Minerals and Cancer: Overview of the Possible Diagnostic Value
Abstract
:Simple Summary
Abstract
1. Introduction
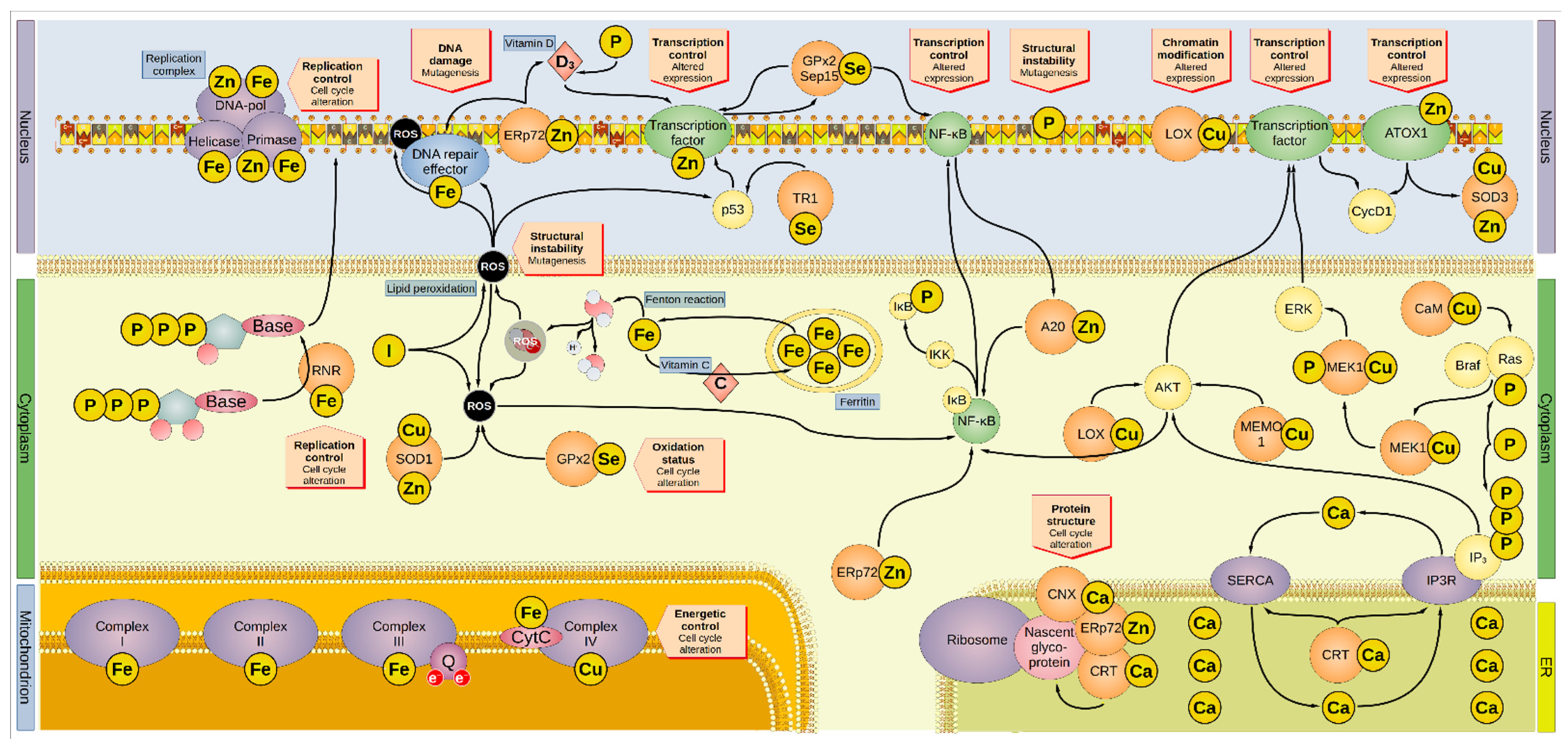
2. Copper and Zinc
3. Selenium
4. Phosphorus
5. Iron and Iodine
6. Calcium
7. Discussion
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 4E-BP1 | eukaryotic translation initiation factor 4E binding protein 1 |
| A20 | zinc finger protein A20 |
| AKT | Ak strain transforming |
| APC | adenomatous polyposis coli |
| AR | average requirement |
| AscH− | ascorbate |
| ATP7A | ATPase copper transporter 7A |
| ATOX1 | antioxidant-1 |
| Braf | rapidly accelerated fibrosarcoma isoform B |
| CaM | calmodulin |
| CI | confidence interval |
| CNX | calnexin |
| Cr | correlation |
| CRC | colorectal carcinoma |
| CRT | calreticulin |
| CytC | cytochrome c |
| DMT-1 | divalent metal transporter 1 |
| DNA-pol | DNA polymerase |
| EAR | estimated average requirement |
| ER | endoplasmic reticulum |
| Erp72 | endoplasmic reticulum resident protein 72 |
| ERK | extracellular signal-regulated kinase |
| GPx | glutathione peroxidase |
| HCC | hepatocellular carcinoma |
| HIF-lα | hypoxia-inducible factor 1α |
| HR | hazard ratio |
| IκB | NF-κB inhibitor |
| IKK | IκB kinase |
| IP3 | inositol 1,4,5-trisphosphate |
| IP3R | inositol trisphosphate receptor |
| K-Ras | Kirsten rat sarcoma |
| LOX | lysyl oxidase |
| MAPK | mitogen-activated protein kinase |
| MAPKK | mitogen-activated protein kinase kinase |
| MD | Menkes disease |
| MEK | MAPK/ERK kinase |
| MEMO1 | mediator of cell motility 1 |
| mTORC1 | mammalian target of rapamycin complex 1 |
| MURR1 | mouse U2af1-rs1 region 1 |
| NADH | nicotinamide adenine dinucleotide hydride |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| OR | odds ratio |
| OS | overall survival |
| PI3K | phosphoinositide 3-kinase |
| PRI | population reference intake |
| ppm | parts per million |
| Q | coenzyme Q |
| Qt | quantitative comparison of levels between groups |
| Raf | rapidly accelerated fibrosarcoma |
| Ras | rat sarcoma virus |
| RDA | recommended daily allowance |
| RNR | ribonucleotide reductase |
| ROS | reactive oxygen species |
| RR | relative risk |
| Sec | selenocysteine |
| SECIS | Sec insertion sequence |
| Sep15 | selenoprotein of 15-kDa |
| SERCA | sarco/endoplasmic reticulum calcium ATPase |
| SOD | superoxide dismutase |
| SPARC | secreted protein acidic and rich in cysteine |
| TR1 | thioredoxin reductase 1 |
| UL | tolerable upper intake level |
| ULK | Unc-51-like autophagy activating kinase |
| Wnt | wingless/int-1 |
| XIAP | X-linked inhibitor of apoptosis |
References
- Swinburn, B.; Sacks, G.; Ravussin, E. Increased Food Energy Supply Is More than Sufficient to Explain the US Epidemic of Obesity. Am. J. Clin. Nutr. 2009, 90, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The Global Obesity Pandemic: Shaped by Global Drivers and Local Environments. Lancet Lond. Engl. 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Thomas, D. A Study on the Mineral Depletion of the Foods Available to Us as a Nation over the Period 1940 to 1991. Nutr. Health 2003, 17, 85–115. [Google Scholar] [CrossRef]
- Thomas, D. The Mineral Depletion of Foods Available to Us as a Nation (1940–2002)—A Review of the 6th Edition of McCance and Widdowson. Nutr. Health 2007, 19, 21–55. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.-M.B.; Trenchard, L.; Rayns, F. Historical Changes in the Mineral Content of Fruit and Vegetables in the UK from 1940 to 2019: A Concern for Human Nutrition and Agriculture. Int. J. Food Sci. Nutr. 2021, 1–12. [Google Scholar] [CrossRef]
- Muthayya, S.; Rah, J.H.; Sugimoto, J.D.; Roos, F.F.; Kraemer, K.; Black, R.E. The Global Hidden Hunger Indices and Maps: An Advocacy Tool for Action. PLoS ONE 2013, 8, e67860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesalski, H.K.; Tinz, J. Multivitamin/Mineral Supplements: Rationale and Safety—A Systematic Review. Nutrition 2017, 33, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Shenkin, A. Micronutrients in Health and Disease. Postgrad. Med. J. 2006, 82, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Feeding the Immune System. Proc. Nutr. Soc. 2013, 72, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-Boosting Role of Vitamins D, C, E, Zinc, Selenium and Omega-3 Fatty Acids: Could They Help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The Concept of Immune Surveillance against Tumors. The First Theories. Oncotarget 2017, 8, 7175–7180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority (EFSA). Summary of Tolerable Upper Intake Levels—Version 4. Overview on Tolerable Upper Intake Levels as Derived by the Scientific Committee on Food (SCF) and the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). 2018; p. 2. Available online: https://www.efsa.europa.eu/sites/default/files/assets/UL_Summary_tables.pdf (accessed on 21 February 2022).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Iron. EFSA J. 2014, 13, 4254. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Summary of Dietary Reference Values—Version 4. Overview on Dietary Reference Values for the EU Population as Derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). 2017; p. 5. Available online: https://www.efsa.europa.eu/sites/default/files/assets/DRV_Summary_tables_jan_17.pdf (accessed on 21 February 2022).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef] [Green Version]
- Yokokawa, H.; Fukuda, H.; Saita, M.; Miyagami, T.; Takahashi, Y.; Hisaoka, T.; Naito, T. Serum zinc concentrations and characteristics of zinc deficiency/marginal deficiency among Japanese subjects. J. Gen. Fam. Med. 2020, 21, 248–255. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Phosphorus. EFSA J. 2014, 13, 4185. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Calcium. EFSA J. 2015, 13, 4101. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Copper. EFSA J. 2015, 13, 4253. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Iodine. EFSA J. 2014, 12, 3660. [Google Scholar] [CrossRef] [Green Version]
