.; Korones, D.N.; L-N, H.; Walter, K.; Hussain, A. Ewing’s sarcoma presenting as a cervical intradural extramedullary tumor in a 42 year old: Report of a case. Interdiscip. Neurosurg. Adv. Tech. Case Manag. 2020, 20, 100634. [Google Scholar] [CrossRef]
- Benesch, M.; Sperl, D.; Von Bueren, A.O.; Schmid, I.; Von Hoff, K.; Warmuth-Metz, M.; Ferrari, R.; Lassay, L.; Kortmann, R.D.; Pietsch, T.; et al. Primary central nervous system primitive neuroectodermal tumors (CNS-PNETs) of the spinal cord in children: Four cases from the German HIT database with a critical review of the literature. J. Neurooncol. 2011, 104, 279–286. [Google Scholar] [CrossRef]
- Takahashi, S.; Takayanagi, S.; Ikemura, M.; Nejo, T.; Nomura, M.; Yanagisawa, S.; Tanaka, S.; Mukasa, A.; Saito, N. Rare-02. Primary Spinal Cord Ewing’s Sarcoma in 50-year old woman: A case report. Neuro. Oncol. 2017, 19, vi212. [Google Scholar] [CrossRef][Green Version]
- Albrecht, C.F.; Weiss, E.; Schulz-Schaeffer, W.J.; Albrecht, T.; Fauser, S.; Wickboldt, J.; Hess, C.F. Primary intraspinal primitive neuroectodermal tumor: Report of two cases and review of the literature. J. Neurooncol. 2003, 61, 113–120. [Google Scholar] [CrossRef]
- Yavuz, A.A.; Yaris, N.; Yavuz, M.N.; Sari, A.; Reis, A.K.; Aydin, F. Primary intraspinal primitive neuroectodermal tumor: Case report of a tumor arising from the sacral spinal nerve root and review of the literature. Am. J. Clin. Oncol. Cancer Clin. Trials 2002, 25, 135–139. [Google Scholar] [CrossRef]
- Izycka-Swieszewska, E.; Stefanowicz, J.; Debiec-Rychter, M.; Rzepko, R.; Borowska-Lehman, J. Peripheral primitive neuroectodermal tumor within the spinal epidural space. Neuropathology 2001, 21, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Jin, B.H.; Kim, T.S.; Cho, Y.E. Primary intraspinal primitive neuroectodermal tumor at conus medullaris. Yonsei Med. J. 2004, 45, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Nutman, A.; Postovsky, S.; Zaidman, I.; Elhasid, R.; Vlodavsky, E.; Kreiss, Y.; Ben Arush, M.W. Primary intraspinal primitive neuroectodermal tumor treated with autologous stem cell transplantation: Case report and review of the literature. Pediatr. Hematol. Oncol. 2007, 24, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Rutz, H.P.; Lomax, A.J.; Schneider, U.; Lombriser, N.; Zenhausern, R.; Goitein, G. First spinal axis segment irradiation with spot-scanning proton beam delivered in the treatment of a lumbar primitive neuroectodermal tumour. Clin. Oncol. 2004, 16, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Gollard, R.P.; Rosen, L.; Anson, J.; Mason, J.; Khoury, J. Intramedullary PNET of the spine: Long-term survival after combined modality therapy and subsequent relapse. J. Pediatr. Hematol. Oncol. 2011, 33, 107–112. [Google Scholar] [CrossRef]
- Alexander, H.S.; Koleda, C.; Hunn, M.K. Peripheral Primitive Neuroectodermal Tumour (pPNET) in the cervical spine. J. Clin. Neurosci. 2010, 17, 259–261. [Google Scholar] [CrossRef]
- Kaspers, G.J.; Kamphorst, W.; van de Graaff, M.; van Alphen, H.A.; Veerman, A.J. Primary spinal epidural extraosseous Ewing’s sarcoma. Cancer 1991, 68, 648–654. [Google Scholar] [CrossRef]
- Kumar, R.; Reddy, S.J.; Wani, A.A.; Pal, L. Primary spinal primitive neuroectodermal tumor: Case series and review of the literature. Pediatr. Neurosurg. 2006, 43, 1–6. [Google Scholar] [CrossRef]
