Immune Checkpoint Inhibitors in Advanced Prostate Cancer: Current Data and Future Perspectives
Abstract
:Simple Summary
Abstract
1. Introduction
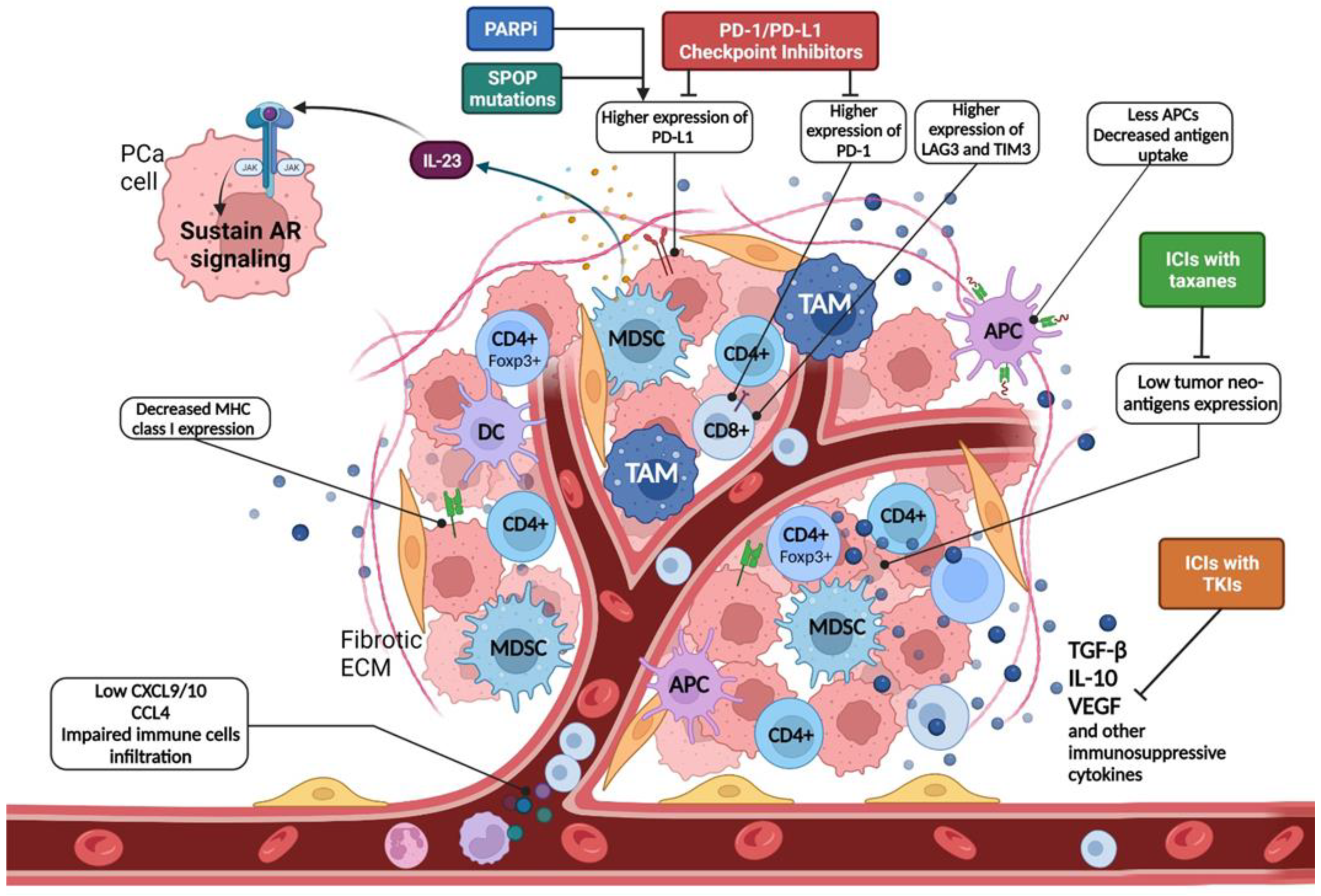
2. Immunobiology of Prostate Cancer
3. Immune Checkpoint Inhibitors in Hormone-Sensitive Prostate Cancer
3.1. Current Evidence
3.2. Ongoing Trials
4. Immune Checkpoint Inhibitors in Castration-Resistant Prostate Cancer
4.1. Current Evidence: Monotherapy
4.2. Current Evidence: Combination Therapies
4.3. Ongoing Trials
5. Clinical Trial on Molecularly Selected PCa Patients
5.1. Molecular Pathways
5.2. PD-L1 Expression
5.3. DNA Damage Repair Genes
5.4. Tumor Mutational Burden
5.5. Mismatch Repair Pathway/Microsatellite Instability
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [CrossRef]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II—2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Achard, V.; Putora, P.M.; Omlin, A.; Zilli, T.; Fischer, S. Metastatic Prostate Cancer: Treatment Options. Oncology 2021, 100, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Bansal, D.; Reimers, M.; Knoche, E.; Pachynski, R. Immunotherapy and Immunotherapy Combinations in Metastatic Castration-Resistant Prostate Cancer. Cancers 2021, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Guo, C.; Gurel, B.; De Marzo, A.M.; Sfanos, K.S.; Mani, R.; Gil, J.; Drake, C.G.; Alimonti, A. Prostate carcinogenesis: Inflammatory storms. Nat. Cancer 2020, 20, 455–469. [Google Scholar] [CrossRef]
- Krueger, T.E.; Thorek, D.L.J.; Meeker, A.K.; Isaacs, J.T.; Brennen, W.N. Tumor-infiltrating mesenchymal stem cells: Drivers of the immunosuppressive tumor microenvironment in prostate cancer? Prostate 2018, 79, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Calcinotto, A.; Spataro, C.; Zagato, E.; Di Mitri, D.; Gil, V.; Crespo, M.; De Bernardis, G.; Losa, M.; Mirenda, M.; Pasquini, E.; et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 2018, 559, 363–369. [Google Scholar] [CrossRef]
- Lu, X.; Horner, J.W.; Paul, E.; Shang, X.; Troncoso, P.; Deng, P.; Jiang, S.; Chang, Q.; Spring, D.J.; Sharma, P.; et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 2017, 543, 728–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, J.T.W.; Bryant, R.J.; Parkes, E.E. The tumor microenvironment and immune responses in prostate cancer patients. Endocr.-Relat. Cancer 2021, 28, T95–T107. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Berger, A.; Bindea, G.; Meatchi, T.; Bruneval, P.; Trajanoski, Z.; Fridman, W.-H.; Pagès, F.; et al. Histopathologic-Based Prognostic Factors of Colorectal Cancers Are Associated With the State of the Local Immune Reaction. J. Clin. Oncol. 2011, 29, 610–618. [Google Scholar] [CrossRef]
- Kaur, H.B.; Guedes, L.B.; Lu, J.; Maldonado, L.; Reitz, L.; Barber, J.R.; De Marzo, A.M.; Tosoian, J.J.; Tomlins, S.A.; Schaeffer, E.M.; et al. Association of tumor-infiltrating T-cell density with molecular subtype, racial ancestry and clinical outcomes in prostate cancer. Mod. Pathol. 2018, 31, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Attwood, K.; Bshara, W.; Mohler, J.L.; Guru, K.; Xu, B.; Kalinski, P.; Chatta, G. High intratumoral CD8+ T-cell infiltration is associated with improved survival in prostate cancer patients undergoing radical prostatectomy. Prostate 2021, 81, 20–28. [Google Scholar] [CrossRef]