- Blockhuys, S.; Wittung-Stafshede, P. Roles of Copper-Binding Proteins in Breast Cancer. Int. J. Mol. Sci. 2017, 18, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y. Copper Homeostasis: Emerging Target for Cancer Treatment. IUBMB Life 2020, 72, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.D.; Dameron, C.T. Molecular Mechanisms of Copper Metabolism and the Role of the Menkes Disease Protein. J. Biochem. Mol. Toxicol. 1999, 13, 93–106. [Google Scholar] [CrossRef]
- Michalczyk, K.; Cymbaluk-Płoska, A. The Role of Zinc and Copper in Gynecological Malignancies. Nutrients 2020, 12, 3732. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cheng, J.; Zheng, N.; Zhang, X.; Dai, X.; Zhang, L.; Hu, C.; Wu, X.; Jiang, Q.; Wu, D.; et al. Copper Promotes Tumorigenesis by Activating the PDK1-AKT Oncogenic Pathway in a Copper Transporter 1 Dependent Manner. Adv. Sci. 2021, 8, e2004303. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.; Mumper, R.J. Elevated Copper and Oxidative Stress in Cancer Cells as a Target for Cancer Treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.A.; de Luca, A.; Squitti, R.; Rongioletti, M.; Rossi, L.; Machado, C.M.L.; Cerchiaro, G. Copper in tumors and the use of copper-based compounds in cancer treatment. J. Inorg. Biochem. 2022, 226, 111634. [Google Scholar] [CrossRef]
- Stepien, M.; Jenab, M.; Freisling, H.; Becker, N.-P.; Czuban, M.; Tjønneland, A.; Olsen, A.; Overvad, K.; Boutron-Ruault, M.-C.; Mancini, F.R.; et al. Pre-Diagnostic Copper and Zinc Biomarkers and Colorectal Cancer Risk in the European Prospective Investigation into Cancer and Nutrition Cohort. Carcinogenesis 2017, 38, 699–707. [Google Scholar] [CrossRef]
- Gupta, S.K.; Singh, S.P.; Shukla, V.K. Copper, Zinc, and Cu/Zn Ratio in Carcinoma of the Gallbladder. J. Surg. Oncol. 2005, 91, 204–208. [Google Scholar] [CrossRef]
- Dìez, M.; Cerdàn, F.J.; Arroyo, M.; Balibrea, J.L. Use of the Copper/Zinc Ratio in the Diagnosis of Lung Cancer. Cancer 1989, 63, 726–730. [Google Scholar] [CrossRef]
- Feng, Y.; Zeng, J.-W.; Ma, Q.; Zhang, S.; Tang, J.; Feng, J.-F. Serum Copper and Zinc Levels in Breast Cancer: A Meta-Analysis. J. Trace Elem. Med. Biol. Organ. Soc. Miner. Trace Elem. GMS 2020, 62, 126629. [Google Scholar] [CrossRef]
- Atakul, T.; Altinkaya, S.O.; Abas, B.I.; Yenisey, C. Serum Copper and Zinc Levels in Patients with Endometrial Cancer. Biol. Trace Elem. Res. 2020, 195, 46–54. [Google Scholar] [CrossRef]
- Naidu, M.S.K.; Suryakar, A.N.; Swami, S.C.; Katkam, R.V.; Kumbar, K.M. Oxidative Stress and Antioxidant Status in Cervical Cancer Patients. Indian J. Clin. Biochem. 2007, 22, 140–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burstein, E.; Ganesh, L.; Dick, R.D.; van De Sluis, B.; Wilkinson, J.C.; Klomp, L.W.J.; Wijmenga, C.; Brewer, G.J.; Nabel, G.J.; Duckett, C.S. A Novel Role for XIAP in Copper Homeostasis through Regulation of MURR1. EMBO J. 2004, 23, 244–254. [Google Scholar] [CrossRef] [Green Version]
- van De Sluis, B.; Rothuizen, J.; Pearson, P.L.; van Oost, B.A.; Wijmenga, C. Identification of a New Copper Metabolism Gene by Positional Cloning in a Purebred Dog Population. Hum. Mol. Genet. 2002, 11, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Blockhuys, S.; Celauro, E.; Hildesjö, C.; Feizi, A.; Stål, O.; Fierro-González, J.C.; Wittung-Stafshede, P. Defining the Human Copper Proteome and Analysis of Its Expression Variation in Cancers. Met. Integr. Biometal Sci. 2017, 9, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigiracciolo, D.C.; Scarpelli, A.; Lappano, R.; Pisano, A.; Santolla, M.F.; De Marco, P.; Cirillo, F.; Cappello, A.R.; Dolce, V.; Belfiore, A.; et al. Copper Activates HIF-1α/GPER/VEGF Signalling in Cancer Cells. Oncotarget 2015, 6, 34158–34177. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Ge, G. Lysyl Oxidase, Extracellular Matrix Remodeling and Cancer Metastasis. Cancer Microenviron. Off. J. Int. Cancer Microenviron. Soc. 2012, 5, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [Green Version]
- Arnold, S.A.; Brekken, R.A. SPARC: A Matricellular Regulator of Tumorigenesis. J. Cell Commun. Signal. 2009, 3, 255–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorokin, A.V.; Chen, J. MEMO1, a New IRS1-Interacting Protein, Induces Epithelial-Mesenchymal Transition in Mammary Epithelial Cells. Oncogene 2013, 32, 3130–3138. [Google Scholar] [CrossRef] [PubMed]
- Iturbide, A.; de Herreros, A.G.; Peiró, S. A New Role for LOX and LOXL2 Proteins in Transcription Regulation. FEBS J. 2015, 282, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.C.; Crowe, M.S.; Turski, M.L.; Hobbs, G.A.; Yao, X.; Chaikuad, A.; Knapp, S.; Xiao, K.; Campbell, S.L.; Thiele, D.J.; et al. Copper Is Required for Oncogenic BRAF Signalling and Tumorigenesis. Nature 2014, 509, 492–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemieux, E.; Bergeron, S.; Durand, V.; Asselin, C.; Saucier, C.; Rivard, N. Constitutively Active MEK1 Is Sufficient to Induce Epithelial-to-Mesenchymal Transition in Intestinal Epithelial Cells and to Promote Tumor Invasion and Metastasis. Int. J. Cancer 2009, 125, 1575–1586. [Google Scholar] [CrossRef]
- Turski, M.L.; Brady, D.C.; Kim, H.J.; Kim, B.-E.; Nose, Y.; Counter, C.M.; Winge, D.R.; Thiele, D.J. A Novel Role for Copper in Ras/Mitogen-Activated Protein Kinase Signaling. Mol. Cell. Biol. 2012, 32, 1284–1295. [Google Scholar] [CrossRef] [Green Version]
- Kunz, M.; Vera, J. Modelling of Protein Kinase Signaling Pathways in Melanoma and Other Cancers. Cancers 2019, 11, 465. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.A.; Assoian, R.K. Transcriptional Regulation of the Cyclin D1 Gene at a Glance. J. Cell Sci. 2008, 121, 3853–3857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, S.; Ozumi, K.; Kim, H.W.; Nakagawa, O.; McKinney, R.D.; Folz, R.J.; Zelko, I.N.; Ushio-Fukai, M.; Fukai, T. Novel Mechanism for Regulation of Extracellular SOD Transcription and Activity by Copper: Role of Antioxidant-1. Free Radic. Biol. Med. 2009, 46, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Ozumi, K.; Sudhahar, V.; Kim, H.W.; Chen, G.-F.; Kohno, T.; Finney, L.; Vogt, S.; McKinney, R.D.; Ushio-Fukai, M.; Fukai, T. Role of Copper Transport Protein Antioxidant 1 in Angiotensin II-Induced Hypertension: A Key Regulator of Extracellular Superoxide Dismutase. Hypertension 2012, 60, 476–486. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lee, J.; Xie, C. Autophagy Regulation on Cancer Stem Cell Maintenance, Metastasis, and Therapy Resistance. Cancers 2022, 14, 381. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Interactions between Reactive Oxygen Species and Autophagy: Special Issue: Death Mechanisms in Cellular Homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119041. [Google Scholar] [CrossRef]
