The Effect of Hypoxia on Relative Biological Effectiveness and Oxygen Enhancement Ratio for Cells Irradiated with Grenz Rays
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Irradiation Geometries and Materials
2.2. Determination of Secondary-Electron Fluence
2.3. Monte Carlo Damage Simulation (MCDS)
2.4. Calculation of DSB Induction
2.5. Statistical Methods
2.6. RMF Model
2.7. RBE for DSB Induction and Cell Survival
2.8. Oxygen Enhancement Ratio (OER)
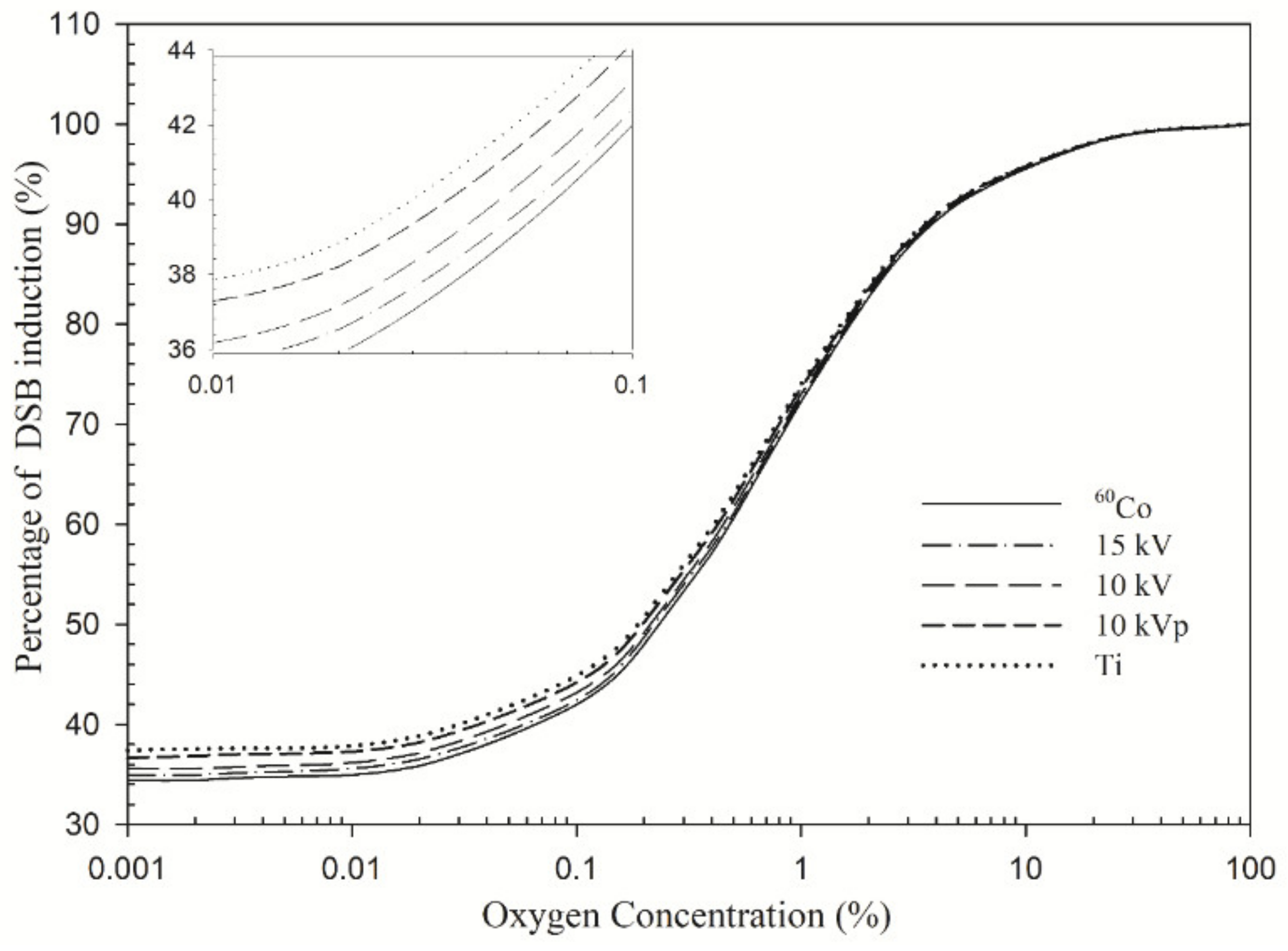
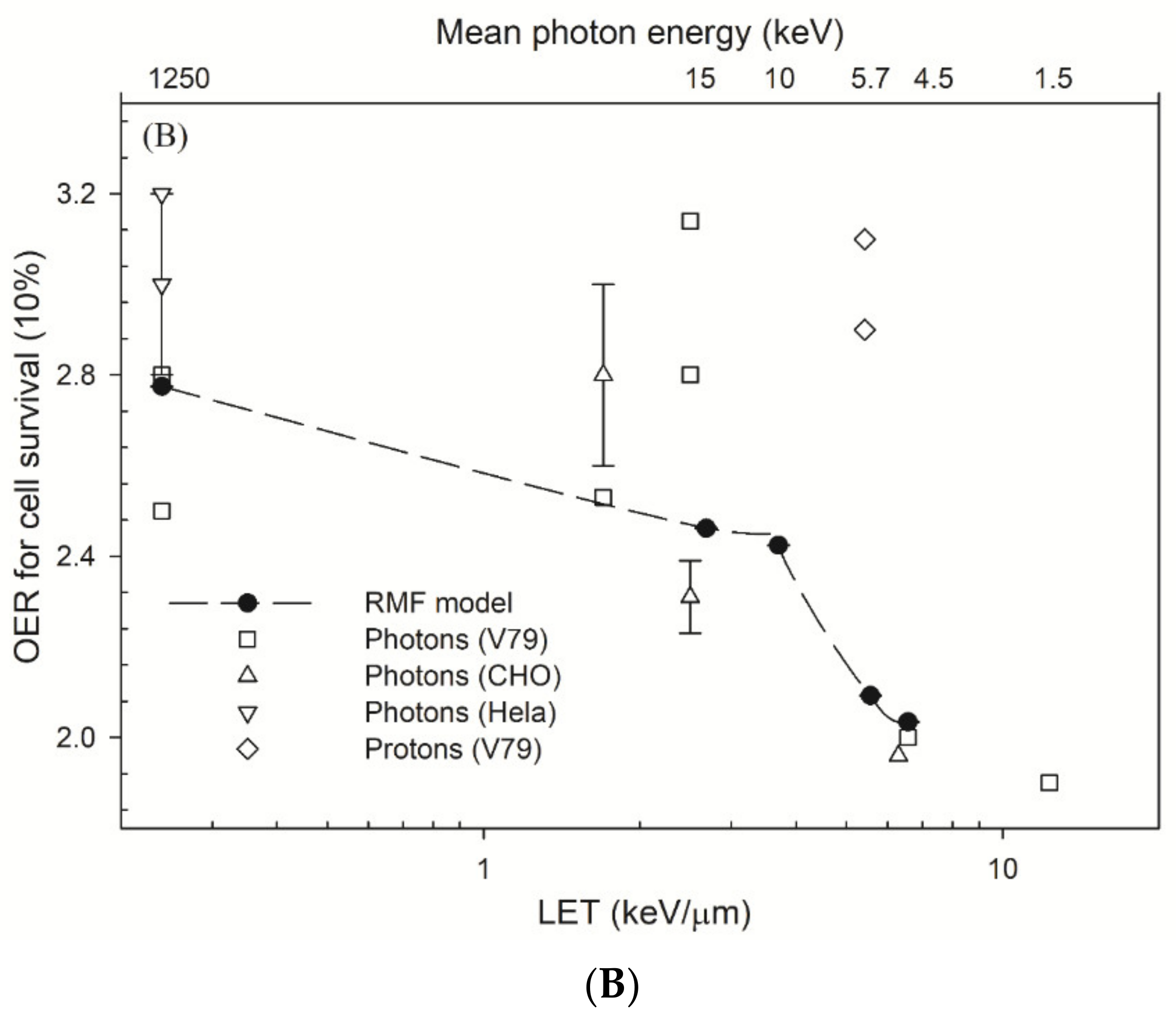
3. Results
4. Discussion
4.1. DNA Damage Profile
4.2. OER and Parameters α
4.3. Comparison of RBE
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panizzon, R.G. Grenz rays: An alternative treatment for superficial skin cancers in elderly patients. Aging Health 2009, 5, 495–496. [Google Scholar] [CrossRef]
- Panizzon, R.G.; Seegenschmiedt, M.H. Radiation Treatment and Radiation Reactions in Dermatology; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Hanlon, A. A Practical Guide to Skin Cancer; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Drakensjö, I.R.T.; Rosen, E.; Frohm Nilsson, M.; Girnita, A. Ten-year follow-up study of grenz ray treatment for lentigo maligna and early lentigo maligna melanoma. Acta Derm. Venereol. 2020, 100, adv00282. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, A.; Cozzio, A.; Plasswilm, L.; Panje, C.M. Radiotherapy for lentigo maligna and lentigo maligna melanoma—A systematic review. Radiat. Oncol. 2020, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Hedblad, M.A.; Mallbris, L. Grenz ray treatment of lentigo maligna and early lentigo maligna melanoma. J. Am. Acad. Dermatol. 2012, 67, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Farshad, A.; Burg, G.; Panizzon, R.; Dummer, R. A retrospective study of 150 patients with lentigo maligna and lentigo maligna melanoma and the efficacy of radiotherapy using grenz or soft X-rays. Br. J. Dermatol. 2002, 146, 1042–1046. [Google Scholar] [CrossRef]
- Lazarevic, D.; Ramelyte, E.; Dummer, R.; Imhof, L. Radiotherapy in periocular cutaneous malignancies: A retrospective study. Dermatology 2019, 235, 234–239. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.H.; Brunner, B.; Konz, B.; Kaudewitz, P.; Wendtner, C.M.; Peter, R.U.; Plewig, G.; Volkenandt, M. Fractionated radiotherapy of lentigo maligna and lentigo maligna melanoma in 64 patients. J. Am. Acad. Dermatol. 2000, 43, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, G.B.; Hong, A.; Economides, A.; Guitera, P. Experience with treating lentigo maligna with definitive radiotherapy. Dermatol. Res. Pract. 2018, 2018, 7439807. [Google Scholar] [CrossRef]
- Breneman, J.C.; Goldschmidt, H.; Gorson, R.O.; Panizzon, R.G.; Lindelöf, B. Modern Dermatologic Radiation Therapy; Springer: New York, NY, USA, 2012. [Google Scholar]
- Frentz, G. Grenz ray-induced nonmelanoma skin cancer. J. Am. Acad. Dermatol. 1989, 21, 475–478. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Goodhead, D.T.; Thacker, J. Inactivation and mutation of cultured mammalian cells by aluminium characteristic ultrasoft X-rays. I. Properties of aluminium X-rays and preliminary experiments with chinese hamster cells. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1977, 31, 541–559. [Google Scholar] [CrossRef]