- Rodrigues, D.N.; Rescigno, P.; Liu, D.; Yuan, W.; Carreira, S.; Lambros, M.B.; Seed, G.; Mateo, J.; Riisnaes, R.; Mullane, S.; et al. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer. J. Clin. Investig. 2018, 128, 4441–4453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, L.; Kriner, M.; Coleman, I.; Morrissey, C.; Roudier, M.; True, L.D.; Gulati, R.; Plymate, S.R.; Zhou, Z.; Birditt, B.; et al. Inter- and intra-tumor heterogeneity of metastatic prostate cancer determined by digital spatial gene expression profiling. Nat. Commun. 2021, 12, 1426. [Google Scholar] [CrossRef]
- He, M.X.; Cuoco, M.S.; Crowdis, J.; Bosma-Moody, A.; Zhang, Z.; Bi, K.; Kanodia, A.; Su, M.-J.; Ku, S.-Y.; Garcia, M.M.; et al. Transcriptional mediators of treatment resistance in lethal prostate cancer. Nat. Med. 2021, 27, 426–433. [Google Scholar] [CrossRef]
- Vitkin, N.; Nersesian, S.; Siemens, D.R.; Koti, M. The Tumor Immune Contexture of Prostate Cancer. Front. Immunol. 2019, 10, 603. [Google Scholar] [CrossRef] [Green Version]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Toso, A.; Revandkar, A.; DI Mitri, D.; Guccini, I.; Proietti, M.; Sarti, M.; Pinton, S.; Zhang, J.; Kalathur, M.; Civenni, G.; et al. Enhancing Chemotherapy Efficacy in Pten -Deficient Prostate Tumors by Activating the Senescence-Associated Antitumor Immunity. Cell Rep. 2014, 9, 75–89. [Google Scholar] [CrossRef]
- Blattner, M.; Liu, D.; Robinson, B.D.; Huang, D.; Poliakov, A.; Gao, D.; Nataraj, S.; Deonarine, L.D.; Augello, M.A.; Sailer, V.; et al. SPOP Mutation Drives Prostate Tumorigenesis In Vivo through Coordinate Regulation of PI3K/mTOR and AR Signaling. Cancer Cell 2017, 31, 436–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, G.P.; Massague, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.H.; Dewal, N.; Sokol, E.; Mathew, P.; Whitehead, R.; Millis, S.Z.; Frampton, G.M.; Bratslavsky, G.; Pal, S.K.; Lee, R.J.; et al. Prospective Comprehensive Genomic Profiling of Primary and Metastatic Prostate Tumors. JCO Precis. Oncol. 2019, 3, 1–23. [Google Scholar] [CrossRef]
- Rescigno, P.; Aversa, C.; Crespo, M.; Seed, G.; Lambros, M.; Gurel, B.; Figueiredo, I.; Bianchini, D.; Riisnaes, R.; Pereira, R.; et al. Elucidating Durable Responses to Immune Checkpoint Inhibition. Eur. Urol. 2020, 78, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, P.; Gurel, B.; Pereira, R.; Crespo, M.; Rekowski, J.; Rediti, M.; Barrero, M.; Mateo, J.; Bianchini, D.; Messina, C.; et al. Characterizing CDK12-Mutated Prostate Cancers. Clin. Cancer Res. 2021, 27, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Giunta, E.F.; Annaratone, L.; Bollito, E.; Porpiglia, F.; Cereda, M.; Banna, G.L.; Mosca, A.; Marchiò, C.; Rescigno, P. Molecular Characterization of Prostate Cancers in the Precision Medicine Era. Cancers 2021, 13, 4771. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.E.; Hurley, P.J.; Tran, P.T.; Rowe, S.P.; Benzon, B.; Neal, T.O.; Chapman, C.; Harb, R.; Milman, Y.; Trock, B.J.; et al. A pilot trial of pembrolizumab plus prostatic cryotherapy for men with newly diagnosed oligometastatic hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis. 2020, 23, 184–193. [Google Scholar] [CrossRef]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; Van den Eertwegh, A.J.M.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef] [Green Version]