- Tsang, T.; Posimo, J.M.; Gudiel, A.A.; Cicchini, M.; Feldser, D.M.; Brady, D.C. Copper Is an Essential Regulator of the Autophagic Kinases ULK1/2 to Drive Lung Adenocarcinoma. Nat. Cell Biol. 2020, 22, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Zischka, H.; Kroemer, G. Copper—A Novel Stimulator of Autophagy. Cell Stress 2020, 4, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Evanics, F.; Maurmann, L.; Yang, W.W.; Bose, R.N. Nuclear Magnetic Resonance Structures of the Zinc Finger Domain of Human DNA Polymerase-Alpha. Biochim. Biophys. Acta 2003, 1651, 163–171. [Google Scholar] [CrossRef]
- Baranovskiy, A.G.; Zhang, Y.; Suwa, Y.; Babayeva, N.D.; Gu, J.; Pavlov, Y.I.; Tahirov, T.H. Crystal Structure of the Human Primase. J. Biol. Chem. 2015, 290, 5635–5646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [Green Version]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and Anti-Inflammatory Effects of Zinc. Zinc-Dependent NF-ΚB Signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, R.; Baltimore, D. Multiple Nuclear Factors Interact with the Immunoglobulin Enhancer Sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-ΚB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, P.S.; Shu, Y.; Bi, W.X.; Zheng, X.B.; Feng, W.J.; He, L.Y.; Li, J.S. Emerging Role of Zinc Finger Protein A20 as a Suppressor of Hepatocellular Carcinoma. J. Cell. Physiol. 2019, 234, 21479–21484. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc: Mechanisms of Host Defense. J. Nutr. 2007, 137, 1345–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide Dismutase Multigene Family: A Comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) Gene Structures, Evolution, and Expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [Green Version]
- Laskey, J.; Webb, I.; Schulman, H.M.; Ponka, P. Evidence That Transferrin Supports Cell Proliferation by Supplying Iron for DNA Synthesis. Exp. Cell Res. 1988, 176, 87–95. [Google Scholar] [CrossRef]
- Radi, R. Oxygen Radicals, Nitric Oxide, and Peroxynitrite: Redox Pathways in Molecular Medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, Ł.; Kepinska, M.; Milnerowicz, H. The Copper-Zinc Superoxide Dismutase Activity in Selected Diseases. Eur. J. Clin. Investig. 2019, 49, e13036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren, O.; Tako, E. Chronic Dietary Zinc Deficiency Alters Gut Microbiota Composition and Function. Proceedings 2020, 61, 16. [Google Scholar]
- Collins, D.; Hogan, A.M.; Winter, D.C. Microbial and Viral Pathogens in Colorectal Cancer. Lancet Oncol. 2011, 12, 504–512. [Google Scholar] [CrossRef]
- Rusch, P.; Hirner, A.V.; Schmitz, O.; Kimmig, R.; Hoffmann, O.; Diel, M. Zinc Distribution within Breast Cancer Tissue of Different Intrinsic Subtypes. Arch. Gynecol. Obstet. 2021, 303, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Secchi, D.G.; Aballay, L.R.; Galíndez, M.F.; Piccini, D.; Lanfranchi, H.; Brunotto, M. Red Meat, Micronutrients and Oral Squamous Cell Carcinoma of Argetine Adult Patients. Nutr. Hosp. 2015, 32, 1214–1221. [Google Scholar]
- Fang, A.-P.; Chen, P.-Y.; Wang, X.-Y.; Liu, Z.-Y.; Zhang, D.-M.; Luo, Y.; Liao, G.-C.; Long, J.-A.; Zhong, R.-H.; Zhou, Z.-G.; et al. Serum Copper and Zinc Levels at Diagnosis and Hepatocellular Carcinoma Survival in the Guangdong Liver Cancer Cohort. Int. J. Cancer 2019, 144, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Elango, S.; Samuel, S.; Khashim, Z.; Subbiah, U. Selenium Influences Trace Elements Homeostasis, Cancer Biomarkers in Squamous Cell Carcinoma Patients Administered with Cancerocidal Radiotherapy. Asian Pac. J. Cancer Prev. 2018, 19, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Lener, M.R.; Scott, R.J.; Wiechowska-Kozłowska, A.; Serrano-Fernández, P.; Baszuk, P.; Jaworska-Bieniek, K.; Sukiennicki, G.; Marciniak, W.; Muszyńska, M.; Kładny, J.; et al. Serum Concentrations of Selenium and Copper in Patients Diagnosed with Pancreatic Cancer. Cancer Res. Treat. 2016, 48, 1056–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wandzilak, A.; Czyzycki, M.; Radwanska, E.; Adamek, D.; Geraki, K.; Lankosz, M. X-ray Fluorescence Study of the Concentration of Selected Trace and Minor Elements in Human Brain Tumours. Spectrochim. Acta Part. B At. Spectrosc. 2015, 114, 52–57. [Google Scholar] [CrossRef]
- Dehnhardt, M.; Zoriy, M.V.; Khan, Z.; Reifenberger, G.; Ekström, T.J.; Sabine Becker, J.; Zilles, K.; Bauer, A. Element Distribution Is Altered in a Zone Surrounding Human Glioblastoma Multiforme. J. Trace Elem. Med. Biol. Organ. Soc. Miner. Trace Elem. GMS 2008, 22, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Turecký, L.; Kalina, P.; Uhlíková, E.; Námerová, S.; Krizko, J. Serum Ceruloplasmin and Copper Levels in Patients with Primary Brain Tumors. Klin. Wochenschr. 1984, 62, 187–189. [Google Scholar] [CrossRef]
- Tamai, Y.; Iwasa, M.; Eguchi, A.; Shigefuku, R.; Sugimoto, K.; Hasegawa, H.; Takei, Y. Serum Copper, Zinc and Metallothionein Serve as Potential Biomarkers for Hepatocellular Carcinoma. PLoS ONE 2020, 15, e0237370. [Google Scholar] [CrossRef]
- Schwarz, K.; Foltz, C.M. Factor 3 Activity of Selenium Compounds. J. Biol. Chem. 1958, 233, 245–251. [Google Scholar] [CrossRef]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium Stimulates the Antitumour Immunity: Insights to Future Research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Selenius, M.; Rundlöf, A.-K.; Olm, E.; Fernandes, A.P.; Björnstedt, M. Selenium and the Selenoprotein Thioredoxin Reductase in the Prevention, Treatment and Diagnostics of Cancer. Antioxid. Redox Signal. 2010, 12, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H.E. Selenium Deficiency Risk Predicted to Increase under Future Climate Change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef] [Green Version]