- Raju, M.R.; Carpenter, S.G.; Chmielewski, J.J.; Schillaci, M.E.; Wilder, M.E.; Freyer, J.P.; Johnson, N.F.; Schor, P.L.; Sebring, R.J.; Goodhead, D.T. Radiobiology of ultrasoft X rays. I. Cultured hamster cells (V79). Radiat. Res. 1987, 110, 396–412. [Google Scholar] [CrossRef]
- De Lara, C.M.; Hill, M.A.; Jenner, T.J.; Papworth, D.; O’Neill, P. Dependence of the yield of DNA double-strand breaks in chinese hamster V79–4 cells on the photon energy of ultrasoft X rays. Radiat. Res. 2001, 155, 440–448. [Google Scholar] [CrossRef]
- Raju, M.R.; Amols, H.I.; Bain, E.; Carpenter, S.G.; Cox, R.A.; Robertson, J.B. A heavy particle comparative study. Part III: OER and RBE. Br. J. Radiol. 1978, 51, 712–719. [Google Scholar] [CrossRef]
- Hill, M.A. The variation in biological effectiveness of X-rays and gamma rays with energy. Radiat. Prot. Dosim. 2004, 112, 471–481. [Google Scholar] [CrossRef]
- Cox, R.; Thacker, J.; Goodhead, D.T. Inactivation and mutation of cultured mammalian cells by aluminium characteristic ultrasoft X-rays. II. Dose-responses of chinese hamster and human diploid cells to aluminium X-rays and radiations of different LET. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1977, 31, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Goodhead, D.T.; Nikjoo, H. Current status of ultrasoft X rays and track structure analysis as tools for testing and developing biophysical models of radiation action. Radiat. Prot. Dosim. 1990, 31, 343–350. [Google Scholar] [CrossRef]
- Hannam, S.; Webster, M.R.; Nixon, R.L. Radiotherapy in the treatment of hand eczema. In Textbook of Hand Eczema; Alikhan, A., Lachapelle, J., Maibach, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 353–359. [Google Scholar]
- Fenton, L.; Dawe, R.S. Six years’ experience of grenz ray therapy for the treatment of inflammatory skin conditions. Clin. Exp. Dermatol. 2016, 41, 864–870. [Google Scholar] [CrossRef]
- Ramelyte, E.; Bylaite-Bucinskiene, M.; Dummer, R.; Imhof, L. Successful use of grenz rays for disseminated superficial actinic porokeratosis: Report of 8 cases. Dermatology 2017, 233, 217–222. [Google Scholar] [CrossRef]
- Elsässer, T.; Weyrather, W.K.; Friedrich, T.; Durante, M.; Iancu, G.; Krämer, M.; Kragl, G.; Brons, S.; Winter, M.; Weber, K.J.; et al. Quantification of the relative biological effectiveness for ion beam radiotherapy: Direct experimental comparison of proton and carbon ion beams and a novel approach for treatment planning. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1177–1183. [Google Scholar] [CrossRef]
- Friedrich, T.; Scholz, U.; Elsässer, T.; Durante, M.; Scholz, M. Calculation of the biological effects of ion beams based on the microscopic spatial damage distribution pattern. Int. J. Radiat. Biol. 2012, 88, 103–107. [Google Scholar] [CrossRef]