- Fizazi, K.; Drake, C.G.; Beer, T.M.; Kwon, E.D.; Scher, H.I.; Gerritsen, W.R.; Bossi, A.; Eertwegh, A.J.V.D.; Krainer, M.; Houede, N.; et al. Final Analysis of the Ipilimumab Versus Placebo Following Radiotherapy Phase III Trial in Postdocetaxel Metastatic Castration-resistant Prostate Cancer Identifies an Excess of Long-term Survivors. Eur. Urol. 2020, 78, 822–830. [Google Scholar] [CrossRef]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef]
- Hansen, A.R.; Massard, C.; Ott, P.A.; Haas, N.B.; Lopez, J.S.; Ejadi, S.; Wallmark, J.M.; Keam, B.; Delord, J.-P.; Aggarwal, R.; et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann. Oncol. 2018, 29, 1807–1813. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.P.; Loriot, Y.; Shaffer, D.R.; Braiteh, F.; Powderly, J.; Harshman, L.C.; Conkling, P.; Delord, J.-P.; Gordon, M.; Kim, J.W.; et al. Safety and Clinical Activity of Atezolizumab in Patients with Metastatic Castration-Resistant Prostate Cancer: A Phase I Study. Clin. Cancer Res. 2021, 27, 3360–3369. [Google Scholar] [CrossRef]
- Powles, T.; Yuen, K.C.; Gillessen, S.; Kadel, E.E.; Rathkopf, D.; Matsubara, N.; Drake, C.G.; Fizazi, K.; Piulats, J.M.; Wysocki, P.J.; et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: A randomized phase 3 trial. Nat. Med. 2022, 28, 144–153. [Google Scholar] [CrossRef]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Fléchon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020, 38, 489–499.e3. [Google Scholar] [CrossRef] [PubMed]
- Shenderov, E.; Boudadi, K.; Fu, W.; Wang, H.; Sullivan, R.; Jordan, A.; Dowling, D.; Harb, R.; Schonhoft, J.; Jendrisak, A.; et al. Nivolumab plus ipilimumab, with or without enzalutamide, in AR-V7-expressing metastatic castration-resistant prostate cancer: A phase-2 nonrandomized clinical trial. Prostate 2021, 81, 326–338. [Google Scholar] [CrossRef]
- Fizazi, K.; Mella, P.G.; Castellano, D.; Minatta, J.N.; Kalebasty, A.R.; Shaffer, D.; Limón, J.C.V.; López, H.M.S.; Armstrong, A.J.; Horvath, L.; et al. Nivolumab plus docetaxel in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer: Results from the phase II CheckMate 9KD trial. Eur. J. Cancer 2021, 160, 61–71. [Google Scholar] [CrossRef]
- Petrylak, D.; Perez-Gracia, J.; Lacombe, L.; Bastos, D.; Mahammedi, H.; Kwan, E.; Zschäbitz, S.; Armstrong, A.; Pachynski, R.; Goh, J.; et al. 579MO CheckMate 9KD cohort A2 final analysis: Nivolumab (NIVO) + rucaparib for chemotherapy (CT)-naïve metastatic castration-resistant prostate cancer (mCRPC). Ann. Oncol. 2021, 32, S629–S630. [Google Scholar] [CrossRef]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Molavi, O.; Kahroba, H.; Hejazi, M.S.; Maleki-Dizaji, N.; Barghi, S.; Kiaie, S.H.; Jadidi-Niaragh, F. Clinical application of immune checkpoints in targeted immunotherapy of prostate cancer. Cell. Mol. Life Sci. 2020, 77, 3693–3710. [Google Scholar] [CrossRef] [PubMed]