- Vinceti, M.; Filippini, T.; Wise, L.A. Environmental Selenium and Human Health: An Update. Curr. Environ. Health Rep. 2018, 5, 464–485. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-Chain Selenium and Human Health: Emphasis on Intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients 2021, 13, 1649. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.S.; Lei, X.G. Selenium. Adv. Nutr. 2016, 7, 415–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of Mammalian Selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, M.; Schweizer, U. Unveiling the Molecular Mechanisms behind Selenium-Related Diseases through Knockout Mouse Studies. Antioxid. Redox Signal. 2010, 12, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and Selenocysteine: Roles in Cancer, Health, and Development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Holmgren, A. The Thioredoxin Antioxidant System. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Urig, S.; Becker, K. On the Potential of Thioredoxin Reductase Inhibitors for Cancer Therapy. Semin. Cancer Biol. 2006, 16, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Yu, S.; Zhao, G. Recent Advances in the Development of Thioredoxin Reductase Inhibitors as Anticancer Agents. Curr. Drug Targets 2012, 13, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Kasaikina, M.V.; Fomenko, D.E.; Labunskyy, V.M.; Lachke, S.A.; Qiu, W.; Moncaster, J.A.; Zhang, J.; Wojnarowicz, M.W.J.; Natarajan, S.K.; Malinouski, M.; et al. Roles of the 15-KDa Selenoprotein (Sep15) in Redox Homeostasis and Cataract Development Revealed by the Analysis of Sep 15 Knockout Mice. J. Biol. Chem. 2011, 286, 33203–33212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonska, E.; Gromadzinska, J.; Sobala, W.; Reszka, E.; Wasowicz, W. Lung Cancer Risk Associated with Selenium Status Is Modified in Smoking Individuals by Sep15 Polymorphism. Eur. J. Nutr. 2008, 47, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Irons, R.; Tsuji, P.A.; Carlson, B.A.; Ouyang, P.; Yoo, M.-H.; Xu, X.-M.; Hatfield, D.L.; Gladyshev, V.N.; Davis, C.D. Deficiency in the 15-KDa Selenoprotein Inhibits Tumorigenicity and Metastasis of Colon Cancer Cells. Cancer Prev. Res. 2010, 3, 630–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kipp, A.P.; Müller, M.F.; Göken, E.M.; Deubel, S.; Brigelius-Flohé, R. The Selenoproteins GPx2, TrxR2 and TrxR3 Are Regulated by Wnt Signalling in the Intestinal Epithelium. Biochim. Biophys. Acta 2012, 1820, 1588–1596. [Google Scholar] [CrossRef]
- Kipp, A.P. Selenium in Colorectal and Differentiated Thyroid Cancer. Horm. Athens Greece 2020, 19, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Coates, R.J.; Weiss, N.S.; Daling, J.R.; Morris, J.S.; Labbe, R.F. Serum Levels of Selenium and Retinol and the Subsequent Risk of Cancer. Am. J. Epidemiol. 1988, 128, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Brodin, O.; Hackler, J.; Misra, S.; Wendt, S.; Sun, Q.; Laaf, E.; Stoppe, C.; Björnstedt, M.; Schomburg, L. Selenoprotein P as Biomarker of Selenium Status in Clinical Trials with Therapeutic Dosages of Selenite. Nutrients 2020, 12, 1067. [Google Scholar] [CrossRef] [Green Version]
- Callejón-Leblic, B.; Rodríguez-Moro, G.; Arias-Borrego, A.; Pereira-Vega, A.; Gómez-Ariza, J.L.; García-Barrera, T. Absolute Quantification of Selenoproteins and Selenometabolites in Lung Cancer Human Serum by Column Switching Coupled to Triple Quadrupole Inductively Coupled Plasma Mass Spectrometry. J. Chromatogr. A 2020, 1619, 460919. [Google Scholar] [CrossRef]
- Pietrzak, S.; Wójcik, J.; Scott, R.J.; Kashyap, A.; Grodzki, T.; Baszuk, P.; Bielewicz, M.; Marciniak, W.; Wójcik, N.; Dębniak, T.; et al. Influence of the Selenium Level on Overall Survival in Lung Cancer. J. Trace Elem. Med. Biol. Organ. Soc. Miner. Trace Elem. GMS 2019, 56, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-M.; Lin, Y.-C.; Huang, Y.-L.; Shiue, H.-S.; Pu, Y.-S.; Huang, C.-Y.; Chung, C.-J. Effect of Plasma Selenium, Red Blood Cell Cadmium, Total Urinary Arsenic Levels, and EGFR on Renal Cell Carcinoma. Sci. Total Environ. 2021, 750, 141547. [Google Scholar] [CrossRef]
- Çelen, İ.; Müezzinoğlu, T.; Ataman, O.Y.; Bakırdere, S.; Korkmaz, M.; Neşe, N.; Şenol, F.; Lekili, M. Selenium, Nickel, and Calcium Levels in Cancerous and Non-Cancerous Prostate Tissue Samples and Their Relation with Some Parameters. Environ. Sci. Pollut. Res. Int. 2015, 22, 13070–13076. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.K.; Franke, A.A.; Steven Morris, J.; Cooney, R.V.; Wilkens, L.R.; Le Marchand, L.; Goodman, M.T.; Henderson, B.E.; Kolonel, L.N. Association of Selenium, Tocopherols, Carotenoids, Retinol, and 15-Isoprostane F(2t) in Serum or Urine with Prostate Cancer Risk: The Multiethnic Cohort. Cancer Causes Control 2009, 20, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.-W.; Bae, S.-M.; Kim, Y.-W.; Liu, H.-B.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; Chaturvedi, P.K.; Battogtokh, G.; Ahn, W.S. Serum Selenium Levels in Korean Hepatoma Patients. Biol. Trace Elem. Res. 2012, 148, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.J.; Fedirko, V.; Jenab, M.; Schomburg, L.; Méplan, C.; Freisling, H.; Bueno-de-Mesquita, H.B.; Hybsier, S.; Becker, N.-P.; Czuban, M.; et al. Selenium Status Is Associated with Colorectal Cancer Risk in the European Prospective Investigation of Cancer and Nutrition Cohort. Int. J. Cancer 2015, 136, 1149–1161. [Google Scholar] [CrossRef]
- Xie, B.; Lin, J.; Sui, K.; Huang, Z.; Chen, Z.; Hang, W. Differential Diagnosis of Multielements in Cancerous and Non-Cancerous Esophageal Tissues. Talanta 2019, 196, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Gül-Klein, S.; Haxhiraj, D.; Seelig, J.; Kästner, A.; Hackler, J.; Sun, Q.; Heller, R.A.; Lachmann, N.; Pratschke, J.; Schmelzle, M.; et al. Serum Selenium Status as a Diagnostic Marker for the Prognosis of Liver Transplantation. Nutrients 2021, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- Sandsveden, M.; Nilsson, E.; Borgquist, S.; Rosendahl, A.H.; Manjer, J. Prediagnostic Serum Selenium Levels in Relation to Breast Cancer Survival and Tumor Characteristics. Int. J. Cancer 2020, 147, 2424–2436. [Google Scholar] [CrossRef] [PubMed]
