Triple Negative Breast Cancer: Updates on Classification and Treatment in 2021
Abstract
Simple Summary
Abstract
1. Introduction
2. Triple-Negative Breast Cancer Molecular Subtyping
3. Chemotherapy for Triple Negative Breast Cancer
4. Detecting PDL-1 Expression in TNBC
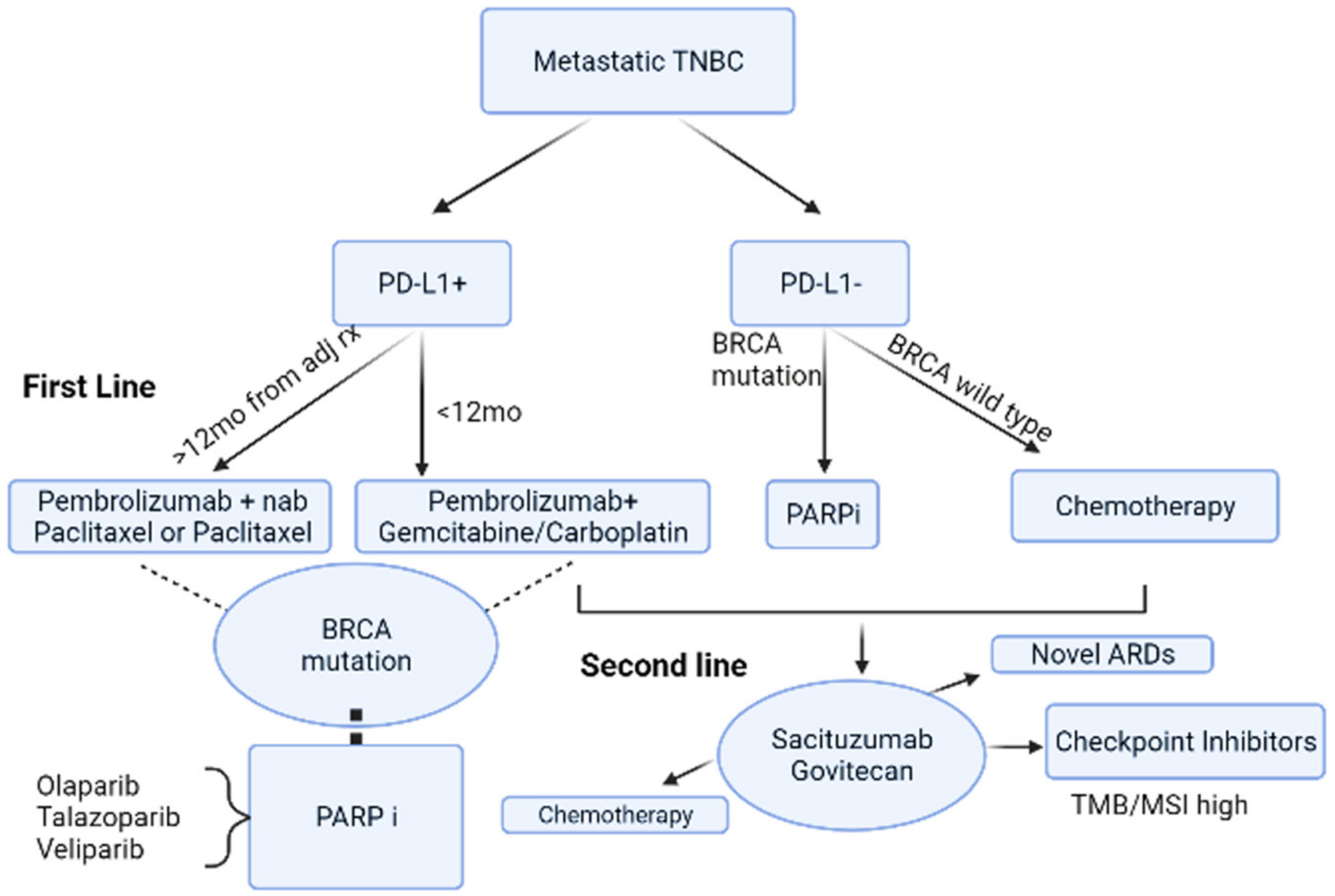
5. Beyond Chemotherapy for Metastatic Triple Negative Breast Cancer
5.1. Antibody Drug Conjugates-Sacituzumab Govitecan
5.2. Immune Check-Point Inhibitors
5.2.1. Atezolizumab
5.2.2. Pembrolizumab
5.3. Poly-Adenosine Diphosphate Ribose (ADP) Polymerase Inhibitors (PARPi)
5.3.1. Olaparib
5.3.2. Veliparib
5.4. Androgen Receptor Targeted Agents
5.4.1. Bicalutamide
5.4.2. Abiraterone
5.4.3. Enzalutamide
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A | doxorubicin |
| ACT | adriamycin, cyclophosphamide, and paclitaxel (taxol) |
| AR | androgen receptor |
| AUC | area under the curve |
| BC | Breast Cancer |
| Bev | bevacizumab |
| BL1 | basal-like 1 |
| BL2 | basal-like 2 |
| BLIA | basal-like immune-activated |
| BLIS | basal-like immunosuppressed |
| C | cyclophosphamide |
| CBR | clinical benefit rate |
| DC | ductal carcinoma |
| dd | dose-dense |
| ddAC | Dose-Dense Adjuvant Doxorubicin and Cyclophosphamide |
| E | epirubicin |
| ECM | extracellular matrix |
| ER | estrogen receptor |
| FUSCC | Fudan University Shanghai Cancer Center |
| G | gemcitabine |
| GO | Gene Ontology |
| HER2 | human epidermal growth factor receptor 2 |
| IM | immunomodulatory |
| LAR | luminal androgen receptor |
| lncRNAs | long noncoding RNAs |
| M | mesenchymal |
| MES | mesenchymal-like subtype |
| MSL | mesenchymal stem-like |
| MUC1 | cell-surface mucin |
| N/A | not applicable |
| nP | nab-Paclitaxel |
| NPLD | non-pegylated liposomal doxorubicin |
| NST | neoadjuvant systemic treatment |
| OS | overall survival |
| P | paclitaxel |
| pCR | pathological complete response |
| PDGFRα | platelet-derived growth factor receptor α |
| PR | progesterone receptor |
| RCTs | randomized controlled trials |
| RFS | relapse-free survival |
| RT | radiotherapy |
| T | docetaxel |
| TGF | transforming growth factor |
| TNBC | Triple-negative breast cancer |
References
- Bilani, N.; Zabor, E.C.; Elson, L.; Elimimian, E.B.; Nahleh, Z. Breast cancer in the United States: A cross-sectional overview. J. Cancer Epidemiol. 2020, 2020, 6387378. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.; Gelber, R.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J.; Albain, K.S.; André, F.; Bergh, J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.J.; Naidu, S.; Topham, A.K.; Guiles, F.; Xu, Y.; McCue, P.; Schwartz, G.F.; Park, P.K.; Rosenberg, A.L.; Brill, K. Differences in breast carcinoma characteristics in newly diagnosed African–American and Caucasian patients: A single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and end results database. