Exploratory Study of Superparamagnetic Iron Oxide Dose Optimization in Breast Cancer Sentinel Lymph Node Identification Using a Handheld Magnetic Probe and Iron Quantitation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Materials
2.3. Procedure
2.4. Statistical Analysis
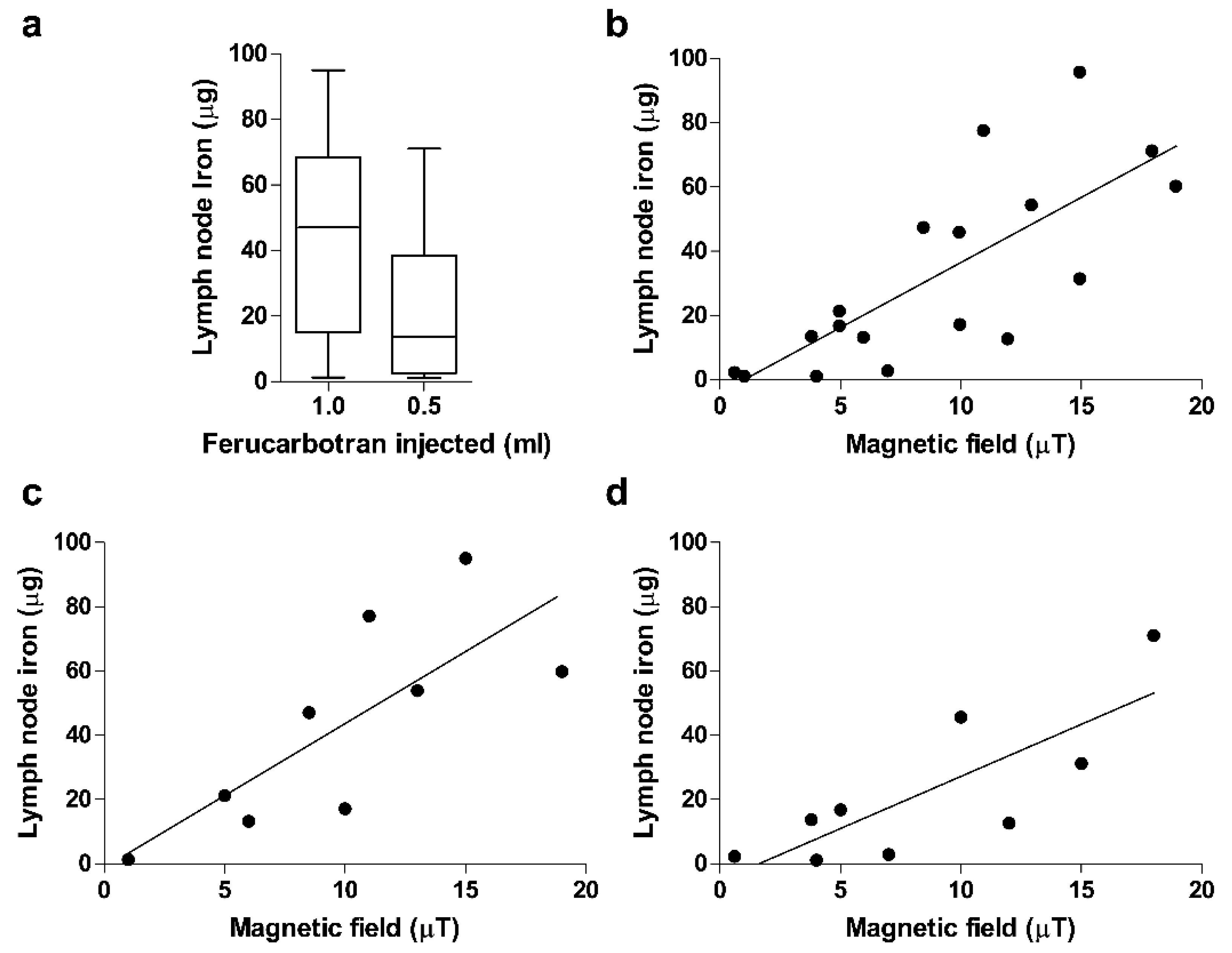
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giuliano, A.E.; Kirgan, D.M.; Guenther, J.M.; Morton, D.L. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann. Surg. 1994, 220, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Pesek, S.; Ashikaga, T.; Krag, L.E.; Krag, D. The false-negative rate of sentinel node biopsy in patients with breast cancer: A meta-analysis. World J. Surg. 2012, 36, 2239–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrenk, P.; Rieger, R.; Shamiyeh, A.; Wayand, W. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer 2000, 88, 608–614. [Google Scholar] [CrossRef]
- Thill, M.; Kurylcio, A.; Welter, R.; van Haasteren, V.; Grosse, B.; Berclaz, G.; Polkowski, W.; Hauser, N. The Central-European SentiMag study: Sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast 2014, 23, 175–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douek, M.; Klaase, J.; Monypenny, I.; Kothari, A.; Zechmeister, K.; Brown, D.; Wyld, L.; Drew, P.; Garmo, H.; Agbaje, O.; et al. Sentinel node biopsy using a magnetic tracer versus standard technique: The SentiMAG multicentre trial. Ann. Surg. Oncol. 2014, 21, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Houpeau, J.-L.; Chauvet, M.-P.; Guillemin, F.; Bendavid-Athias, C.; Charitansky, H.; Kramar, A.; Giard, S. Sentinel lymph node identification using superparamagnetic iron oxide particles versus radioisotope: The French Sentimag feasibility trial. J. Surg. Oncol. 2016, 113, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, A.; Christiansen, P.M.; Fischer, L.; Hedin, C.; Pistioli, L.; Sund, M.; Rasmussen, N.R.; Jørnsgård, H.; Tegnelius, D.; Eriksson, S.; et al. The Nordic SentiMag trial: A comparison of super paramagnetic iron oxide (SPIO) nanoparticles versus Tc(99) and patent blue in the detection of sentinel node (SN) in patients with breast cancer and a meta-analysis of earlier studies. Breast Cancer Res. Treat. 2016, 157, 281–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekino, M.; Kuwahata, A.; Ookubo, T.; Shiozawa, M.; Ohashi, K.; Kaneko, M.; Saito, I.; Inoue, Y.; Ohsaki, H.; Takei, H.; et al. Handheld magnetic probe with permanent magnet and Hall sensor for identifying sentinel lymph nodes in breast cancer patients. Sci. Rep. 2018, 19, 1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taruno, K.; Kurita, T.; Kuwahata, A.; Yanagihara, K.; Enokido, K.; Katayose, Y.; Nakmura, S.; Takei, H.; Sekino, M.; Kusakabe, M. Multicenter clinical trial on sentinel lymph node biopsy using superparamagnetic iron oxide nanoparticles and a novel handheld magnetic probe. J. Surg. Oncol. 2019, 120, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Krischer, B.; Forte, S.; Niemann, T.; Kubik-Huch, R.A.; Leo, C. Feasibility of breast MRI after sentinel procedure for breast cancer with superparamagnetic tracers. Eur. J. Surg. Oncol. 2018, 44, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.T.; Rodriguez-Revuelto, R.; Espinosa-Bravo, M.; Siso, C.; Rivero, J.; Esgueva, A. A randomized study comparing different doses of superparamagnetic iron oxide tracer for sentinel lymph node biopsy in breast cancer: The SUNRISE study. Eur. J. Surg. Oncol. 2020, 46, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.; Argáez, C. Magnetic Localization System for Sentinel Lymph Node Biopsy: A Review of the Diagnostic Accuracy, Cost-Effectiveness, and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562944/ (accessed on 1 October 2021).