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Jakubowska, A.; Debniak, T.; Falco, M.; Kladny, J.; et al. Serum Selenium Levels Predict Survival after Breast Cancer. Breast Cancer Res. Treat. 2018, 167, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Berner, Y.N.; Shike, M. Consequences of Phosphate Imbalance. Annu. Rev. Nutr. 1988, 8, 121–148. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Tucker, K.L. Is Phosphorus Intake That Exceeds Dietary Requirements a Risk Factor in Bone Health? Ann. N. Y. Acad. Sci. 2013, 1301, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding Sources of Dietary Phosphorus in the Treatment of Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 519–530. [Google Scholar] [CrossRef]
- Takeda, E.; Yamamoto, H.; Yamanaka-Okumura, H.; Taketani, Y. Increasing Dietary Phosphorus Intake from Food Additives: Potential for Negative Impact on Bone Health. Adv. Nutr. 2014, 5, 92–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheard, P.R.; Nute, G.R.; Richardson, R.I.; Perry, A.; Taylor, A.A. Injection of Water and Polyphosphate into Pork to Improve Juiciness and Tenderness after Cooking. Meat Sci. 1999, 51, 371–376. [Google Scholar] [CrossRef]
- Shimada, M.; Shutto-Uchita, Y.; Yamabe, H. Lack of Awareness of Dietary Sources of Phosphorus Is a Clinical Concern. Vivo Athens Greece 2019, 33, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.J.B. Potential Health Concerns of Dietary Phosphorus: Cancer, Obesity, and Hypertension. Ann. N. Y. Acad. Sci. 2013, 1301, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S. Dietary Phosphorus, Calcium Metabolism and Bone. J. Nutr. 1993, 123, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Serna, J.; Bergwitz, C. Importance of Dietary Phosphorus for Bone Metabolism and Healthy Aging. Nutrients 2020, 12, 3001. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Leischner, C.; Helling, T.; Burkard, M.; Marongiu, L. Vitamins as Possible Cancer Biomarkers: Significance and Limitations. Nutrients 2021, 13, 3914. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Dietary Influences of 1,25(OH)2 Vitamin D in Relation to Prostate Cancer: A Hypothesis. Cancer Causes Control 1998, 9, 567–582. [Google Scholar] [CrossRef]
- Brown, R.B. Vitamin D, Cancer, and Dysregulated Phosphate Metabolism. Endocrine 2019, 65, 238–243. [Google Scholar] [CrossRef]
- Kapur, S. A Medical Hypothesis: Phosphorus Balance and Prostate Cancer. Cancer Investig. 2000, 18, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, M.; Hope, B.; Krause, L.; Morrison, M.; Protani, M.M.; Zakrzewski, M.; Neale, R.E. Vitamin D and the Gut Microbiome: A Systematic Review of In Vivo Studies. Eur. J. Nutr. 2019, 58, 2895–2910. [Google Scholar] [CrossRef] [PubMed]
- Papaloucas, C.D.; Papaloucas, M.D.; Kouloulias, V.; Neanidis, K.; Pistevou-Gompaki, K.; Kouvaris, J.; Zygogianni, A.; Mystakidou, K.; Papaloucas, A.C. Measurement of Blood Phosphorus: A Quick and Inexpensive Method for Detection of the Existence of Cancer in the Body. Too Good to Be True, or Forgotten Knowledge of the Past? Med. Hypotheses 2014, 82, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Kyle, M.M.; Smith, M.S.; Nagy, J.D. Biological Stoichiometry in Human Cancer. PLoS ONE 2007, 2, e1028. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Xu, C.-X.; Lim, H.-T.; Park, S.-J.; Shin, J.-Y.; Chung, Y.-S.; Park, S.-C.; Chang, S.-H.; Youn, H.-J.; Lee, K.-H.; et al. High Dietary Inorganic Phosphate Increases Lung Tumorigenesis and Alters Akt Signaling. Am. J. Respir. Crit. Care Med. 2009, 179, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.-H.; Yu, K.N.; Lee, Y.-S.; An, G.-H.; Beck, G.R.J.; Colburn, N.H.; Lee, K.-H.; Cho, M.-H. Elevated Inorganic Phosphate Stimulates Akt-ERK1/2-Mnk1 Signaling in Human Lung Cells. Am. J. Respir. Cell Mol. Biol. 2006, 35, 528–539. [Google Scholar] [CrossRef] [Green Version]
- Wargovich, M.J.; Eng, V.W.; Newmark, H.L. Calcium Inhibits the Damaging and Compensatory Proliferative Effects of Fatty Acids on Mouse Colon Epithelium. Cancer Lett. 1984, 23, 253–258. [Google Scholar] [CrossRef]
- Wargovich, M.J.; Eng, V.W.; Newmark, H.L.; Bruce, W.R. Calcium Ameliorates the Toxic Effect of Deoxycholic Acid on Colonic Epithelium. Carcinogenesis 1983, 4, 1205–1207. [Google Scholar] [CrossRef]
- Lipkin, M.; Newmark, H. Effect of Added Dietary Calcium on Colonic Epithelial-Cell Proliferation in Subjects at High Risk for Familial Colonic Cancer. N. Engl. J. Med. 1985, 313, 1381–1384. [Google Scholar] [CrossRef]
- Chan, J.M.; Pietinen, P.; Virtanen, M.; Malila, N.; Tangrea, J.; Albanes, D.; Virtamo, J. Diet and Prostate Cancer Risk in a Cohort of Smokers, with a Specific Focus on Calcium and Phosphorus (Finland). Cancer Causes Control 2000, 11, 859–867. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, C.; Hu, B.; Yuan, D.; Chen, W.; Wang, W.; Su, J.; Liu, Z.; Jiao, K.; Chen, X.; et al. Dietary Phosphorus Intake and Serum Prostate-Specific Antigen in Non-Prostate Cancer American Adults: A Secondary Analysis of the National Health and Nutrition Examination Survey (NHANES), 2003–2010. Asia Pac. J. Clin. Nutr. 2020, 29, 322–333. [Google Scholar] [CrossRef]
- Kesse, E.; Boutron-Ruault, M.-C.; Norat, T.; Riboli, E.; Clavel-Chapelon, F. Dietary Calcium, Phosphorus, Vitamin D, Dairy Products and the Risk of Colorectal Adenoma and Cancer among French Women of the E3N-EPIC Prospective Study. Int. J. Cancer 2005, 117, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkman, M.T.; Buntinx, F.; Kellen, E.; Dagnelie, P.C.; Van Dongen, M.C.J.M.; Muls, E.; Zeegers, M.P. Dietary Intake of Micronutrients and the Risk of Developing Bladder Cancer: Results from the Belgian Case-Control Study on Bladder Cancer Risk. Cancer Causes Control 2011, 22, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Tavani, A.; Bertuccio, P.; Bosetti, C.; Talamini, R.; Negri, E.; Franceschi, S.; Montella, M.; La Vecchia, C. Dietary Intake of Calcium, Vitamin D, Phosphorus and the Risk of Prostate Cancer. Eur. Urol. 2005, 48, 27–33. [Google Scholar] [CrossRef]
- Wilson, K.M.; Shui, I.M.; Mucci, L.A.; Giovannucci, E. Calcium and Phosphorus Intake and Prostate Cancer Risk: A 24-y Follow-up Study. Am. J. Clin. Nutr. 2015, 101, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Phipps, O.; Brookes, M.J.; Al-Hassi, H.O. Iron Deficiency, Immunology, and Colorectal Cancer. Nutr. Rev. 2021, 79, 88–97. [Google Scholar] [CrossRef]