- Hawkins, R.B. A microdosimetric-kinetic model of cell death from exposure to ionizing radiation of any LET, with experimental and clinical applications. Int. J. Radiat. Biol. 1996, 69, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.B. A microdosimetric-kinetic theory of the dependence of the RBE for cell death on LET. Med. Phys. 1998, 25, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.B.; Inaniwa, T. A microdosimetric-kinetic model for cell killing by protracted continuous irradiation including dependence on LET I: Repair in cultured mammalian cells. Radiat. Res. 2013, 180, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.D.; Carlson, D.J.; Butkus, M.P.; Hawkins, R.; Friedrich, T.; Scholz, M. A comparison of mechanism-inspired models for particle relative biological effectiveness (RBE). Med. Phys. 2018, 45, e925–e952. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.; Monini, C.; Testa, E.; Beuve, M. Nanox, a new model to predict cell survival in the context of particle therapy. Phys. Med. Biol. 2017, 62, 1248. [Google Scholar] [CrossRef]
- Carlson, D.J.; Stewart, R.D.; Semenenko, V.A.; Sandison, G.A. Combined use of monte carlo DNA damage simulations and deterministic repair models to examine putative mechanisms of cell killing. Radiat. Res. 2008, 169, 447–459. [Google Scholar] [CrossRef]
- Frese, M.C.; Yu, V.K.; Stewart, R.D.; Carlson, D.J. A mechanism-based approach to predict the relative biological effectiveness of protons and carbon ions in radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 442–450. [Google Scholar] [CrossRef]
- Streitmatter, S.W.; Stewart, R.D.; Jenkins, P.A.; Jevremovic, T. DNA double strand break (DSB) induction and cell survival in iodine-enhanced computed tomography (CT). Phys. Med. Biol. 2017, 62, 6164–6184. [Google Scholar] [CrossRef]
- Mara, E.; Clausen, M.; Khachonkham, S.; Deycmar, S.; Pessy, C.; Dörr, W.; Kuess, P.; Geord, D.; Gruber, S. Investigating the impact of alpha/beta and LETd on relative biological effectiveness in scanned proton beams: An in vitro study based on human cell lines. Med. Phys. 2020, 47, 3691–3702. [Google Scholar] [CrossRef]
- Kamp, F.; Carlson, D.J.; Wilkens, J.J. Rapid implementation of the repair-misrepair-fixation (RMF) model facilitating online adaption of radiosensitivity parameters in ion therapy. Phys. Med. Biol. 2017, 62, N285–N296. [Google Scholar] [CrossRef][Green Version]
- Mairani, A.; Dokic, I.; Magro, G.; Tessonnier, T.; Kamp, F.; Carlson, D.J.; Ciocca, M.; Cerutti, F.; Sala, P.R.; Ferrari, A.; et al. Biologically optimized helium ion plans: Calculation approach and its in vitro validation. Phys. Med. Biol. 2016, 61, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, R.; Furusawa, Y.; Fukawa, T.; Ando, K. Repair kinetics of DNA-DSB induced by X-rays or carbon ions under oxic and hypoxic conditions. J. Radiat. Res. 2005, 46, 325–332. [Google Scholar] [CrossRef]
- Tinganelli, W.; Ma, N.Y.; Von Neubeck, C.; Maier, A.; Schicker, C.; Kraft-Weyrather, W.; Durante, M. Influence of acute hypoxia and radiation quality on cell survival. J. Radiat. Res. 2013, 54, 23–30. [Google Scholar] [CrossRef]
- Prise, K.M.; Folkard, M.; Davies, S.; Michael, B.D. The irradiation of V79 mammalian cells by protons with energies below 2 MeV. Part II. Measurement of oxygen enhancement ratios and DNA damage. Int. J. Radiat. Biol. 1990, 58, 261–277. [Google Scholar] [CrossRef]
- Hsiao, Y.; Stewart, R.D. Monte Carlo simulation of DNA damage induction by X-rays and selected radioisotopes. Phys. Med. Biol. 2008, 53, 233–244. [Google Scholar] [CrossRef]