- Vogin, G.; Biston, M.C.; Marchesi, V.; Amessis, M.; De Marzi, L.; Lacroix, F.; Leroy, A.; Gassa, F.; Zefkili, S.; Helfre, S. Sarcomes d’Ewing localisés du rachis chez l’enfant: Étude préliminaire d’escalade de dose comparant les techniques innovantes. Cancer/Radiotherapie 2013, 17, 26–33. [Google Scholar] [CrossRef]
- Triarico, S.; Maurizi, P.; Mastrangelo, S.; Attinà, G.; Capozza, M.A.; Ruggiero, A. Improving the brain delivery of chemotherapeutic drugs in childhood brain tumors. Cancers 2019, 11, 824. [Google Scholar] [CrossRef]
- Jacus, M.O.; Daryani, V.M.; Harstead, K.E.; Patel, Y.T.; Stacy, L.; Stewart, C.F. Pharmacokinetic Properties of Anticancer Agents for the Treatment of Central Nervous System Tumors: Update of the Literature. Clin. Pharmacokinet. 2016, 55, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Haresh, K.P.; Chinikkatti, S.K.; Prabhakar, R.; Rishi, A.; Rath, G.K.; Sharma, D.N.; Julka, P.K. A rare case of intradural extramedullary Ewing’s sarcoma with skip metastasis in the spine. Spinal Cord 2008, 46, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Rud, N.P.; Reiman, H.M.; Pritchard, D.J.; Frassica, F.J.; Smithson, W.A. Extraosseous Ewing’s sarcoma. A study of 42 cases. Cancer 1989, 64, 1548–1553. [Google Scholar] [CrossRef]
- Barbieri, E.; Chiaulon, G.; Bunkeila, F.; Putti, C.; Frezza, G.; Neri, S.; Boriani, S.; Campanacci, M.; Babini, L. Radiotherapy in vertebral tumors. Indications and limits: A report on 28 cases of Ewing’s sarcoma of the spine. Chir. Organi Mov. 1998, 83, 105–111. [Google Scholar]



| Characteristic | Hazard Ratio | 95% Confidence Interval | p | |
|---|---|---|---|---|
| Age (years) | 1.07 | 0.98–1.17 | 0.11 | |
| CSI dose (Gy) | 1.05 | 0.77–1.43 | 0.77 | |
| Boost dose (Gy) | 1.06 | 0.73–1.54 | 0.74 | |
| Characteristic | p-Value in Log-Rank Analysis | Directionality | ||
| Sex | 0.54 | None | ||
| Surgery (STR vs. GTR vs. biopsy only) | 0.71 | None | ||
| Application of boost vs. no boost | 0.03 | Boost application favorable | ||
| Radiologic response | <0.001 | Complete response favorable vs. rest | ||
| Localized vs. multifocal | 0.005 | Unifocal favorable vs. multifocal | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troschel, F.M.; Kröger, K.; Siats, J.J.; Rahbar, K.; Eich, H.T.; Scobioala, S. The Role of Neuroaxis Irradiation in the Treatment of Intraspinal Ewing Sarcoma: A Review and Meta-Analysis. Cancers 2022, 14, 1209. https://doi.org/10.3390/cancers14051209
Troschel FM, Kröger K, Siats JJ, Rahbar K, Eich HT, Scobioala S. The Role of Neuroaxis Irradiation in the Treatment of Intraspinal Ewing Sarcoma: A Review and Meta-Analysis. Cancers. 2022; 14(5):1209. https://doi.org/10.3390/cancers14051209
Chicago/Turabian StyleTroschel, Fabian M., Kai Kröger, Jan J. Siats, Kambiz Rahbar, Hans Theodor Eich, and Sergiu Scobioala. 2022. "The Role of Neuroaxis Irradiation in the Treatment of Intraspinal Ewing Sarcoma: A Review and Meta-Analysis" Cancers 14, no. 5: 1209. https://doi.org/10.3390/cancers14051209
APA StyleTroschel, F. M., Kröger, K., Siats, J. J., Rahbar, K., Eich, H. T., & Scobioala, S. (2022). The Role of Neuroaxis Irradiation in the Treatment of Intraspinal Ewing Sarcoma: A Review and Meta-Analysis. Cancers, 14(5), 1209. https://doi.org/10.3390/cancers14051209