- Xue, X.; Shah, Y.M. Intestinal Iron Homeostasis and Colon Tumorigenesis. Nutrients 2013, 5, 2333–2351. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Cherayil, B.J. Iron and Inflammation—The Gut Reaction. Met. Integr. Biometal Sci. 2017, 9, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Fonseca-Nunes, A.; Jakszyn, P.; Agudo, A. Iron and Cancer Risk--A Systematic Review and Meta-Analysis of the Epidemiological Evidence. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014, 23, 12–31. [Google Scholar] [CrossRef] [Green Version]
- Roughead, Z.K.F.; Zito, C.A.; Hunt, J.R. Inhibitory Effects of Dietary Calcium on the Initial Uptake and Subsequent Retention of Heme and Nonheme Iron in Humans: Comparisons Using an Intestinal Lavage Method. Am. J. Clin. Nutr. 2005, 82, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Pierre, F.; Taché, S.; Petit, C.R.; Van der Meer, R.; Corpet, D.E. Meat and Cancer: Haemoglobin and Haemin in a Low-Calcium Diet Promote Colorectal Carcinogenesis at the Aberrant Crypt Stage in Rats. Carcinogenesis 2003, 24, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Teucher, B.; Olivares, M.; Cori, H. Enhancers of Iron Absorption: Ascorbic Acid and Other Organic Acids. Int. J. Vitam. Nutr. Res. 2004, 74, 403–419. [Google Scholar] [CrossRef]
- Plays, M.; Müller, S.; Rodriguez, R. Chemistry and Biology of Ferritin. Met. Integr. Biometal Sci. 2021, 13, mfab021. [Google Scholar] [CrossRef] [PubMed]
- Paganoni, R.; Lechel, A.; Vujic Spasic, M. Iron at the Interface of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 4097. [Google Scholar] [CrossRef] [PubMed]
- Siegers, C.P.; Bumann, D.; Trepkau, H.D.; Schadwinkel, B.; Baretton, G. Influence of Dietary Iron Overload on Cell Proliferation and Intestinal Tumorigenesis in Mice. Cancer Lett. 1992, 65, 245–249. [Google Scholar] [CrossRef]
- Lund, E.K.; Wharf, S.G.; Fairweather-Tait, S.J.; Johnson, I.T. Increases in the Concentrations of Available Iron in Response to Dietary Iron Supplementation Are Associated with Changes in Crypt Cell Proliferation in Rat Large Intestine. J. Nutr. 1998, 128, 175–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perše, M. Oxidative Stress in the Pathogenesis of Colorectal Cancer: Cause or Consequence? BioMed Res. Int. 2013, 2013, 725710. [Google Scholar] [CrossRef] [Green Version]
- Vissers, M.C.M.; Das, A.B. Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Front. Physiol. 2018, 9, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, L.L.; Gomez-Cabrera, M.-C.; Vina, J. Role of Nuclear Factor KappaB and Mitogen-Activated Protein Kinase Signaling in Exercise-Induced Antioxidant Enzyme Adaptation. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2007, 32, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.F.; Goodyear, L.J. Exercise, MAPK, and NF-KappaB Signaling in Skeletal Muscle. J. Appl. Physiol. 2007, 103, 388–395. [Google Scholar] [CrossRef]
- Read, A.D.; Bentley, R.E.; Archer, S.L.; Dunham-Snary, K.J. Mitochondrial Iron-Sulfur Clusters: Structure, Function, and an Emerging Role in Vascular Biology. Redox Biol. 2021, 47, 102164. [Google Scholar] [CrossRef]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martínez-Pastor, M.T. The Elemental Role of Iron in DNA Synthesis and Repair. Met. Integr. Biometal Sci. 2017, 9, 1483–1500. [Google Scholar] [CrossRef] [Green Version]
- Eklund, H.; Uhlin, U.; Färnegårdh, M.; Logan, D.T.; Nordlund, P. Structure and Function of the Radical Enzyme Ribonucleotide Reductase. Prog. Biophys. Mol. Biol. 2001, 77, 177–268. [Google Scholar] [CrossRef]
- Ruskoski, T.B.; Boal, A.K. The Periodic Table of Ribonucleotide Reductases. J. Biol. Chem. 2021, 297, 101137. [Google Scholar] [CrossRef] [PubMed]
- Brookes, M.J.; Boult, J.; Roberts, K.; Cooper, B.T.; Hotchin, N.A.; Matthews, G.; Iqbal, T.; Tselepis, C. A Role for Iron in Wnt Signalling. Oncogene 2008, 27, 966–975. [Google Scholar] [CrossRef] [Green Version]
- Aceves, C.; Mendieta, I.; Anguiano, B.; Delgado-González, E. Molecular Iodine Has Extrathyroidal Effects as an Antioxidant, Differentiator, and Immunomodulator. Int. J. Mol. Sci. 2021, 22, 1228. [Google Scholar] [CrossRef] [PubMed]
- Smyth, P.P.A. Role of Iodine in Antioxidant Defence in Thyroid and Breast Disease. BioFactors 2003, 19, 121–130. [Google Scholar] [CrossRef]
- Zacharski, L.R.; Chow, B.K.; Howes, P.S.; Shamayeva, G.; Baron, J.A.; Dalman, R.L.; Malenka, D.J.; Ozaki, C.K.; Lavori, P.W. Decreased Cancer Risk after Iron Reduction in Patients with Peripheral Arterial Disease: Results from a Randomized Trial. J. Natl. Cancer Inst. 2008, 100, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.P.; Lee, J.H.; Tai, Y.-P.; Wen, C.; Wu, S.B.; Tsai, M.K.; Hsieh, D.P.H.; Chiang, H.-C.; Hsiung, C.A.; Hsu, C.Y.; et al. High Serum Iron Is Associated with Increased Cancer Risk. Cancer Res. 2014, 74, 6589–6597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Wang, C.; Chai, R.; Dong, Q.; Tu, S. Iron Intake, Serum Iron Indices and Risk of Colorectal Adenomas: A Meta-Analysis of Observational Studies. Eur. J. Cancer Care 2017, 26, e12486. [Google Scholar] [CrossRef]
- Chang, V.C.; Cotterchio, M.; Khoo, E. Iron Intake, Body Iron Status, and Risk of Breast Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2019, 19, 543. [Google Scholar] [CrossRef] [Green Version]
- Sawayama, H.; Iwatsuki, M.; Kuroda, D.; Toihata, T.; Uchihara, T.; Koga, Y.; Yagi, T.; Kiyozumi, Y.; Eto, T.; Hiyoshi, Y.; et al. Total Iron-Binding Capacity Is a Novel Prognostic Marker after Curative Gastrectomy for Gastric Cancer. Int. J. Clin. Oncol. 2018, 23, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Malya, F.U.; Kadioglu, H.; Hasbahceci, M.; Dolay, K.; Guzel, M.; Ersoy, Y.E. The Correlation between Breast Cancer and Urinary Iodine Excretion Levels. J. Int. Med. Res. 2018, 46, 687–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Holle, A.; O’Brien, K.M.; Sandler, D.P.; Janicek, R.; Weinberg, C.R. Association Between Serum Iron Biomarkers and Breast Cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2021, 30, 422–425. [Google Scholar] [CrossRef]