- Friedland, W.; Jacob, P.; Paretzke, H.G.; Merzagora, M.; Ottolenghi, A. Simulation of DNA fragment distributions after irradiation with photons. Radiat. Environ. Biophys. 1999, 38, 39–47. [Google Scholar] [CrossRef]
- Baró, J.; Sempau, J.; Fernández-Vaream, J.M.; Salvat, F. Penelope: An algorithm for Monte Carlo simulation of the penetration and energy loss of electrons and positrons in matter. Nucl. Instrum. Methods Phys. Res. B 1995, 100, 31–46. [Google Scholar] [CrossRef]
- Sempau, J.; Acosta, E.; Baró, J.; Fernández-Varea, J.M.; Salvat, F. An algorithm for Monte Carlo simulation of coupled electron-photon transport. Nucl. Instrum. Methods Phys. Res. B 1997, 132, 377–390. [Google Scholar] [CrossRef]
- Salvat, F.; Fernandez-Varea, J.M.; Acosta, E.; Sempau, J. Penelope—A Code System for Monte Carlo Simulation of Electron and Photon Transport; Nuclear Energy Agency of the OECD (NEA)/Organisation for Economic Co-Operation and Development—Nuclear Energy Agency: Paris, France, 2001. [Google Scholar]
- Falzone, N.; Fernández-Varea, J.M.; Flux, G.; Vallis, K.A. Monte Carlo evaluation of auger electron-emitting theranostic radionuclides. J. Nucl. Med. 2015, 56, 1441–1446. [Google Scholar] [CrossRef]
- Salvat, F.; Fernández-Varea, J.; Sempau, J. Penelope 2011: A Code System for Monte Carlo Simulation of Electron and Photon Transport; OECD Nuclear Energy Agency: Issy-les-Moulineaux, France, 2011. [Google Scholar]
- Salvat, F.; Fernández-Varea, J.; Baró, J.; Sempau, J. Penelope, an Algorithm and Computer Code for Monte Carlo Simulation of Electron-Photon Showers; CIEMAT: Madrid, Spain, 1996. [Google Scholar]
- Roos, H.; Schmid, E. Analysis of chromosome aberrations in human peripheral lymphocytes induced by 5.4 Kev X-rays. Radiat. Environ. Biophys. 1998, 36, 251–254. [Google Scholar] [CrossRef]
- Semenenko, V.A.; Stewart, R.D. A fast Monte Carlo algorithm to simulate the spectrum of DNA damages formed by ionizing radiation. Radiat. Res. 2004, 161, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Semenenko, V.A.; Stewart, R.D. Fast Monte Carlo simulation of DNA damage formed by electrons and light ions. Phys. Med. Biol. 2006, 51, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.D.; Yu, V.K.; Georgakilas, A.G.; Koumenis, C.; Park, J.H.; Carlson, D.J. Effects of radiation quality and oxygen on clustered DNA lesions and cell death. Radiat. Res. 2011, 176, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Nikjoo, H.; Uehara, S.; Wilson, W.E.; Hoshi, M.; Goodhead, D.T. Track structure in radiation biology: Theory and applications. Int. J. Radiat. Biol. 1998, 73, 355–364. [Google Scholar] [CrossRef]
- El Naqa, I.; Pater, P.; Seuntjens, J. Monte Carlo role in radiobiological modelling of radiotherapy outcomes. Phys. Med. Biol. 2012, 57, R75–R97. [Google Scholar] [CrossRef]
- Incerti, S.; Kyriakou, I.; Bernal, M.A.; Bordage, M.C.; Francis, Z.; Guatelli, S.; Ivanchenko, V.; Karamitros, M.; Lampe, N.; Lee, S.B.; et al. Geant4-DNA example applications for track structure simulations in liquid water: A report from the Geant4-DNA project. Med. Phys. 2018, 45, e722–e739. [Google Scholar] [CrossRef]
- Chatzipapas, K.P.; Papadimitroulas, P.; Emfietzoglou, D.; Kalospyros, S.A.; Hada, M.; Georgakilas, A.G. Ionizing radiation and complex DNA damage: Quantifying the radiobiological damage using Monte Carlo simulations. Cancers 2020, 12, 799. [Google Scholar] [CrossRef]