- Peek, M.C.L.; Saeki, K.; Ohashi, K.; Chikaki, S.; Baker, R.; Nakagawa, T.; Kusakabe, M.; Douek, M.; Sekino, M. Optimization of SPIO injection for sentinel lymph node dissection in a rat model. Cancers 2021, 13, 5031. [Google Scholar] [CrossRef] [PubMed]
- Kuwahata, A.; Taruno, K.; Kurita, T.; Makita, M.; Chikaki, S.; Saito, I.; Takei, H.; Nakamura, S.; Kusakabe, M.; Sekino, M. Magnetic nanoparticle detection by utilizing nonlinear magnetization for sentinel lymph nodes of breast cancer patients. IEEE Trans. Magn. 2021, 57, 5300404. [Google Scholar] [CrossRef]
- Kurita, T.; Taruno, K.; Nakamura, S.; Takei, H.; Enokido, K.; Kuwayama, T.; Kanada, Y.; Akashi-Tanaka, S.; Matsuyanagi, M.; Hankyo, M.; et al. Magnetically guided localization using a Guiding-Marker System® and a handheld magnetic probe for nonpalpable breast lesions: A multicenter feasibility study in Japan. Cancers 2021, 13, 2923. [Google Scholar] [CrossRef] [PubMed]
- Grootendorst, D.J.; Jose, J.; Fratila, R.M.; Visscher, M.; Velders, A.H.; Haken, B.T.; Van Leeuwen, T.G.; Steenbergen, W.; Manohar, S.; Ruers, T.J.M. Evaluation of superparamagnetic iron oxide nanoparticles (Endorem®) as a photoacoustic contrast agent for intra-operative nodal staging. Contrast Media Mol. Imaging 2013, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Anninga, B.; Ahmed, M.; Van Hemelrijck, M.; Pouw, J.; Westbroek, D.; Pinder, S.; Ten Haken, B.; Pankhurst, Q.; Douek, M. Magnetic sentinel lymph node biopsy and localization properties of a magnetic tracer in an in vivo porcine model. Breast Cancer Res. Treat. 2013, 141, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Anninga, B.; Pouw, J.J.; Vreemann, S.; Peek, M.; Van Hmelrijck, M.; Pinder, S.; Haken, B.T.; Pankhurst, Q.; Douek, M. Optimising magnetic sentinel lymph node biopsy in an in vivo porcine model. Nanomedicine 2015, 11, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, B.; van den Bosch, C.H.; Wijnen, M.W.H.A.; van Scheltinga, C.E.J.T.; Fiocco, M.F.; van der Steeg, A.F.W. Systematic review and meta-analysis concerning near-infrared imaging with fluorescent agents to identify the sentinel lymph node in oncology patients. Eur. J. Surg. Oncol. 2020, 46, 2011–2022. [Google Scholar] [CrossRef]
- Hersi, A.-F.; Pistiolis, L.; Dussan Luberth, C.; Vikhe-Patil, E.; Nilsson, F.; Mohammed, I.; Olofsson Bagge, R.; Wärnberg, F.; Eriksson, S.; Karakatsanis, A. Optimizing dose and timing in magnetic tracer techniques for sentinel lymph node detection in early breast cancers: The prospective multicenter SentiDose trial. Cancers 2021, 13, 693. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [PubMed]