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 110, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Claus, E.; Sohl, J.; Razzak, A.R.; Arnaout, A.; Winer, E.P. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: High incidence of central nervous system metastases. Cancer 2008, 113, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, L.N.; Wilkinson, K.H.; Kong, A. Triple-negative breast cancer: Who should receive neoadjuvant chemotherapy? Surg. Oncol. Clin. 2018, 27, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Pietenpol, J.A. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J. Pathol. 2014, 232, 142–150. [Google Scholar] [CrossRef]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Jiang, Y.-Z.; Xu, X.-E.; Yu, K.-D.; Jin, X.; Hu, X.; Zuo, W.-J.; Hao, S.; Wu, J.; Liu, G.-Y. Comprehensive transcriptome analysis identifies novel molecular subtypes and subtype-specific RNAs of triple-negative breast cancer. Breast Cancer Res. 2016, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, R.; Mnich, K.; Dolan, S.; Hillis, J.; Almanza, A.; Logue, S.E.; Samali, A.; Gorman, A.M. RIP2 enhances cell survival by activation of NF-ĸB in triple negative breast cancer cells. Biochem. Biophys. Res. Commun. 2018, 497, 115–121. [Google Scholar] [CrossRef]
- Abduljabbar, R.; Al-Kaabi, M.M.; Negm, O.H.; Jerjees, D.; Muftah, A.A.; Mukherjee, A.; Lai, C.F.; Buluwela, L.; Ali, S.; Tighe, P.J. Prognostic and biological significance of peroxisome proliferator-activated receptor-gamma in luminal breast cancer. Breast Cancer Res. Treat. 2015, 150, 511–522. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-c. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Wahba, H.A.; El-Hadaad, H.A. Current approaches in treatment of triple-negative breast cancer. Cancer Biol. Med. 2015, 12, 106. [Google Scholar] [PubMed]
- Ismail-Khan, R.; Bui, M.M. A review of triple-negative breast cancer. Cancer Control. 2010, 17, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Del Mastro, L.; Cinquini, M.; Montemurro, F.; Biganzoli, L.; Cortesi, L.; Zambelli, A.; Criscitiello, C.; Levaggi, A.; Conte, B. Inclusion of platinum agents in neoadjuvant chemotherapy regimens for triple-negative breast cancer patients: Development of grade (Grades of Recommendation, Assessment, Development and Evaluation) recommendation by the Italian Association of Medical Oncology (AIOM). Cancers 2019, 11, 1137. [Google Scholar]
- Birgisdottir, V.; Stefansson, O.A.; Bodvarsdottir, S.K.; Hilmarsdottir, H.; Jonasson, J.G.; Eyfjord, J.E. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006, 8, R38. [Google Scholar] [CrossRef]
