The Continuum of Metastatic Prostate Cancer: Interpreting PSMA PET Findings in Recurrent Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction:
2. Conventional Imaging in Prostate Cancer
3. The New Era of PSMA PET Imaging
3.1. Redefining Prostate Cancer Recurrence with PSMA PET Imaging
3.2. Interpreting PSMA PET Scans to Guide Management
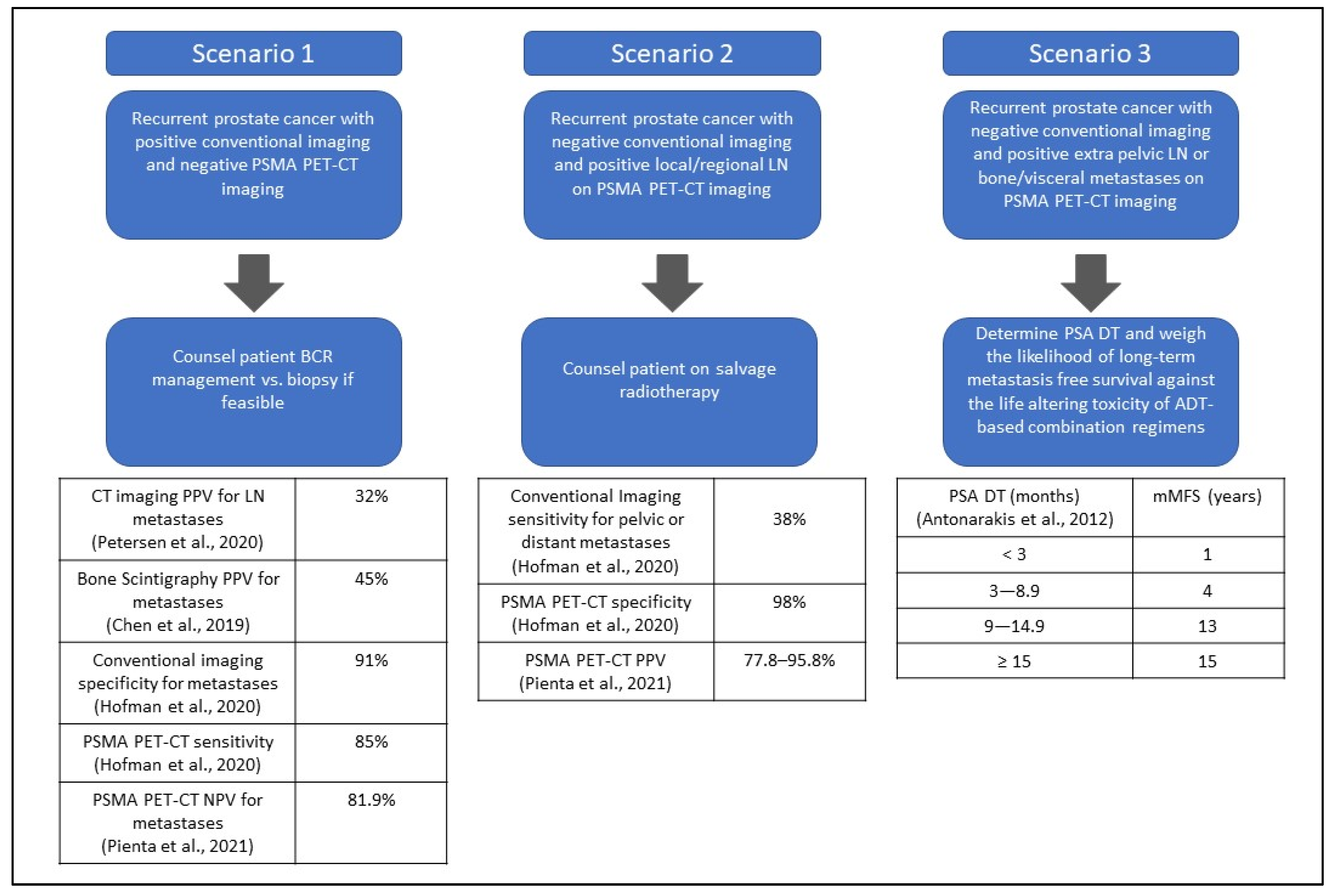
3.2.1. Scenario 1: A Patient Presents with Biochemical Recurrent Prostate Cancer with Positive Conventional Imaging and Negative PSMA PET–CT Imaging
3.2.2. Scenario 2: A Patient Presents with Biochemical Recurrence with Negative Conventional Imaging and Positive Local or Regional Lymph Nodes (N1M0) by PSMA PET–CT Imaging
3.2.3. Scenario 3: Patient Presents with Biochemical Recurrence with Negative Conventional Imaging and Positive Extra-Pelvic Lymph Nodes (M1a), Bone (M1b), or Visceral Metastases (M1c) by PSMA PET–CT Imaging
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Folkvaljon, Y.; Robinson, D.; Lissbrant, I.F.; Egevad, L.; Stattin, P. Evaluation of the 2015 Gleason Grade Groups in a Nationwide Population-based Cohort. Eur. Urol. 2016, 69, 1135–1141. [Google Scholar] [CrossRef]
- Paller, C.J.; Antonarakis, E.S.; Eisenberger, M.A.; Carducci, M.A. Management of Patients with Biochemical Recurrence after Local Therapy for Prostate Cancer. Hematol. Oncol. Clin. N. Am. 2013, 27, 1205–1219. [Google Scholar] [CrossRef]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Parent, E.E.; Schuster, D.M. Update on 18F-Fluciclovine PET for Prostate Cancer Imaging. J. Nucl. Med. 2018, 59, 733–739. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA approves 11C-choline for PET in prostate cancer. J. Nucl. Med. 2012, 53, 11n. [Google Scholar]