- Fan, S.; Li, X.; Zheng, L.; Hu, D.; Ren, X.; Ye, Z. Correlations between the Iodine Concentrations from Dual Energy Computed Tomography and Molecular Markers Ki-67 and HIF-1α in Rectal Cancer: A Preliminary Study. Eur. J. Radiol. 2017, 96, 109–114. [Google Scholar] [CrossRef]
- Hu, M.-J.; He, J.-L.; Tong, X.-R.; Yang, W.-J.; Zhao, H.-H.; Li, G.-A.; Huang, F. Associations between Essential Microelements Exposure and the Aggressive Clinicopathologic Characteristics of Papillary Thyroid Cancer. Biometals 2021, 34, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Bong, A.H.L.; Monteith, G.R. Calcium Signaling and the Therapeutic Targeting of Cancer Cells. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Calcium in Tumour Metastasis: New Roles for Known Actors. Nat. Rev. Cancer 2011, 11, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Groenendyk, J.; Michalak, M. Endoplasmic Reticulum Quality Control and Apoptosis. Acta Biochim. Pol. 2005, 52, 381–395. [Google Scholar] [CrossRef]
- Schrag, J.D.; Bergeron, J.J.; Li, Y.; Borisova, S.; Hahn, M.; Thomas, D.Y.; Cygler, M. The Structure of Calnexin, an ER Chaperone Involved in Quality Control of Protein Folding. Mol. Cell 2001, 8, 633–644. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol Trisphosphate and Calcium Signalling Mechanisms. Biochim. Biophys. Acta 2009, 1793, 933–940. [Google Scholar] [CrossRef] [Green Version]
- Chung, F.-Y.; Lin, S.-R.; Lu, C.-Y.; Yeh, C.-S.; Chen, F.-M.; Hsieh, J.-S.; Huang, T.-J.; Wang, J.-Y. Sarco/Endoplasmic Reticulum Calcium-ATPase 2 Expression as a Tumor Marker in Colorectal Cancer. Am. J. Surg. Pathol. 2006, 30, 969–974. [Google Scholar] [CrossRef]
- Papp, B.; Launay, S.; Gélébart, P.; Arbabian, A.; Enyedi, A.; Brouland, J.-P.; Carosella, E.D.; Adle-Biassette, H. Endoplasmic Reticulum Calcium Pumps and Tumor Cell Differentiation. Int. J. Mol. Sci. 2020, 21, 3351. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, G.; Azeroual, S.; Rosenauer, A.; Määttänen, P.; Denisov, A.Y.; Thomas, D.Y.; Gehring, K. Structure of the Catalytic a(0)a Fragment of the Protein Disulfide Isomerase ERp72. J. Mol. Biol. 2010, 401, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Turano, C.; Gaucci, E.; Grillo, C.; Chichiarelli, S. ERp57/GRP58: A Protein with Multiple Functions. Cell. Mol. Biol. Lett. 2011, 16, 539–563. [Google Scholar] [CrossRef] [PubMed]
- Abdelkarim, H.; Leschinsky, N.; Jang, H.; Banerjee, A.; Nussinov, R.; Gaponenko, V. The Dynamic Nature of the K-Ras/Calmodulin Complex Can Be Altered by Oncogenic Mutations. Curr. Opin. Struct. Biol. 2021, 71, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Villalobo, A.; Berchtold, M.W. The Role of Calmodulin in Tumor Cell Migration, Invasiveness, and Metastasis. Int. J. Mol. Sci. 2020, 21, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, M.G.; Winkler, S.S.; Lentz, S.S.; Berliner, S.H.; Swain, M.F.; Skinner, H.G.; Schwartz, G.G. Serum Calcium and Serum Albumin Are Biomarkers That Can Discriminate Malignant from Benign Pelvic Masses. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2015, 24, 1593–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wulaningsih, W.; Sagoo, H.K.; Hamza, M.; Melvin, J.; Holmberg, L.; Garmo, H.; Malmström, H.; Lambe, M.; Hammar, N.; Walldius, G.; et al. Serum Calcium and the Risk of Breast Cancer: Findings from the Swedish AMORIS Study and a Meta-Analysis of Prospective Studies. Int. J. Mol. Sci. 2016, 17, 1487. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Lan, M.; Peng, A.-F.; Yu, Q.-F.; Chen, W.-Z.; Liu, Z.-L.; Liu, J.-M.; Huang, S.-H. Serum Calcium, Alkaline Phosphotase and Hemoglobin as Risk Factors for Bone Metastases in Bladder Cancer. PLoS ONE 2017, 12, e0183835. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-H.; Zhu, P.-W.; Li, B.; Shi, W.-Q.; Lin, Q.; Min, Y.-L.; Ge, Q.-M.; Yuan, Q.; Shao, Y. Carbohydrate Antigen-125, Calcium, and Hemoglobin as Predictive Clinical Indicator for Ocular Metastasis in Male Liver Cancer Patients. Biosci. Rep. 2020, 40, BSR20194405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohatgi, A. WebPlotDigitizer: Version 4.5. 2021. Available online: https://automeris.io/WebPlotDigitizer/ (accessed on 21 February 2022).
- Erem, S.; Razzaque, M.S. Dietary Phosphate Toxicity: An Emerging Global Health Concern. Histochem. Cell Biol. 2018, 150, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Tulchinsky, T.H. Micronutrient Deficiency Conditions: Global Health Issues. Public Health Rev. 2010, 32, 243–255. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, J.T.; Woteki, C.; Bailey, R.; Britten, P.; Carriquiry, A.; Gaine, P.C.; Miller, D.; Moshfegh, A.; Murphy, M.M.; Smith Edge, M. Fortification: New Findings and Implications. Nutr. Rev. 2014, 72, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Best, C.; Neufingerl, N.; Del Rosso, J.M.; Transler, C.; van den Briel, T.; Osendarp, S. Can Multi-Micronutrient Food Fortification Improve the Micronutrient Status, Growth, Health, and Cognition of Schoolchildren? A Systematic Review. Nutr. Rev. 2011, 69, 186–204. [Google Scholar] [CrossRef]
- Rowe, L.A. Addressing the Fortification Quality Gap: A Proposed Way Forward. Nutrients 2020, 12, 3899. [Google Scholar] [CrossRef]
- Dewi, N.U.; Mahmudiono, T. Effectiveness of Food Fortification in Improving Nutritional Status of Mothers and Children in Indonesia. Int. J. Environ. Res. Public Health 2021, 18, 2133. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Salam, R.A.; Kumar, R.; Bhutta, Z.A. Micronutrient Fortification of Food and Its Impact on Woman and Child Health: A Systematic Review. Syst. Rev. 2013, 2, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Mineral | RDA/PRI (µg/Day) | EAR/AR (µg/Day) | UL (µg/Day) | Serum Levels | Source | References |
|---|---|---|---|---|---|---|
| Iron | 8000 | 6000 | 45,000 | 30 µg/L † | Meat, fish, cereals, beans, nuts. | [12,13,14] |
| Zinc | 8000–11,000 | 9400 | 25,000 | ≥800 µg/L ‡ | Meat, legumes, eggs, fish, grains. | [12,15,16] |
| Selenium | 30–70 | 70 | 300 | 47–145 µg/L | Meat, fish. | [12,17] |
| Phosphorus | 700,000 | 580,000 | n.d. | 0.8–1.5 × 103 µmol/L | Meat, fish. | [12,18] |
| Calcium | 1,000,000 | 750,000 | 2,500,000 | 2500 µmol/L | Milk, fish, legumes. | [12,14,19] |
| Copper | 900 | 1600 | 5000 | 1200 µg/L | Milk, fish, eggs, vegetables. | [12,20] |
| Iodine | 150 | 95 | 600 | 40–80 µg/L | Marine products, eggs, milk, iodized salt. | [12,21] |