- Biswas, T.; Efird, J.T.; Prasad, S.; Jindal, C.; Walker, P.R. The survival benefit of neoadjuvant chemotherapy and pCR among patients with advanced stage triple negative breast cancer. Oncotarget 2017, 8, 112712. [Google Scholar] [CrossRef]
- Isakoff, S.J.; Mayer, E.L.; He, L.; Traina, T.A.; Carey, L.A.; Krag, K.J.; Rugo, H.S.; Liu, M.C.; Stearns, V.; Come, S.E. TBCRC009: A multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J. Clin. Oncol. 2015, 33, 1902. [Google Scholar] [CrossRef]
- Byrski, T.; Gronwald, J.; Huzarski, T.; Grzybowska, E.; Budryk, M.; Stawicka, M.; Mierzwa, T.; Szwiec, M.; Wiśniowski, R.; Siolek, M. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J. Clin. Oncol. 2010, 28, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.P.; Richardson, A.L.; Eklund, A.C.; Wang, Z.C.; Szallasi, Z.; Li, Q.; Juul, N.; Leong, C.-O.; Calogrias, D.; Buraimoh, A. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J. Clin. Oncol. 2010, 28, 1145. [Google Scholar] [CrossRef] [PubMed]
- Byrski, T.; Dent, R.; Blecharz, P.; Foszczynska-Kloda, M.; Gronwald, J.; Huzarski, T.; Cybulski, C.; Marczyk, E.; Chrzan, R.; Eisen, A. Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Res. 2012, 14, R110. [Google Scholar] [CrossRef] [PubMed]
- Sikov, W.M.; Berry, D.A.; Perou, C.M.; Singh, B.; Cirrincione, C.T.; Tolaney, S.M.; Kuzma, C.S.; Pluard, T.J.; Somlo, G.; Port, E.R. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 2015, 33, 13. [Google Scholar]
- Sikov, W.M.; Polley, M.-Y.; Twohy, E.; Perou, C.M.; Singh, B.; Berry, D.A.; Tolaney, S.M.; Somlo, G.; Port, E.R.; Ma, C.X. CALGB (Alliance) 40603: Long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT)+/-carboplatin (Cb) and bevacizumab (Bev) in triple-negative breast cancer (TNBC). Am. Soc. Clin.Oncol. 2019, 37, 591. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 2014, 15, 747–756. [Google Scholar] [CrossRef]
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Pondé, N.; La Valle, G.; Del Mastro, L.; De Azambuja, E.; Lambertini, M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1497–1508. [Google Scholar] [CrossRef]
- Loibl, S.; Sikov, W.; Huober, J.; Rugo, H.S.; Wolmark, N.; O’Shaughnessy, J.; Maag, D.; Untch, M.; Golshan, M.; Lorenzo, J.P.; et al. Event-Free Survival (EFS), Overall Survival (OS), and Safety of Adding Veliparib (V) Plus Carboplatin (Cb) or Carboplatin Alone to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer (TNBC) after ≥4 Years of Follow-Up: BrighTNess, a Randomized Phase III Trial. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-congress-2021/event-free-survival-efs-overall-survival-os-and-safety-of-adding-veliparib-v-plus-carboplatin-cb-or-carboplatin-alone-to-neoadjuvant-chem (accessed on 12 October 2021).