- FDA. Approves New Diagnostic Imaging Agent to Detect Recurrent Prostate Cancer. 2016. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-diagnostic-imaging-agent-detect-recurrent-prostate-cancer (accessed on 16 December 2021).
- Carlucci, G.; Ippisch, R.; Slavik, R.; Mishoe, A.; Blecha, J.; Zhu, S. 68Ga-PSMA-11 NDA Approval: A Novel and Successful Academic Partnership. J. Nucl. Med. 2021, 62, 149–155. [Google Scholar] [CrossRef]
- Song, H.; Iagaru, A.; Rowe, S.P. 18F DCFPyL PET Acquisition, Interpretation and Reporting: Suggestions Post Food and Drug Administration Approval. J. Nucl. Med. 2021, 63. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and End Points of Clinical Trials for Patients with Progressive Prostate Cancer and Castrate Levels of Testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.; Kelly, W.K.; Ma, H.; Zhou, H.; Solomon, N.C.; Fizazi, K.; Tangen, C.M.; Rosenthal, M.; Petrylak, D.P.; Hussain, M.; et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men with Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2016, 34, 1652–1659. [Google Scholar] [CrossRef]
- Anttinen, M.; Ettala, O.; Malaspina, S.; Jambor, I.; Sandell, M.; Kajander, S.; Rinta-Kiikka, I.; Schildt, J.; Saukko, E.; Rautio, P.; et al. A Prospective Comparison of 18F-prostate-specific Membrane Antigen-1007 Positron Emission Tomography Computed Tomography, Whole-body 1.5 T Magnetic Resonance Imaging with Diffusion-weighted Imaging, and Single-photon Emission Computed Tomography/Computed Tomography with Traditional Imaging in Primary Distant Metastasis Staging of Prostate Cancer (PROSTAGE). Eur. Urol. Oncol. 2021, 4, 635–644. [Google Scholar]
- FDA. Approves Second PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. 2021. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-second-psma-targeted-pet-imaging-drug-men-prostate-cancer (accessed on 16 December 2021).