- Calagua, C.; Russo, J.; Sun, Y.; Schaefer, R.; Lis, R.; Zhang, Z.; Mahoney, K.; Bubley, G.J.; Loda, M.; Taplin, M.-E.; et al. Expression of PD-L1 in Hormone-naïve and Treated Prostate Cancer Patients Receiving Neoadjuvant Abiraterone Acetate plus Prednisone and Leuprolide. Clin. Cancer Res. 2017, 23, 6812–6822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gevensleben, H.; Dietrich, D.; Golletz, C.; Steiner, S.; Jung, M.; Thiesler, T.; Majores, M.; Stein, J.; Uhl, B.; Müller, S.; et al. The Immune Checkpoint Regulator PD-L1 Is Highly Expressed in Aggressive Primary Prostate Cancer. Clin. Cancer Res. 2016, 22, 1969–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, D.; Van Allen, E.M.; Wu, Y.-M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.; Kote-Jarai, Z.; Mikropoulos, C.; Eeles, R. Prostate Cancer Germline Variations and Implications for Screening and Treatment. Cold Spring Harb. Perspect. Med. 2017, 8, a030379. [Google Scholar] [CrossRef]
- Mouw, K.W.; Goldberg, M.S.; Konstantinopoulos, P.A.; D’Andrea, A.D. DNA Damage and Repair Biomarkers of Immunotherapy Response. Cancer Discov. 2017, 7, 675–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo, J.; Lord, C.J.; Serra, V.; Tutt, A.; Balmaña, J.; Castroviejo-Bermejo, M.; Cruz, C.; Oaknin, A.; Kaye, S.B.; de Bono, J.S. A decade of clinical development of PARP inhibitors in perspective. Ann. Oncol. 2019, 30, 1437–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, S.; Xia, W.; Yamaguchi, H.; Wei, Y.; Chen, M.-K.; Hsu, J.-M.; Hsu, J.L.; Yu, W.-H.; Du, Y.; Lee, H.-H.; et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin. Cancer Res. 2017, 23, 3711–3720. [Google Scholar] [CrossRef] [Green Version]
- Antonarakis, E.S.; Velho, P.I.; Fu, W.; Wang, H.; Agarwal, N.; Santos, V.S.; Maughan, B.L.; Pili, R.; Adra, N.; Sternberg, C.N.; et al. CDK12-Altered Prostate Cancer: Clinical Features and Therapeutic Outcomes to Standard Systemic Therapies, Poly (ADP-Ribose) Polymerase Inhibitors, and PD-1 Inhibitors. JCO Precis. Oncol. 2020, 4, 370–381. [Google Scholar] [CrossRef]
- Strickler, J.H.; Hanks, B.A.; Khasraw, M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin. Cancer Res. 2021, 27, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- McGrail, D.; Pilié, P.; Rashid, N.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef]
- Berger, M.F.; Lawrence, M.S.; Demichelis, F.; Drier, Y.; Cibulskis, K.; Sivachenko, A.Y.; Sboner, A.; Esgueva, R.; Pflueger, D.; Sougnez, C.; et al. The genomic complexity of primary human prostate cancer. Nature 2011, 470, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiricny, J. The multifaceted mismatch-repair system. Nat. Rev. Mol. Cell Biol. 2006, 7, 335–346. [Google Scholar] [CrossRef]
- O’Connor, M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef] [Green Version]