- Chen, H.; Marsiglia, W.M.; Cho, M.-K.; Huang, Z.; Deng, J.; Blais, S.P.; Gai, W.; Bhattacharya, S.; Neubert, T.A.; Traaseth, N.J. Elucidation of a four-site allosteric network in fibroblast growth factor receptor tyrosine kinases. eLife 2017, 6, e21137. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Moebus, V.; Tesch, H.; Hanusch, C.; Denkert, C.; Luebbe, K.; Huober, J.; Klare, P.; Kuemmel, S.; Untch, M. Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (GeparOcto—GBG 84): A randomised phase III trial. Eur. J. Cancer 2019, 106, 181–192. [Google Scholar]
- Zhang, P.; Yin, Y.; Mo, H.; Zhang, B.; Wang, X.; Li, Q.; Yuan, P.; Wang, J.; Zheng, S.; Cai, R. Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: A randomized phase 2 trial. Oncotarget 2016, 7, 60647. [Google Scholar] [CrossRef]
- Ando, M.; Yamauchi, H.; Aogi, K.; Shimizu, S.; Iwata, H.; Masuda, N.; Yamamoto, N.; Inoue, K.; Ohono, S.; Kuroi, K. Randomized phase II study of weekly paclitaxel with and without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIA breast cancer without HER2 overexpression. Breast Cancer Res. Treat. 2014, 145, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Erber, R.; Hartmann, A. Understanding PD-L1 testing in breast cancer: A practical approach. Breast Care 2020, 15, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Cimadamore, A.; Hartmann, A.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M.; Montironi, R.; Gevaert, T. PD-L1 assessment in urothelial carcinoma: A practical approach. Ann. Transl. Med. 2019, 7, 690. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Ancevski Hunter, K.; Socinski, M.A.; Villaruz, L.C. PD-L1 Testing in Guiding Patient Selection for PD-1/PD-L1 Inhibitor Therapy in Lung Cancer. Mol Diagn 2018, 22, 1–10. [Google Scholar] [CrossRef]
- U.S Food & Drug Administration. List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools). Available online: https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools (accessed on 12 October 2021).
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L. Pembrolizumab for the treatment of non–small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Roach, C.; Zhang, N.; Corigliano, E.; Jansson, M.; Toland, G.; Ponto, G.; Dolled-Filhart, M.; Emancipator, K.; Stanforth, D.; Kulangara, K. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non–small-cell lung cancer. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 392. [Google Scholar] [CrossRef]
- Scheel, A.H.; Dietel, M.; Heukamp, L.C.; Jöhrens, K.; Kirchner, T.; Reu, S.; Rüschoff, J.; Schildhaus, H.-U.; Schirmacher, P.; Tiemann, M. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod. Pathol. 2016, 29, 1165–1172. [Google Scholar] [CrossRef]
- Fenn, K.M.; Kalinsky, K. Sacituzumab govitecan: Antibody-drug conjugate in triple-negative breast cancer and other solid tumors. Drugs Today 2019, 55, 575–585. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Younes, A.; Bartlett, N.L.; Leonard, J.P.; Kennedy, D.A.; Lynch, C.M.; Sievers, E.L.; Forero-Torres, A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 2010, 363, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Tolaney, S.M.; Punie, K.; Loirat, D.; Oliveira, M.; Kalinsky, K.; Zelnak, A.; Aftimos, P.; Dalenc, F.; Sardesai, S.; et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015, 6, 22496–22512. [Google Scholar] [CrossRef]
- Zaman, S.; Jadid, H.; Denson, A.C.; Gray, J.E. Targeting Trop-2 in solid tumors: Future prospects. OncoTargets Ther. 2019, 12, 1781. [Google Scholar] [CrossRef]
- Starodub, A.N.; Ocean, A.J.; Shah, M.A.; Guarino, M.J.; Picozzi, V.J.; Vahdat, L.T.; Thomas, S.S.; Govindan, S.V.; Maliakal, P.P.; Wegener, W.A. First-in-human trial of a novel anti-Trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin. Cancer Res. 2015, 21, 3870–3878. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Heimes, A.S.; Schmidt, M. Atezolizumab for the treatment of triple-negative breast cancer. Expert Opin. Investig. Drugs 2019, 28, 1–5. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Emens, L.A.; Adams, S.; Barrios, C.H.; Diéras, V.; Iwata, H.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Winer, E.P.; Patel, S.; et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann. Oncol. 2021, 32, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Bianchini, G.; Dugo, M.; Huang, C.; Egle, D.; Bermejo, B.; Seitz, R.S.; Nielsen, T.J.J.; Zamagni, C.; Thill, M.; Anton, A.; et al. LBA12-Predictive Value of Gene-Expression Profiles (GEPs) and Their Dynamics during Therapy in the NeoTRIPaPDL1 Trial. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-congress-2021/predictive-value-of-gene-expression-profiles-geps-and-their-dynamics-during-therapy-in-the-neotripapdl1-trial (accessed on 12 October 2021).