- Hupe, M.C.; Philippi, C.; Roth, D.; Kümpers, C.; Ribbat-Idel, J.; Becker, F.; Joerg, V.; Duensing, S.; Lubczyk, V.H.; Kirfel, J.; et al. Expression of Prostate-Specific Membrane Antigen (PSMA) on Biopsies Is an Independent Risk Stratifier of Prostate Cancer Patients at Time of Initial Diagnosis. Front. Oncol. 2018, 8, 623. [Google Scholar] [CrossRef]
- Chang, S.S.; Reuter, V.E.; Heston, W.D.; Bander, N.H.; Grauer, L.S.; Gaudin, P.B. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999, 59, 3192–3198. [Google Scholar]
- Chang, S.S.; O’Keefe, D.S.; Bacich, D.J.; Reuter, V.E.; Heston, W.D.; Gaudin, P.B. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin. Cancer Res. 1999, 5, 2674–2681. [Google Scholar]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Kasperzyk, J.L.; Finn, S.P.; Flavin, R.; Fiorentino, M.; Lis, R.; Hendrickson, W.K.; Clinton, S.K.; Sesso, H.D.; Giovannucci, E.L.; Stampfer, M.J.; et al. Prostate-specific membrane antigen protein expression in tumor tissue and risk of lethal prostate cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2354–2363. [Google Scholar] [CrossRef]
- Pienta, K.J.; Gorin, M.A.; Rowe, S.P.; Carroll, P.R.; Pouliot, F.; Probst, S.; Saperstein, L.; Preston, M.A.; Alva, A.S.; Patnaik, A.; et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J. Urol. 2021, 206, 52–61. [Google Scholar] [CrossRef]
- Durack, J.C.; Alva, A.S.; Preston, M.A.; Pouliot, F.; Saperstein, L.; Carroll, P.R.; Pienta, K.J.; Rowe, S.P.; Patnaik, A.; Probst, S.; et al. A prospective phase II/III study of PSMA-targeted 18F-DCFPyL-PET/CT in patients (pts) with prostate cancer (PCa) (OSPREY): A subanalysis of disease staging changes in PCa pts with recurrence or metastases on conventional imaging. J. Clin. Oncol. 2021, 39, 32. [Google Scholar] [CrossRef]
- Morris, M.J.; Rowe, S.P.; Gorin, M.A.; Saperstein, L.; Pouliot, F.; Josephson, D.; Wong, J.Y.C.; Pantel, A.R.; Cho, S.Y.; Gage, K.L.; et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin. Cancer Res. 2021, 27, 3674–3682. [Google Scholar] [CrossRef] [PubMed]
- Thornbury, J.R. Eugene W. Caldwell Lecture. Clinical efficacy of diagnostic imaging: Love it or leave it. AJR Am. J. Roentgenol. 1994, 162, 1–8. [Google Scholar] [CrossRef]
- Petersen, L.J.; Zacho, H.D. PSMA PET for primary lymph node staging of intermediate and high-risk prostate cancer: An expedited systematic review. Cancer Imaging 2020, 20, 10. [Google Scholar] [CrossRef]
- Chen, B.; Wei, P.; Macapinlac, H.A.; Lu, Y. Comparison of 18F-Fluciclovine PET/CT and 99mTc-MDP bone scan in detection of bone metastasis in prostate cancer. Nucl. Med. Commun. 2019, 40, 940–946. [Google Scholar] [CrossRef]
- Richter, J.A.; Rodríguez, M.; Rioja, J.; Peñuelas, I.; Martí-Climent, J.; Garrastachu, P.; Quincoces, G.; Zudaire, J.; García-Velloso, M.J. Dual tracer 11C-choline and FDG-PET in the diagnosis of biochemical prostate cancer relapse after radical treatment. Mol. Imaging Biol. 2010, 12, 210–217. [Google Scholar] [CrossRef]
- McGeorge, S.; Kwok, M.; Jiang, A.; Emmett, L.; Pattison, D.A.; Thomas, P.A.; Yaxley, J.W.; Roberts, M.J. Dual-Tracer Positron-Emission Tomography Using Prostate-Specific Membrane Antigen and Fluorodeoxyglucose for Staging of Prostate Cancer: A Systematic Review. Adv. Urol. 2021, 2021, 1544208. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu] Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Harmon, S.A.; Mena, E.; Shih, J.H.; Adler, S.; McKinney, Y.; Bergvall, E.; Mehralivand, S.; Sowalsky, A.G.; Couvillon, A.; Madan, R.A.; et al. A comparison of prostate cancer bone metastases on 18F-Sodium Fluoride and Prostate Specific Membrane Antigen: Discordant uptake in the same lesion. Oncotarget 2018, 9, 37676–37688. [Google Scholar] [CrossRef][Green Version]