- Li, G.-M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008, 18, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Antonarakis, E.S.; Shaukat, F.; Velho, P.I.; Kaur, H.; Shenderov, E.; Pardoll, D.M.; Lotan, T. Clinical Features and Therapeutic Outcomes in Men with Advanced Prostate Cancer and DNA Mismatch Repair Gene Mutations. Eur. Urol. 2019, 75, 378–382. [Google Scholar] [CrossRef]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Graham, L.S.; Montgomery, B.; Cheng, H.H.; Yu, E.Y.; Nelson, P.S.; Pritchard, C.; Erickson, S.; Alva, A.; Schweizer, M.T. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS ONE 2020, 15, e0233260. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Morrissey, C.; Kumar, A.; Zhang, X.; Smith, C.; Coleman, I.; Salipante, S.J.; Milbank, J.; Yu, M.; Grady, W.M.; et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat. Commun. 2014, 5, 4988. [Google Scholar] [CrossRef] [Green Version]
- Ritch, E.; Fu, S.Y.; Herberts, C.; Wang, G.; Warner, E.W.; Schönlau, E.; Taavitsainen, S.; Murtha, A.J.; Vandekerkhove, G.; Beja, K.; et al. Identification of Hypermutation and Defective Mismatch Repair in ctDNA from Metastatic Prostate Cancer. Clin. Cancer Res. 2019, 26, 1114–1125. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Yu, E.Y. “Matching” the “Mismatch” Repair–Deficient Prostate Cancer with Immunotherapy. Clin. Cancer Res. 2020, 26, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Linch, M.D.; Wong, Y.N.S.; Jones, R.; Sankey, P.; Josephs, D.H.; Crabb, S.J.; Staffurth, J.; Zarkar, A.; White, L.; Duggan, M.; et al. Abstract LB004: Nivolumab (NIVO) and ipilimumab (IPI) treatment in prostate cancer with an immunogenic signature: Cohort 1 of the NEPTUNES multi-centre, two-stage biomarker-selected Phase II trial. Clin. Res. 2021, 81, LB004. [Google Scholar] [CrossRef]
- Drachenberg, D.E.; Elgamal, A.-A.A.; Rowbotham, R.; Peterson, M.; Murphy, G.P. Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate 1999, 41, 127–133. [Google Scholar] [CrossRef]
- Harshman, L.C.; Wang, V.X.; Hamid, A.A.; Ms, G.S.; Drake, C.G.; Carducci, M.A.; DiPaola, R.S.; Fichorova, R.N.; Sweeney, C.J. Impact of baseline serum IL-8 on metastatic hormone-sensitive prostate cancer outcomes in the Phase 3 CHAARTED trial (E3805). Prostate 2020, 80, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Xu, L.; Wang, Y.; Jiang, Q.; Liu, Z.; Zhang, J.; Zhou, Q.; Zeng, H.; Tong, S.; Wang, T.; et al. Tumor-associated Macrophage-derived Interleukin-23 Interlinks Kidney Cancer Glutamine Addiction with Immune Evasion. Eur. Urol. 2019, 75, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Mohanakrishnan, R.; Beier, S.; Deodhar, A. IL-23 inhibition for the treatment of psoriatic arthritis. Expert Opin. Biol. Ther. 2022, 22, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Wo, Y.J.; Gan, A.S.P.; Lim, X.; Tay, I.S.Y.; Lim, S.; Lim, J.C.T.; Yeong, J.P.S. The Roles of CD38 and CD157 in the Solid Tumor Microenvironment and Cancer Immunotherapy. Cells 2019, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Crespo, M.; Gurel, B.; Dolling, D.; Rekowski, J.; Sharp, A.; Petremolo, A.; Sumanasuriya, S.; Rodrigues, D.N.; Ferreira, A.; et al. CD38 in Advanced