- Booz, J.; Braby, L.; Coyne, J.; Kliauga, P.; Lindborg, L.; Menzel, H.G.; Parmentier, N. ICRU 1983 Microdosimetry Report 36; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 1983. [Google Scholar]
- Kassis, A.I. The amazing world of auger electrons. Int. J. Radiat. Biol. 2004, 80, 789–803. [Google Scholar] [CrossRef]
- Hammond, E.M.; Asselin, M.C.; Forster, D.; O’Connor, J.P.; Senra, J.M.; Williams, K.J. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin. Oncol. 2014, 26, 277–288. [Google Scholar] [CrossRef]
- Rothkamm, K.; Lobrich, M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses. Proc. Natl. Acad. Sci. USA 2003, 100, 5057–5062. [Google Scholar] [CrossRef]
- Sapora, O.; Barone, F.; Belli, M.; Maggi, A.; Quintiliani, M.; Tabocchini, M.A. Relationships between cell killing, mutation induction and DNA damage in X-irradiated V79 cells: The influence of oxygen and DMSO. Int. J. Radiat. Biol. 1991, 60, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.J.; Stewart, R.D.; Semenenko, V.A. Effects of oxygen on intrinsic radiation sensitivity: A test of the relationship between aerobic and hypoxic linear-quadratic (LQ) model parameters. Med. Phys. 2006, 33, 3105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, J.; Deng, X.; Qiu, R.; Wu, Z.; Zhang, H. Development of a DNA damage model that accommodates different cellular oxygen concentrations and radiation qualities. Med. Phys. 2021, 48, 5511–5521. [Google Scholar] [CrossRef] [PubMed]
- Nikjoo, H.; O’Neill, P.; Terrissol, M.; Goodhead, D.T. Quantitative modelling of DNA damage using monte carlo track structure method. Radiat. Environ. Biophys. 1999, 38, 31–38. [Google Scholar] [CrossRef]
- Nikjoo, H.; Emfietzoglou, D.; Liamsuwan, T.; Taleei, R.; Liljequist, D.; Uehara, S. Radiation track, DNA damage and response—A review. Rep. Prog. Phys. 2016, 79, 116601. [Google Scholar] [CrossRef]
- Nikjoo, H.; O’Neill, P.; Goodhead, D.T.; Terrissol, M. Computational modelling of low-energy electron-induced DNA damage by early physical and chemical events. Int. J. Radiat. Biol. 1997, 71, 467–483. [Google Scholar] [CrossRef]
- Cardona, M.; Ley, L. Photoemission in Solids: General Principles; Springer: Berlin/Heidelberg, Germany, 1978. [Google Scholar]
- Watanabe, R.; Rahmanian, S.; Nikjoo, H. Spectrum of radiation-induced clustered non-dsb damage—A Monte Carlo track structure modeling and calculations. Radiat. Res. 2015, 183, 525–540. [Google Scholar] [CrossRef]
- Forster, J.C.; Douglass, M.J.J.; Phillips, W.M.; Bezak, E. Monte Carlo simulation of the oxygen effect in DNA damage induction by ionizing radiation. Radiat. Res. 2018, 190, 248–261. [Google Scholar] [CrossRef]
- Sunada, S.; Hirakawa, H.; Fujimori, A.; Uesaka, M.; Okayasu, R. Oxygen enhancement ratio in radiation-induced initial DSBs by an optimized flow cytometry-based gamma-H2AX analysis in A549 human cancer cells. Radiat. Res. 2017, 188, 591–594. [Google Scholar] [CrossRef]
- Ling, C.C.; Michaels, H.B.; Gerweck, L.E.; Epp, E.R.; Peterson, E.C. Oxygen sensitization of mammalian cells under different irradiation conditions. Radiat. Res. 1981, 86, 325–340. [Google Scholar] [CrossRef]