- Emmett, L.; van Leeuwen, P.J.; Nandurkar, R.; Scheltema, M.J.; Cusick, T.; Hruby, G.; Kneebone, A.; Eade, T.; Fogarty, G.; Jagavkar, R.; et al. Treatment Outcomes from 68Ga-PSMA PET/CT—Informed Salvage Radiation Treatment in Men with Rising PSA After Radical Prostatectomy: Prognostic Value of a Negative PSMA PET. J. Nucl. Med. 2017, 58, 1972–1976. [Google Scholar] [CrossRef]
- Tulsyan, S.; Das, C.J.; Tripathi, M.; Seth, A.; Kumar, R.; Bal, C. Comparison of 68Ga-PSMA PET/CT and multiparametric MRI for staging of high-risk prostate cancer68Ga-PSMA PET and MRI in prostate cancer. Nucl. Med. Commun. 2017, 38, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Horn, T.; Krönke, M.; Rauscher, I.; Haller, B.; Robu, S.; Wester, H.J.; Schottelius, M.; van Leeuwen, F.W.B.; van der Poel, H.G.; Heck, M.; et al. Single Lesion on Prostate-specific Membrane Antigen-ligand Positron Emission Tomography and Low Prostate-specific Antigen Are Prognostic Factors for a Favorable Biochemical Response to Prostate-specific Membrane Antigen-targeted Radioguided Surgery in Recurrent Prostate Cancer. Eur. Urol. 2019, 76, 517–523. [Google Scholar] [PubMed]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; de Santana Gomes, A.J.P.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in Patients with Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J. Clin. Oncol. 2021, 39, 2294–2303. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Feng, Z.; Trock, B.J.; Humphreys, E.B.; Carducci, M.A.; Partin, A.W.; Walsh, P.C.; Eisenberger, M.A. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: Long-term follow-up. BJU Int. 2012, 109, 32–39. [Google Scholar] [CrossRef]
- Loblaw, A.; Bassett, J.; D’Este, C.; Pond, G.R.; Cheung, P.; Millar, J.L.; Frydenberg, M.; King, M.T.; Lukka, H.; Malone, S.; et al. Timing of androgen deprivation therapy for prostate cancer patients after radiation: Planned combined analysis of two randomized phase 3 trials. J. Clin. Oncol. 2018, 36, 5018. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kase, A.M.; Tan, W.; Copland, J.A., III; Cai, H.; Parent, E.E.; Madan, R.A. The Continuum of Metastatic Prostate Cancer: Interpreting PSMA PET Findings in Recurrent Prostate Cancer. Cancers 2022, 14, 1361. https://doi.org/10.3390/cancers14061361
Kase AM, Tan W, Copland JA III, Cai H, Parent EE, Madan RA. The Continuum of Metastatic Prostate Cancer: Interpreting PSMA PET Findings in Recurrent Prostate Cancer. Cancers. 2022; 14(6):1361. https://doi.org/10.3390/cancers14061361
Chicago/Turabian StyleKase, Adam M., Winston Tan, John A. Copland, III, Hancheng Cai, Ephraim E. Parent, and Ravi A. Madan. 2022. "The Continuum of Metastatic Prostate Cancer: Interpreting PSMA PET Findings in Recurrent Prostate Cancer" Cancers 14, no. 6: 1361. https://doi.org/10.3390/cancers14061361
APA StyleKase, A. M., Tan, W., Copland, J. A., III, Cai, H., Parent, E. E., & Madan, R. A. (2022). The Continuum of Metastatic Prostate Cancer: Interpreting PSMA PET Findings in Recurrent Prostate Cancer. Cancers, 14(6), 1361. https://doi.org/10.3390/cancers14061361