- FDA. FDA Approves Atezolizumab for PD-L1 Positive Unresectable Locally Advanced or Metastatic Triple-Negative Breast Cancer. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative (accessed on 12 October 2021).
- Staff, A.P. Update on U.S. Indication for Atezolizumab in PD-L1–Positive Metastatic Triple-Negative Breast Cancer. Available online: https://ascopost.com/issues/september-25-2021/update-on-us-indication-for-atezolizumab-in-pd-l1-positive-metastatic-triple-negative-breast-cancer/ (accessed on 12 October 2021).
- Alva, A.S.; Mangat, P.K.; Garrett-Mayer, E.; Halabi, S.; Hansra, D.; Calfa, C.J.; Khalil, M.F.; Ahn, E.R.; Cannon, T.L.; Crilley, P.; et al. Pembrolizumab in Patients With Metastatic Breast Cancer With High Tumor Mutational Burden: Results From the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. J. Clin. Oncol. 2021, 39, 2443–2451. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Rugo, H.S.; Cortés, J.; Cescon, D.W.; Im, S.; Yusof, M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. LBA16-KEYNOTE-355: Final Results from a Randomized, Double-Blind Phase III Study of First-Line Pembrolizumab + Chemotherapy vs. Placebo + Chemotherapy for Metastatic TNBC. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-congress-2021/keynote-355-final-results-from-a-randomized-double-blind-phase-iii-study-of-first-line-pembrolizumab-chemotherapy-vs-placebo-chemotherapy-for (accessed on 22 September 2021).
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.; Hui, R. VP7-2021: KEYNOTE-522: Phase III study of neoadjuvant pembrolizumab+ chemotherapy vs. placebo+ chemotherapy, followed by adjuvant pembrolizumab vs. placebo for early-stage TNBC. Ann. Oncol. 2021, 32, 1198–1200. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Mangat, P.K.; Halabi, S.; Bruinooge, S.S.; Garrett-Mayer, E.; Alva, A.; Janeway, K.A.; Stella, P.J.; Voest, E.; Yost, K.J.; Perlmutter, J.; et al. Rationale and Design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. JCO Precis. Oncol. 2018, 2, 1–14. [Google Scholar] [CrossRef]
- Yu, J. April 27–29, 2021: Meeting of the Oncologic Drugs Advisory Committee Meeting Announcement. Available online: https://www.fda.gov/advisory-committees/advisory-committee-calendar/april-27-29-2021-meeting-oncologic-drugs-advisory-committee-meeting-announcement-04272021-04292021 (accessed on 20 August 2021).
- News, C.O. Keytruda Approved to Treat High-Risk, Early-Stage TNBC. Available online: https://www.clinicaloncology.com/FDA-Watch/Article/08-21/Keytruda-Approved-to-Treat-High-Risk-Early-Stage-TNBC-/64351 (accessed on 12 October 2021).