- Sapozink, M.D. Oxygen enhancement ratios in synchronous hela cells exposed to low-LET radiation. Radiat. Res. 1977, 69, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.J.; Howell, R.L. Combined radiation-protective and radiation-sensitizing agents: II. Radiosensitivity of hypoxic or aerobic Chinese hamster fibroblasts in the presence of cysteamine and misonidazole: Implications for the “oxygen effect” (with appendix on calculation of dose-modifying factors). Radiat. Res. 1981, 87, 265–283. [Google Scholar] [PubMed]
- Watts, M.E.; Hodgkiss, R.J.; Jones, N.R.; Fowler, J.F. Radiosensitization of chinese hamster cells by oxygen and misonidazole at low X-ray doses. Int. J. Radiat.Biol. 1986, 50, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Spadinger, I.; Palcic, B. The relative biological effectiveness of 60Co γ-rays, 55 kVp X-rays, 250 kVp X-rays, and 11 MeV electrons at low doses. Int. J. Radiat. Biol. 1992, 61, 345–353. [Google Scholar] [CrossRef]
- Goodhead, D.T.; Thacker, J.; Cox, R. Is selective absorption of ultrasoft X-rays biologically important in mammalian cells? Phys. Med. Biol. 1981, 26, 1115–1127. [Google Scholar] [CrossRef]
- Lehnert, A.; Dörr, W.; Lessmann, E.; Pawelke, J. RBE of 10 kV X rays determined for the human mammary epithelial cell line MCF-12A. Radiat. Res. 2008, 169, 330–336. [Google Scholar] [CrossRef]
- Streitmatter, S.W.; Stewart, R.D.; Moffitt, G.; Jevremovic, T. Mechanistic modeling of the relative biological effectiveness of boron neutron capture therapy. Cells 2020, 9, 2302. [Google Scholar] [CrossRef]
- Hsiao, Y.Y.; Chen, F.H.; Chan, C.C.; Tsai, C.C. Monte Carlo simulation of double-strand break induction and conversion after ultrasoft X-rays irradiation. Int. J. Mol. Sci. 2021, 22, 11713. [Google Scholar] [CrossRef]
- Luo, W.R.; Chen, F.H.; Huang, R.J.; Chen, Y.P.; Hsiao, Y.Y. Effects of indirect actions and oxygen on relative biological effectiveness: Estimate of DSB inductions and conversions induced by therapeutic proton beams. Int. J. Radiat. Biol. 2020, 96, 187–196. [Google Scholar] [CrossRef]
- Chan, C.C.; Chen, F.H.; Hsiao, Y.Y. Impact of hypoxia on relative biological effectiveness and oxygen enhancement ratio for a 62-MeV therapeutic proton beam. Cancers 2021, 13, 2997. [Google Scholar] [CrossRef]
- Chan, C.C.; Hsiao, Y.Y. The effects of dimethylsulfoxide and oxygen on DNA damage induction and repair outcomes for cells irradiated by 62 MeV proton and 3.31 MeV helium ions. J. Pers. Med. 2021, 11, 286. [Google Scholar] [CrossRef]
- Hsiao, Y.Y.; Tai, F.C.; Chan, C.C.; Tsai, C.C. A computational method to estimate the effect of gold nanoparticles on X-ray induced dose enhancement and double-strand break yields. IEEE Access 2021, 9, 62745–62751. [Google Scholar] [CrossRef]
- Hill, M.A.; Vecchia, M.D.; Townsend, K.M.; Goodhead, D.T. Production and dosimetry of copper L ultrasoft X-rays for biological and biochemical investigations. Phys. Med. Biol. 1998, 43, 351–363. [Google Scholar] [CrossRef]
- Berger, M.; Coursey, J.; Zucker, M. ESTAR, PSTAR, and ASTAR: Computer Programs for Calculating Stopping-Power and Range Tables for Electrons, Protons, and Helium Ions. 1999. Available online: http://physics.nist.gov/Star (accessed on 20 December 2020).