| Mineral | Organ | Sample | Association * | Measure † | Reference |
|---|---|---|---|---|---|
| Zinc | Breast | Tissue | Direct | Qt | [70] |
| Brain | Tissue | Inverse | Qt | [76] | |
| Intake | Inverse | Qt | [75] | ||
| Mouth | Intake | None | Qt | [71] | |
| Serum | Inverse | Qt | [73] | ||
| Liver | Serum | None | HR | [72] | |
| Serum | Direct | Qt | [78] | ||
| Colon | Tissue | Inverse | OR | [29] | |
| Copper | Liver | Serum | Direct | HR | [72] |
| Serum | Direct | Qt | [78] | ||
| Mouth | Serum | Direct | Qt | [73] | |
| Colon | Tissue | Direct | OR | [29] | |
| Brain | Tissue | Inverse | Qt | [76] | |
| Serum | Direct | Qt | [77] | ||
| Intake | Inverse | Qt | [75] | ||
| Pancreas | Serum | Direct | Qt | [74] | |
| Selenium | Esophagus | Tissue | Direct | Qt | [107] |
| Prostate | Tissue | None | Qt | [103] | |
| Serum | None ║ | Qt | [104] | ||
| Any | Serum | None | Qt | [98] | |
| Serum | Inverse | Qt | [99] | ||
| Liver | Serum | Inverse | Qt | [105] | |
| Serum | Inverse | Qt | [108] | ||
| Colon | Serum | None ¶ | IR | [106] | |
| Pancreas | Serum | Inverse | OR | [74] | |
| Breast | Serum | Inverse | HR | [109] | |
| Serum | Inverse | HR | [110] | ||
| Lung | Serum | None | Qt | [100] | |
| Lung | Serum | Direct | HR | [101] | |
| Kidney | Serum | Inverse | OR | [102] | |
| Mouth | Intake | Direct | Qt | [71] | |
| Phosphorus | Brain | Intake | Direct | Qt | [75] |
| Prostate | Intake | None | RR | [133] | |
| Intake | Direct | Cr | [133] | ||
| Intake | Direct | OR | [137] | ||
| Intake | None | OR | [136] | ||
| Colon | Intake | Inverse # | RR | [134] | |
| Bladder | Intake | None | OR | [135] | |
| Mouth | Intake | Direct | Qt | [71] | |
| Calcium | Prostate | Tissue | Direct | Qt | [103] |
| Brain | Intake | Direct | Qt | [75] | |
| Ovary | Serum | Direct | Qt | [180] | |
| Breast | Serum | Inverse | HR | [181] | |
| Mouth | Serum | Direct | Qt | [73] | |
| Iron | Any | Serum | Direct/Inverse | HR | [161] |
| Intake | Direct | HR | [160] | ||
| Stomach | Tissue | Direct | Qt | [164] | |
| Brain | Intake | Direct | Qt | [75] | |
| Mouth | Intake | Direct | Qt | [71] | |
| Serum | Direct | Qt | [73] | ||
| Breast | Serum | None | HR | [166] | |
| Thyroid | Urine | Direct | OR | [168] | |
| Iodine | Breast | Urine | Direct | Qt | [165] |
| Rectum | Tissue | Direct | Cr | [167] |
| Mineral | Cancer Entity | Sample | Case Group (Cancer) Mean Mineral Content Number of Patients | Control Group (Healthy) Mean Mineral Content Number of Patients | Reference |
|---|---|---|---|---|---|
| Zinc | Breast | Tissue † | 3.5–19.5 ppm (n = 26) | 0.8–11.4 ppm (n = 26) | [70] |
| Glioblastoma | Tissue | 0.0403 µg/cm2 (n = 11) | 0.0285 µg/cm2 (n = 11) | [75] | |
| Tissue † | 0.20 g/kg (n = 6) | 0.27 g/kg (n = 6) | [76] | ||
| Colon | Serum | 96.4 µg/dL (n = 966) | 97.1 µg/dL (n = 966) | [29] | |
| Copper | Glioblastoma | Tissue | 0.0090 µg/cm2 (n = 11) | 0.0079 µg/cm2 (n = 11) | [75] |
| Tissue † | 0.48 g/kg (n = 6) | 1.26 g/kg (n = 6) | [76] | ||
| Serum | 27.5 µmol/L (n = 52) ‡ | 19.7 µmol/L (n = 52) ‡ | [77] | ||
| Colon | Serum | 138.6 µg/dL (n = 966) | 135.8 µg/dL (n = 966) | [29] | |
| Pancreas | Serum | 1432 µg/L (n = 100) | 1098 µg/L (n = 100) | [74] | |
| Selenium | Any | Serum | 58.8 µg/L * | 84.8 µg/L (n = 966) | [99,106] |
| Esophageal | Tissue † | 0.73 µg/g (n = 30) ‡ | 0.59 µg/g (n = 30) ‡ | [107] | |
| Prostate | Tissue | 191 µg/kg (n = 49) | 168 µg/kg (n = 49) | [103] | |
| Serum | 0.13 µg/g (n = 467) | 0.14 µg/g (n = 936) | [104] | ||
| Breast | Serum | 90.5 ng/mL (n = 100) | 91.3 ng/mL (n = 1186) | [109] | |
| Liver | Serum | 67.47 µg/L (n = 187) | 108.38 µg/L (n = 120) | [105] | |
| Colon | Serum | 84.0 µg/L (n = 966) | 85.6 µg/L (n = 966) | [106] | |
| Pancreas | Serum | 60.0 µg/L (n = 100) | 76.0 µg/L (n = 100) | [74] | |
| Lung | Serum | 166.00 ng/g (n = 48) | 144.74 ng/g (n = 39) | [100] | |
| Renal | Serum | 161.7 µg/L (n = 401) | 288.8 µg/L (n = 774) | [102] | |
| Phosphorus | Glioblastoma | Tissue | 1.71 µg/cm2 (n = 11) | 3.01 µg/cm2 (n = 11) | [75] |
| Iron | Glioblastoma | Tissue | 0.037 µg/cm2 (n = 11) | 0.118 µg/cm2 (n = 11) | [75] |
| Oral | Serum | 194.6 µg/dL * | 128.6 µg/dL * | [73] | |
| Calcium | Prostate | Tissue | 657 mg/kg (n = 50) | 1431 mg/kg (n = 49) | [103] |
| Oral | Serum | 14.7 mEq/L * | 9.4 mEq/L * | [73] | |
| Ovary | Serum | 9.34 mg/dL (n = 170) | 9.31 mg/dL (n = 344) | [180] |
| Mineral | Cancer Entity | Case Group (Cancer) Mean Mineral Intake Number of Patients | Control Group (Healthy) Mean Mineral Intake Number of Patients | Reference |
|---|---|---|---|---|
| Zinc | Oral | 12,851 µg/day (n = 27) | 11,788 µg/day (n = 86) | [71] |
| Bladder | 14.5 mg/day (n = 198) | 14.7 mg/day (n = 377) | [135] | |
| Copper | Bladder | 2.5 mg/day (n = 198) | 2.8 mg/day (n = 377) | [135] |
| Selenium | Oral | 142.9 µg/day (n = 27) | 166.7 µg/day (n = 86) | [71] |
| Phosphorus | Oral | 1761 mg/day (n = 27) | 1431 mg/day (n = 86) | [71] |
| Bladder | 1898.3 mg/day (n = 198) | 1940.4 mg/day (n = 377) | [135] | |
| Iron | Oral | 22.4 mg/day (n = 27) | 18.9 mg/day (n = 86) | [71] |
| Bladder | 21.3 mg/day (n = 198) | 23.1 mg/day (n = 377) | [135] | |
| Calcium | Bladder | 1127.2 mg/day (n = 198) | 1194.5 mg/day (n = 377) | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venturelli, S.; Leischner, C.; Helling, T.; Renner, O.; Burkard, M.; Marongiu, L. Minerals and Cancer: Overview of the Possible Diagnostic Value. Cancers 2022, 14, 1256. https://doi.org/10.3390/cancers14051256
Venturelli S, Leischner C, Helling T, Renner O, Burkard M, Marongiu L. Minerals and Cancer: Overview of the Possible Diagnostic Value. Cancers. 2022; 14(5):1256. https://doi.org/10.3390/cancers14051256
Chicago/Turabian StyleVenturelli, Sascha, Christian Leischner, Thomas Helling, Olga Renner, Markus Burkard, and Luigi Marongiu. 2022. "Minerals and Cancer: Overview of the Possible Diagnostic Value" Cancers 14, no. 5: 1256. https://doi.org/10.3390/cancers14051256
APA StyleVenturelli, S., Leischner, C., Helling, T., Renner, O., Burkard, M., & Marongiu, L. (2022). Minerals and Cancer: Overview of the Possible Diagnostic Value. Cancers, 14(5), 1256. https://doi.org/10.3390/cancers14051256