- Geenen, J.J.J.; Linn, S.C.; Beijnen, J.H.; Schellens, J.H.M. PARP Inhibitors in the Treatment of Triple-Negative Breast Cancer. Clin. Pharm. 2018, 57, 427–437. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.A.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: An updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016, 17, 1579–1589. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 2015, 33, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J. Adjuvant Olaparib for Patients with BRCA1-or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Swisher, E.M.; Lin, K.K.; Oza, A.M.; Scott, C.L.; Giordano, H.; Sun, J.; Konecny, G.E.; Coleman, R.L.; Tinker, A.V.; O’Malley, D.M.; et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 75–87. [Google Scholar] [CrossRef]
- Tung, N.M.; Robson, M.E.; Ventz, S.; Santa-Maria, C.A.; Nanda, R.; Marcom, P.K.; Shah, P.D.; Ballinger, T.J.; Yang, E.S.; Vinayak, S.; et al. TBCRC 048: Phase II Study of Olaparib for Metastatic Breast Cancer and Mutations in Homologous Recombination-Related Genes. J. Clin. Oncol. 2020, 38, 4274–4282. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 2016, 16, 110–120. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Antolín, A.A.; Mestres, J. Linking off-target kinase pharmacology to the differential cellular effects observed among PARP inhibitors. Oncotarget 2014, 5, 3023. [Google Scholar] [CrossRef][Green Version]
- Sharma, P.; Rodler, E.; Barlow, W.E.; Gralow, J.; Huggins-Puhalla, S.L.; Anders, C.K.; Goldstein, L.J.; Brown-Glaberman, U.A.; Huynh, T.-T.; Szyarto, C.S.; et al. Results of a phase II randomized trial of cisplatin +/- veliparib in metastatic triple-negative breast cancer (TNBC) and/or germline BRCA-associated breast cancer (SWOG S1416). J. Clin. Oncol. 2020, 38, 1001. [Google Scholar] [CrossRef]
- Han, H.; Arun, B.; Kaufman, B.; Wildiers, H.; Friedlander, M.; Ayoub, J.; Puhalla, S.; Bach, B.; Kundu, M.; Khandelwal, N. Veliparib monotherapy following carboplatin/paclitaxel plus veliparib combination therapy in patients with germline BRCA-associated advanced breast cancer: Results of exploratory analyses from the phase III BROCADE3 trial. Ann. Oncol. 2021, 33, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Diéras, V.; Han, H.S.; Kaufman, B.; Wildiers, H.; Friedlander, M.; Ayoub, J.P.; Puhalla, S.L.; Bondarenko, I.; Campone, M.; Jakobsen, E.H.; et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1269–1282. [Google Scholar] [CrossRef]
- Doane, A.S.; Danso, M.; Lal, P.; Donaton, M.; Zhang, L.; Hudis, C.; Gerald, W.L. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006, 25, 3994–4008. [Google Scholar] [CrossRef] [PubMed]
- Sanga, S.; Broom, B.M.; Cristini, V.; Edgerton, M.E. Gene expression meta-analysis supports existence of molecular apocrine breast cancer with a role for androgen receptor and implies interactions with ErbB family. BMC Med. Genom. 2009, 2, 59. [Google Scholar] [CrossRef]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef]
- Danila, D.C.; Morris, M.J.; de Bono, J.S.; Ryan, C.J.; Denmeade, S.R.; Smith, M.R.; Taplin, M.E.; Bubley, G.J.; Kheoh, T.; Haqq, C.; et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J. Clin. Oncol. 2010, 28, 1496–1501. [Google Scholar] [CrossRef]