- Tsai, J.Y.; Chen, F.H.; Hsieh, T.Y.; Hsiao, Y.Y. Effects of indirect actions and oxygen on relative biological effectiveness: Estimate of DSB induction and conversion induced by gamma rays and helium ions. J. Radiat. Res. 2015, 56, 691–699. [Google Scholar] [CrossRef]
- Hirayama, R.; Uzawa, A.; Matsumoto, Y.; Noguchi, M.; Kase, Y.; Takase, N.; Ito, A.; Koike, S.; Ando, K.; Okayasu, R.; et al. Induction of DNA DSB and its rejoining in clamped and non-clamped tumours after exposure to carbon ion beams in comparison to X rays. Radiat. Prot. Dosim. 2011, 143, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, S.J.; McMillan, T.J. Oxygen effect for DNA double-strand break induction determined by pulsed-field gel electrophoresis. Int. J. Radiat. Biol. 1992, 61, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Kundrát, P.; Friedland, W.; Becker, J.; Eidemüller, M.; Ottolenghi, A.; Baiocco, G. Analytical formulas representing track-structure simulations on DNA damage induced by protons and light ions at radiotherapy-relevant energies. Sci. Rep. 2020, 10, 15775. [Google Scholar] [CrossRef]
- Chaudhary, P.; Marshall, T.I.; Perozziello, F.M.; Manti, L.; Currell, F.J.; Hanton, F.; McMahon, S.J.; Kavanagh, J.N.; Cirrone, G.A.; Romano, F.; et al. Relative biological effectiveness variation along monoenergetic and modulated bragg peaks of a 62-MeV therapeutic proton beam: A preclinical assessment. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 27–35. [Google Scholar] [CrossRef]
- Tsang, R.W.; Liu, F.F.; Wells, W.; Payne, D.G. Lentigo maligna of the head and neck. Results of treatment by radiotherapy. Arch. Dermatol. 1994, 130, 1008–1012. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours—Implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef]
- Tremi, I.; Spyratou, E.; Souli, M.; Efstathopoulos, E.P.; Makropoulou, M.; Georgakilas, A.G.; Sihver, L. Requirements for designing an effective metallic nanoparticle (NP)-boosted radiation therapy (RT). Cancers 2021, 13, 3185. [Google Scholar] [CrossRef]



| MCDS | Track Structure b | LETe (keV/μm) | BD e (%) | SSB e (%) | SSB+ e (%) | 2SSB e (%) | DSB e (%) | DSB+ e (%) | DSB++ e (%) | Total SSB e (Gy−1 Gbp−1) | Total DSB e (Gy−1 Gbp−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 eV d | 21.47 | 44.1 | 32.9 | 8.3 | 3.77 | 4.72 | 3.19 | 3.02 | 102.4 ± 0.1 | 24.9 ± 0.1 | |
| 100 eV | 21.47 | 73.9 | 22.4 | 1.9 | 0.09 | 1.39 | 0.27 | 0.02 | 162.5 | 9.8 | |
| 300 eV d | 16.95 | 55.2 | 33.0 | 4.9 | 1.52 | 3.33 | 1.42 | 0.65 | 139.5 ± 0.1 | 19.1 ± 0.1 | |
| 300 eV a | 16.95 | 66.4 | 26.6 | 3.3 | 0.43 | 2.38 | 0.85 | 0.09 | 162.5 | 15.0 | |
| 500 eV d | 14.12 | 59.5 | 32.1 | 3.6 | 0.92 | 2.67 | 0.88 | 0.29 | 154.2 ± 0.1 | 16.1 ± 0.1 | |
| 500 eV b | 14.12 | 68.7 | 24.4 | 2.8 | 0.47 | 1.86 | 0.79 | 0.07 | 162.5 | 13.0 | |
| 1000 eV d | 10.28 | 63.5 | 30.9 | 2.5 | 0.50 | 2.00 | 0.48 | 0.11 | 168.6 ± 0.1 | 12.9 ± 0.1 | |
| 1000 eV b | 10.28 | 68.9 | 25.2 | 2.8 | 0.50 | 1.81 | 0.71 | 0.08 | 156.0 | 13.0 | |
| 1500 eV d | 8.25 | 65.1 | 30.3 | 2.1 | 0.37 | 1.74 | 0.36 | 0.07 | 174.3 ± 0.1 | 11.5 ± 0.1 | |
| 1500 eV a | 8.25 | 70.5 | 24.3 | 2.4 | 0.40 | 1.69 | 0.63 | 0.07 | 156.0 | 11.7 | |
| 4500 eV c | 4.08 | 66.2 | 29.9 | 1.8 | 0.20 | 1.60 | 0.30 | 0.05 | 123.5 | 7.7 | |
| 4500 eV a | 4.08 | 71.4 | 24.1 | 2.1 | 0.29 | 1.47 | 0.55 | 0.04 | 123.5 | 7.8 |
| Photon Energy | BD d (%) | SSB d (%) | SSB+ d (%) | 2SSB d (%) | DSB d (%) | DSB+ d (%) | DSB++ d (%) | Total SSB d (Gy−1 Gbp−1) | Total DSB d (Gy−1 Gbp−1) |