Prostate Cancers. Eur. Urol. 2021, 79, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, I.S.; Casneuf, T.; Van Velzen, J.; Van Kessel, B.; Axel, A.E.; Syed, K.; Groen, R.; Van Duin, M.; Sonneveld, P.; Minnema, M.C.; et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood 2016, 128, 959–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Available Clinical Trials | Number of Patients Patients’ Characteristics | Pretreatment | Study Interventional Method/Drug | Primary Endpoint | Results |
|---|---|---|---|---|---|
| Single-arm, single-institution pilot trial [30] | n = 12 Oligometastatic patients (≤5 extra-pelvic metastases) | Treatment-naïve | Whole prostate cryoablation + short-term ADT (8 months) + pembrolizumab (6 cycles) | Number of patients with 1 y PSA < 0.6 ng/mL Frequency of AEs | 1 y-PSA < 0.6 ng/mL: 42% mPFS: 14 months mSTFS: 17.5 months AEs grade ≤ 2: 100% |
| Ongoing Clinical Trials (Name/NCT, Phase) | Planned Number of Patients Patients’ Characteristics | Pretreatment | Study Interventional Method/Drug | Primary Endpoint | Estimated Primary Completion Date (Month/Year) |
| PROSTRATEGY NCT03879122 Phase II/III | n = 135 High-volume disease (CHAARTED criteria) | Treatment-naïve (ADT < 120 days allowed) | Control arm: ADT + docetaxel (6 cycles) Experimental arm 1: ADT + docetaxel + nivolumab Experimental arm 2: ADT + ipilimumab alternating with docetaxel Experimental arm 3: ADT + ipilimumab alternating with docetaxel and with nivolumab | OS | July 2022 |
| KEYNOTE-991 NCT04191096 Phase III | n = 1232 At least 2 bone lesions +/− visceral disease | Treatment-naïve (6 cycles docetaxel allowed) | Experimental arm: ADT + enzalutamide + pembrolizumab Control arm: ADT + enzalutamide + placebo | rPFS OS | July 2026 |
| CABIOS NCT04477512 Phase Ib | n = 22 | Treatment-naïve | Experimental level 1: cabozantinib 20 mg + abiraterone acetate + nivolumab Experimental level 2: cabozantinib 40 mg + abiraterone acetate + nivolumab Experimental expansion: cabozantinib + abiraterone acetate + nivolumab | DLTs | January 2022 |
| REGN2810 NCT03951831 Phase II | n = 20 | Treatment-naïve | ADT + cemiplimab + docetaxel (max 6 cycles) | Percentage of subjects achieving undetectable PSA at 6 months after combination treatment | September 2020 |
| NCT04126070 Phase II | n = 60 Cohort 1: somatic or germline homozygous deletions and/or deleterious mutations in a DDR gene, MMRd, or MSI-H Cohort 2: PD-L1 positive and/or CD8+ T cell inflamed using ImmunoProfile without the presence of DDRD Cohort 3: negative for DDRD and PD-L1 with low CD8+ T-cell infiltration | Treatment-naïve | ADT + nivolumab + docetaxel (max 6 cycles) | Number of patients 1 y PSA ≤ 0.2 ng/mL | June 2023 |
| POSTCARD NCT03795207 Phase II | n = 96 Oligometatastatic disease (≤5 bone/lymph node metastases detected only on FCH-PET/CT or Ga-PSMA PET/CT) | Biochemical recurrence after RT or RP | Experimental arm: SBRT + durvalumab (1 year) Control arm: SBRT 3 fractions (32 patients will be enrolled in this arm) | 2 y PFS | November 2023 |
| Clinical Trial (Name, NCT, Phase) | Planned Number of Patients Patients’ Characteristics | Pretreatment | Study Drug | Primary Endpoint | Estimated Completion Date |