- Vogelzang, N.J. Words of wisdom. Re: Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. Eur. Urol. 2009, 56, 220. [Google Scholar] [CrossRef]
- Bonnefoi, H.; Grellety, T.; Tredan, O.; Saghatchian, M.; Dalenc, F.; Mailliez, A.; L’Haridon, T.; Cottu, P.; Abadie-Lacourtoisie, S.; You, B.; et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1). Ann. Oncol. 2016, 27, 812–818. [Google Scholar] [CrossRef]
- Traina, T.A.; Miller, K.; Yardley, D.A.; Eakle, J.; Schwartzberg, L.S.; O’Shaughnessy, J.; Gradishar, W.; Schmid, P.; Winer, E.; Kelly, C.; et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J. Clin. Oncol. 2018, 36, 884–890. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Scoggins, M.E.; Hess, K.R.; Adrada, B.E.; Murthy, R.K.; Damodaran, S.; DeSnyder, S.M.; Brewster, A.M.; Barcenas, C.H.; Valero, V.; et al. Neoadjuvant Talazoparib for Patients With Operable Breast Cancer With a Germline BRCA Pathogenic Variant. J. Clin. Oncol. 2020, 38, 388–394. [Google Scholar] [CrossRef] [PubMed]
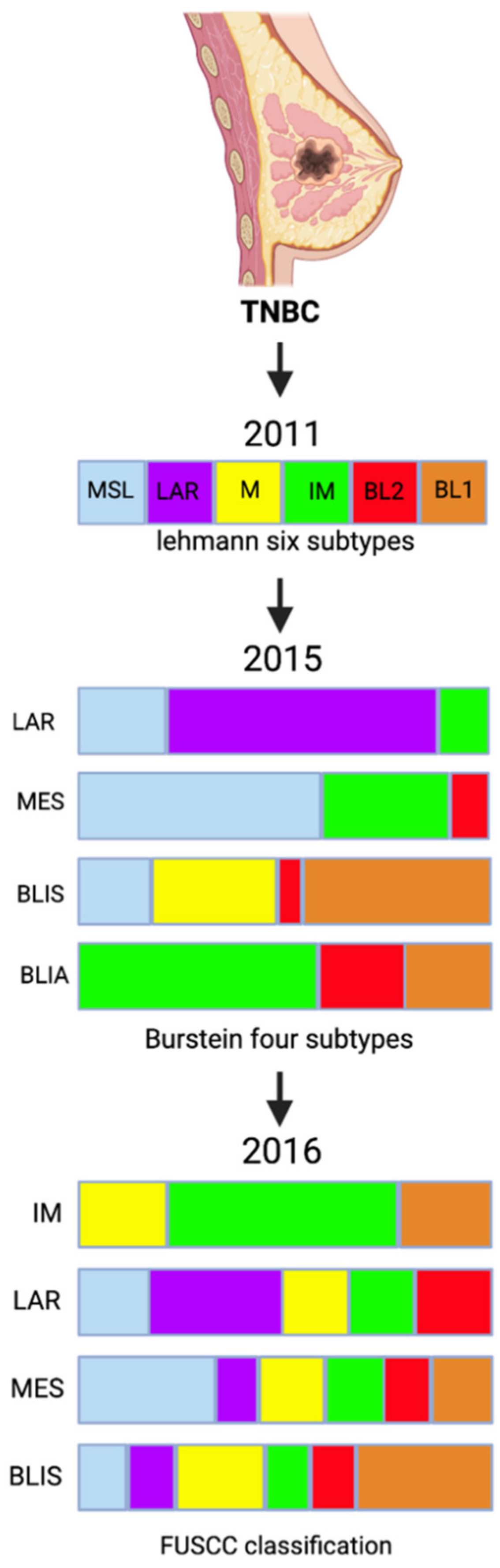
| FUSCC Classification | Pathways | |
|---|---|---|
| IM (immunomodulatory) | ↑ |
|
| LAR (luminal androgen receptor) | ↑ |
|
| MES (mesenchymal-like) | ↑ |
|
| BLIS (basal-like and immune suppressed) | ↑ |
|
| ↓ |
| |
| Trials (References) | Regimen 1 (R1) | Nb. of Patients | Regimen 2 (R2) | Nb of Patients | pCR Rate (R1 vs. R2) | p-Value |
|---|---|---|---|---|---|---|
| GeparOcto GBG84 [30] | P + NPLD + Cb; q1w for 18 weeks | 203 | E then P then C; q2w/3 cycles over 18 weeks | 200 | 51.7% vs. 48.5% | 0.518 |
| GALGB40603 Alliance [24] | (P q1w for 12 weeks then ddAC q2w/4 cycles) + (Cb q3w/4 cycles ± Bev. q2w/9cycles) | 221 | P q1w for 12 weeks then ddAC q2w/4 cycle | 212 | 54% vs. 41% | 0.0029 |
| GeparSixto GBG66 [26] | (P q1w for 18 weeks + NPLD q1w for 18 weeks + Bev. q3w/6 cycles) + Cb q1w for 18 weeks | 158 | P q1w for 18 weeks + NPLD q1w for 18 weeks + Bev. q3w/6 cycles | 157 | 53.2% vs. 36.9% | 0.005 |
| Zhang et al. [31] | P + Cb q3w/4–6 cycles | 47 | P + E q3w/4–6 cycles | 44 | 38.6% vs. 14.0% | 0.014 |
| Ando et al. [32] | (P q2w/2 cycles then CEF q2w/4 cycles) + Cb q3w/4 cycles | 37 | P q2w/2 cycles then CEF q2w/4 cycles | 38 | 61.2% vs. 6.3% | 0.003 |
| Trials (References) | Drug & Approval Date | Indication/Inclusion Criteria | Dosage |
|---|---|---|---|
| OlympiA [72] | Olaparib November 2021 |
| 300 mg PO cycled q28days/1 year twice daily ± food |
| KEYNOTE-522 [64] | Pembrolizumab + Chemotherapy July 2021 |
| 200 mg IV q21 days/8 cycles + chemotherapy pre-operatively followed by 200 mg IV q21 days/9 cycles as single agent post-operatively |
| IMMU-132-01 [77] | Sacituzumab govitecan-hziy April 2021 |
| 10 mg/kg on days 1 & 8 q21 days IV until disease progression/unacceptable adverse events |
| IMpassion130 [52] | Atezolizumab + nab-paclitaxel April 2019 |
| Atezolizumab 840 mg IV day 1 & 15 + 100 mg/m2 on day 1, 8 & 15 nab-paclitaxel q28 days/6 cycle or until disease progression/unacceptable adverse events |
| Regimen | Summary | Primary Endpoint |
|---|---|---|
| Antibody Drug Conjugates | ||
| Sacituzumab Govitecan (SG) [44] |
| ASCENT: Improved median PFS with SG (4.8 months) compared to SOC chemotherapy (1.7 months) (p < 0.001) |