|---|---|---|---|---|---|---|---|---|---|
| Monoenergetic 15 kV (15 kV a,c) | 66.9 | 29.4 | 1.6 | 0.3 | 1.4 | 0.3 | 0.1 | 184.5 ± 0.1 | 8.9 ± 0.1 |
| Monoenergetic 10 kV (10 kV a,c) | 66.0 | 29.7 | 1.9 | 0.4 | 1.6 | 0.3 | 0.1 | 182.7 ± 0.1 | 9.3 ± 0.1 |
| 10 kVp (5.7 kV a,c) | 64.4 | 30.3 | 2.3 | 0.5 | 1.8 | 0.5 | 0.2 | 179.1 ± 0.1 | 10.2 ± 0.1 |
| 4.55 kV (4.55 kV a,c) | 63.5 | 30.5 | 2.6 | 0.6 | 2.0 | 0.6 | 0.2 | 176.9 ± 0.1 | 10.8 ± 0.1 |
| 4.55 kV b | 75.2 | 21.9 | 1.3 | 0.4 | 0.9 | 0.2 | 0.0 | - | - |
| 60Co (1250 kV a,c) | 68.4 | 28.8 | 1.3 | 0.2 | 1.1 | 0.2 | 0.0 | 187.4 ± 0.1 | 8.1 ± 0.1 |
| Photon Energy | LETc (keV/μm) | Measured DSBs c (per Gbp per Gy)b | RBE c | MCDS c DSB c s (per Gbp per Gy) | RBE c |
|---|---|---|---|---|---|
| Monoenergetic 15 kV (15 kV a) | 2.7 | - | - | 8.9 ± 0.1 | 1.1 ± 0.0 |
| Monoenergetic 10 kV (10 kV a) | 3.7 | - | - | 9.3 ± 0.1 | 1.2 ± 0.0 |
| 10 kVp (5.7 kV a) | 5.6 | - | - | 10.2 ± 0.1 | 1.3 ± 0.0 |
| 4.55 kV (4.55 kV a) | 6.6 | 10.4 a | 1.4 | 10.8 ± 0.1 | 1.3 ± 0.0 |
| 60Co (1250 kV a) | 0.24 | 7.6 a | 1.0 | 8.1 ± 0.1 | - |
| Photon Energy | BD c (%) | SSB c (%) | SSB+ c (%) | 2SSB c (%) | DSB c (%) | DSB+ c (%) | DSB++ c (%) | Total SSB c (Gy−1 Gbp−1) | Total DSB c (Gy−1 Gbp−1) |
|---|---|---|---|---|---|---|---|---|---|
| Monoenergetic 15 kV (15 kV a,b) | 67.6 | 29.1 | 1.5 | 0.2 | 1.3 | 0.2 | 0.1 | 171.1 ± 0.1 | 7.5 ± 0.1 |
| Monoenergetic 10 kV (10 kV a,b) | 66.8 | 29.4 | 1.7 | 0.3 | 1.4 | 0.3 | 0.1 | 169.7 ± 0.1 | 7.9 ± 0.1 |
| 10 kVp (5.7 kV a,b) | 65.2 | 30.0 | 2.1 | 0.4 | 1.7 | 0.4 | 0.2 | 166.8 ± 0.1 | 8.7 ± 0.1 |
| 4.55 kV (4.55 kV a,b) | 64.4 | 30.3 | 2.3 | 0.5 | 1.8 | 0.5 | 0.2 | 165.0 ± 0.1 | 9.2 ± 0.1 |
| 60Co (1250 kV a,b) | 69.0 | 28.5 | 1.2 | 0.2 | 1.0 | 0.1 | 0.0 | 173.5 ± 0.1 | 6.9 ± 0.1 |
| Photon Energy | BD c (%) | SSB c (%) | SSB+ c (%) | 2SSB c (%) | DSB c (%) | DSB+ c (%) | DSB++ c (%) | Total SSB c (Gy−1 Gbp−1) | Total DSB c (Gy−1 Gbp−1) |
|---|---|---|---|---|---|---|---|---|---|
| Monoenergetic 15 kV (15 kV a,b) | 69.8 | 28.1 | 1.0 | 0.1 | 0.9 | 0.1 | 0.0 | 126.0 ± 0.1 | 3.8 ± 0.1 |
| Monoenergetic 10 kV (10 kV a,b) | 69.1 | 28.3 | 1.2 | 0.2 | 1.0 | 0.2 | 0.0 | 125.4 ± 0.1 | 4.1 ± 0.1 |
| 10 kVp (5.7 kV a,b) | 67.8 | 28.8 | 1.5 | 0.3 | 1.2 | 0.3 | 0.1 | 124.3 ± 0.1 | 4.6 ± 0.1 |
| 4.55 kV (4.55 kV a,b) | 67.1 | 29.2 | 1.7 | 0.3 | 1.4 | 0.3 | 0.1 | 123.8 ± 0.1 | 4.9 ± 0.1 |
| 60Co (1250 kV a,b) | 70.9 | 27.5 | 0.8 | 0.1 | 0.7 | 0.1 | 0.0 | 127.0 ± 0.1 | 3.5 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.-C.; Chen, F.-H.; Hsueh, K.-L.; Hsiao, Y.-Y. The Effect of Hypoxia on Relative Biological Effectiveness and Oxygen Enhancement Ratio for Cells Irradiated with Grenz Rays. Cancers 2022, 14, 1262. https://doi.org/10.3390/cancers14051262
Chan C-C, Chen F-H, Hsueh K-L, Hsiao Y-Y. The Effect of Hypoxia on Relative Biological Effectiveness and Oxygen Enhancement Ratio for Cells Irradiated with Grenz Rays. Cancers. 2022; 14(5):1262. https://doi.org/10.3390/cancers14051262
Chicago/Turabian StyleChan, Chun-Chieh, Fang-Hsin Chen, Kuang-Lung Hsueh, and Ya-Yun Hsiao. 2022. "The Effect of Hypoxia on Relative Biological Effectiveness and Oxygen Enhancement Ratio for Cells Irradiated with Grenz Rays" Cancers 14, no. 5: 1262. https://doi.org/10.3390/cancers14051262
APA StyleChan, C.-C., Chen, F.-H., Hsueh, K.-L., & Hsiao, Y.-Y. (2022). The Effect of Hypoxia on Relative Biological Effectiveness and Oxygen Enhancement Ratio for Cells Irradiated with Grenz Rays. Cancers, 14(5), 1262. https://doi.org/10.3390/cancers14051262