|---|---|---|---|---|---|
| Molecular-Selected Patients | |||||
| CHOMP trial NCT04104893 Phase II | n = 30 MMD or somatic biallelic inactivation of CDK12 | One 2nd generation hormonal therapy for mCSPC, M0CRPC and/or mCRPC setting (i.e., abiraterone acetate, enzalutamide, apalutamide or darolutamide) | Pembrolizumab | PSA50 ORR | March 2023 |
| PERSEUS1 NCT03506997 Phase II | n = 100 High mutational load (≥11 mutations per targeted panel) on NGS and/or DNA repair defect including MMD | ≥1 approved treatment for mCRPC (i.e., abiraterone acetate, enzalutamide, docetaxel, cabazitaxel, radium-233) | Pembrolizumab | PSA50 ORR | September 2023 |
| INSPIRE NCT04717154 Phase II | n = 75 Immunogenic phenotype: MMD and/or high TMB (>7 mutations/Mb (cluster A); BRCA2 inactivation or BRCAness signature (cluster B); a tandem duplication signature and/or CDK12 biallelic inactivation (cluster C) | - | Nivolumab + ipilimumab for 4 cycles and nivolumab as maintenance (up to 1 year) | DCR | January 2026 |
| IMPACT NCT03570619 Phase II | n = 40 Patients with metastatic cancers and CDK12 mutations: mCRPC (cohort A), metastatic solid tumors (non-prostate) (cohort B) | Patients must be ≥2 weeks from most recent systemic therapy or most recent radiation therapy | Nivolumab + ipilimumab for 4 cycles and nivolumab as maintenance (up to 1 year) | PSA50 ORR | September 2021 |
| ImmunoProst trial NCT03040791 Phase II | n = 38 Patients with germline and somatic DRD (including HR and MMRd) | Documented prostate cancer progression, during treatment with docetaxel | Nivolumab | PSA response rate | January 2022 |
| Neptunes NCT03061539 Phase II | n = 175 mCRPC patients with immunogenic biomarker positive disease (DRD–MMRd–high tumor-infiltrating lymphocyte) | 1 or more lines of systemic treatment for mCRPC | Nivolumab + ipilimumab for 4 cycles and nivolumab as maintenance (up to 1 year) | PSA50 Radiological response conversion of CTC count | April 2022 |
| NCT03248570 Phase II | n = 50 Patients with mCRPC with or without DNA damage repair defects | Patients must have received prior 2nd hormonal therapy (abiraterone, enzalutamide and/or apalutamide) | Pembrolizumab | rPFS | July 2023 |
| NCT 04019964 Phase II | n = 15 Patients with at least one of the following genetic alterations: MMRd, MSIh, TMBh, inactivating mutation of CDK12 | Prior local therapy with prostatectomy or EBRT/brachytherapy is required. Prior salvage or adjuvant radiation therapy is allowed but not mandated. Radiation therapy must have been completed for at least 6 months | Nivolumab | PSA50 | January 2025 |
| ICI + TKI combination | |||||
| NCT04159896 Phase II | n = 49 | 2nd generation hormonal agent (i.e., abiraterone acetate, enzalutamide) and chemotherapy (docetaxel and/or cabazitaxel) | ESK981 (pan-VEGFR/TIE2 TKI) + nivolumab | PSA50 AEs | March 2022 |
| CONTACT-02 NCT04446117 Phase III | n = 580 | One 2nd generation hormonal therapy (i.e., abiraterone, apalutamide, darolutamide, or enzalutamide) for mCSPC, M0CRPC, mCRPC | Experimental arm: atezolizumab + cabozantinib Control arm: abiraterone acetate/enzalutamide | PFS OS | March 2022 |