| Immune-checkpoint inhibitors | ||
| Atezolizumab [46,50,51,52,53,54,55,56,66] |
| IMpassion130: 9.5 months increase in OS with Atezolizumab + nP compared to Placebo + nP (p = 0.08) IMpassion131: no significantly improved in PFS with Atezolizumab + paclitaxel without nab vs. placebo + paclitaxel (p = 0.20) IMpassion031: improved pCR with Atezolizumab + chemotherapy vs. placebo + chemotherapy (Rate difference: 17%) (p = 0·0044) |
| Pembrolizumab [60,61,64] |
| Keynote-355: Prolonged PFS with pembrolizumab + chemotherapy (9.7 months) vs. chemotherapy alone (5.6 months) (p = 0.0012) Keynote-522: Better pCR with Pembrolizumab + chemotherapy (64.8%) compared to placebo + chemotherapy (51.2%) (p < 0.001) TAPUR: Stable disease for ≥16 weeks was achieved in 37% with Pembrolizumab monotherapy in high-tumor burden disease |
| Poly Adenosine diphosphate-ribose PARP inhibitors | ||
| Olaparib [69,70,71,75] |
| OlympiAD: Prolonged PFS with Olaparib (7.0 months) compared to standard treatment (4.2 months) (p < 0.001) TBCRC 048: Significant Objective Response Rate (ORR) seen with germline PABL2 (ORR: 82%) and somatic BRCA1/2 (ORR: 50%) Olympia: Improved 3-year invasive DFS with olaparib (85.9%) as compared to placebo (77.1%) (p < 0.001) |
| Talazoparib [73,91] |
| EMBRACA: Prolonged median PFS and higher ORR with Talazoparib (PFS: 8.6 months; ORR: 62.6%) compared to SOC chemotherapy (5.6 months; ORR: 27.2%) (p < 0.001) |
| Veliparib [79] |
| SWOG S1416: Improved PFS with Veliparib (5.7 months) compared to placebo (4.3 months) (p = 0.023) in BRCA-like mutation group, but not germline mutated BRCA group |
| Androgen receptor targeted agents | ||
| Bicalutamide [86] |
| Multicenter phase II trial: CBR of 19% (95% CI: 7%–39%) and PFS of 12 weeks (95% CI: 11–22 weeks) were observed |
| Abiraterone [85,86,87] |
| Multicenter phase II trial: Primary endpoint of 25% CBR 6 months not met |
| Enzalutamide [88,89,90] |
| Multicenter phase II trial: assessment of CBR at 16 weeks showed 25% of the intention to treat group and 33% of the evaluable patients |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bou Zerdan, M.; Ghorayeb, T.; Saliba, F.; Allam, S.; Bou Zerdan, M.; Yaghi, M.; Bilani, N.; Jaafar, R.; Nahleh, Z. Triple Negative Breast Cancer: Updates on Classification and Treatment in 2021. Cancers 2022, 14, 1253. https://doi.org/10.3390/cancers14051253
Bou Zerdan M, Ghorayeb T, Saliba F, Allam S, Bou Zerdan M, Yaghi M, Bilani N, Jaafar R, Nahleh Z. Triple Negative Breast Cancer: Updates on Classification and Treatment in 2021. Cancers. 2022; 14(5):1253. https://doi.org/10.3390/cancers14051253
Chicago/Turabian StyleBou Zerdan, Maroun, Tala Ghorayeb, Fares Saliba, Sabine Allam, Morgan Bou Zerdan, Marita Yaghi, Nadeem Bilani, Rola Jaafar, and Zeina Nahleh. 2022. "Triple Negative Breast Cancer: Updates on Classification and Treatment in 2021" Cancers 14, no. 5: 1253. https://doi.org/10.3390/cancers14051253
APA StyleBou Zerdan, M., Ghorayeb, T., Saliba, F., Allam, S., Bou Zerdan, M., Yaghi, M., Bilani, N., Jaafar, R., & Nahleh, Z. (2022). Triple Negative Breast Cancer: Updates on Classification and Treatment in 2021. Cancers, 14(5), 1253. https://doi.org/10.3390/cancers14051253