| New drugs | |||||
| NCT04631601 Phase I–II | n = 159 | - | Multiple experimental arm: acapatamab + enzalutamide; acapatamab + abiraterone; acapatamab + AMG404 (anti PD1); AMG 404 monotherapy; acapatamab monotherapy | DLTs TEAEs | November 2022 |
| NCT03792841 Phase I | n = 288 | Second-generation hormonal therapy (abiraterone, enzalutamide, and/or apalutamide) and 1–2 (or unfit/refuses) taxane regimens for mCRPC | Acapatamab ± pembrolizumab, etanercept, or a CP450 cocktail | DLTs TEAEs | December 2025 |
| NCT04633252 Phase I–II | n = 86 Patients with mCSPC and mCRPC | Second-generation hormonal therapy (abiraterone, enzalutamide, apalutamide, or darolutamide) Must have not had progression while on docetaxel if given for mCSPC or within 3 months of completing docetaxel for mCSPC | Docetaxel + M9241 (tumor-targeting immunocytokine) Docetaxel + M9241 (tumor-targeting immunocytokine) + Bintrafusp alfa (M7824) | DLTs AEs PFS | December 2022 |
| PRO-MERIT NCT04382898 Phase I–II | n = 130 | 2–3 lines of systemic therapy for mCSPC and mCRPC setting | W_pro1 (BNT112) Cemiplimab + W_pro1 (BNT112) | DLTs TEAEs ORR | July 2023 |
| Immune combination with standard therapies | |||||
| Rad2Nivo NCT04109729 Phase Ib–II | n = 36 | - | Nivolumab (up 2 years) + radium-233 (6 cycles) | Phase 1b: safety Phase 2: ctDNA reduction after 6 weeks | June 2022 |
| Checkmate 7DX NCT04100018 Phase III | n = 984 | 1–2 s generation hormonal therapies (no ≥1 s generation hormonal therapy in the mCRPC setting) | Experimental arm: nivolumab + docetaxel Control arm: placebo + docetaxel | rPFS OS | April 2023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebuzzi, S.E.; Rescigno, P.; Catalano, F.; Mollica, V.; Vogl, U.M.; Marandino, L.; Massari, F.; Pereira Mestre, R.; Zanardi, E.; Signori, A.; et al. Immune Checkpoint Inhibitors in Advanced Prostate Cancer: Current Data and Future Perspectives. Cancers 2022, 14, 1245. https://doi.org/10.3390/cancers14051245
Rebuzzi SE, Rescigno P, Catalano F, Mollica V, Vogl UM, Marandino L, Massari F, Pereira Mestre R, Zanardi E, Signori A, et al. Immune Checkpoint Inhibitors in Advanced Prostate Cancer: Current Data and Future Perspectives. Cancers. 2022; 14(5):1245. https://doi.org/10.3390/cancers14051245
Chicago/Turabian StyleRebuzzi, Sara Elena, Pasquale Rescigno, Fabio Catalano, Veronica Mollica, Ursula Maria Vogl, Laura Marandino, Francesco Massari, Ricardo Pereira Mestre, Elisa Zanardi, Alessio Signori, and et al. 2022. "Immune Checkpoint Inhibitors in Advanced Prostate Cancer: Current Data and Future Perspectives" Cancers 14, no. 5: 1245. https://doi.org/10.3390/cancers14051245
APA StyleRebuzzi, S. E., Rescigno, P., Catalano, F., Mollica, V., Vogl, U. M., Marandino, L., Massari, F., Pereira Mestre, R., Zanardi, E., Signori, A., Buti, S., Bauckneht, M., Gillessen, S., Banna, G. L., & Fornarini, G. (2022). Immune Checkpoint Inhibitors in Advanced Prostate Cancer: Current Data and Future Perspectives. Cancers, 14(5), 1245. https://doi.org/10.3390/cancers14051245