- Pouw, J.J.; Grootendorst, M.R.; Bezooijen, R.; Klaze, C.A.H.; De Bruin, W.I.; Klasse, J.M.; Hall-Craggs, M.A.; Douek, M.; Ten Haken, B. Pre-operative sentinel lymph node localization in breast cancer with superparamagnetic iron oxide MRI: The SentiMAG Multicentre Trial imaging subprotocol. Br. J. Radiol. 2015, 88, 20150634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Cai, Z.; Ai, H. Stimulus-responsive nanoparticle magnetic resonance imaging contrast agents: Design considerations and applications. Adv. Healthc. Mater. 2021, 10, e2001091. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, J.T.; Stark, D.D. Iron oxide-enhanced MR imaging of the liver and spleen: Review of the first 5 years. AJR Am. J. Roentgenol. 1990, 155, 943–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manca, G.; Volterrani, D.; Mazzarri, S.; Duce, V.; Svirydenka, A.; Giuliano, A.; Mariani, G. Sentinel lymph node mapping in breast cancer: A critical reappraisal of the internal mammary chain issue. Q J. Nucl. Med. Mol. Imaging 2014, 58, 114–126. [Google Scholar] [PubMed]
- Qiu, P.-F.; Cong, B.-B.; Zhao, R.-R.; Yang, G.-R.; Liu, Y.-B.; Chen, P.; Wang, Y.-S. Internal mammary sentinel lymph node biopsy with modified injection technique: High visualization rate and accurate staging. Medicine 2015, 94, e1790. [Google Scholar] [CrossRef] [PubMed]
- Urano, M.; Denewar, F.A.; Murai, T.; Mizutani, M.; Kitase, M.; Ohashi, K.; Shiraki, N.; Shibamoto, Y. Internal mammary lymph node metastases in breast cancer: What should radiologists know? Jpn. J. Radiol. 2018, 36, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Kjellman, P.; Zandt, R.; Fredriksson, S.; Strand, S.E. Optimizing retention of multimodal imaging nanostructures in sentinel lymph nodes by nanoscale size tailoring. Nanomedicine 2014, 10, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Woo, T.; Ohashi, K.; Suzuki, T.; Kaneko, A.; Hoshino, A.; Zada, A.; Baker, R.; Douek, M.; Kusakabe, M.; et al. Magnetic sentinel lymph node biopsy in a murine tumour model. Nanomedicine 2016, 12, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.W.; Tan, S.-M.; Zheng, Q.; Shi, L. Network meta-analysis of novel and conventional sentinel lymph node biopsy techniques in breast cancer. BJS Open 2019, 3, 445–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | Ferucarbotran Injected | Total | |
|---|---|---|---|
| 1 mL | 0.5 mL | ||
| N | 9 | 9 | 18 |
| Age (median [range]) (years) | 54 (43–78) | 66 (44–84) | 59 (43–84) |
| BMI (median [range]) (kg/m) | 21.3 (19.4–29.1) | 20.3 (17.9–26.3) | 20.9 (17.9–29.1) |
| Menopausal state (n) | |||
| Premenopausal | 4 | 2 | 6 |
| Perimenopausal | 5 | 7 | 12 |
| Carcinoma type (n) | |||
| Invasive carcinoma | 9 | 7 | 16 |
| Ductal carcinoma in situ | 0 | 2 | 2 |
| Tumor location (n) | |||
| Upper outer quadrant | 4 | 5 | 9 |
| Upper inner quadrant | 1 | 2 | 3 |
| Lower inner quadrant | 1 | 1 | 2 |
| Lower outer quadrant | 1 | 0 | 1 |
| Central | 1 | 1 | 2 |
| Surgery type (n) | |||
| Total mastectomy | 5 | 7 | 12 |
| Partial mastectomy | 4 | 2 | 6 |
| Pathological tumor size (n) | |||
| pTis | 0 | 2 | 2 |
| pT1 | 6 | 4 | 10 |
| pT2 | 2 | 3 | 5 |
| pT3 | 1 | 0 | 1 |
| Pathological lymph node status (n) | |||
| pN0 | 5 | 9 | |
| pNi+ | 0 | 0 | |
| pN1mi | 2 | 0 | |
| pN1a | 2 | 0 | |
| Cases with metastatic lymph nodes/number of macrometastatic lymph nodes (percentage) | 2 cases/2 nodes (10.0%) | ||
| Hormone receptor status (n) | |||
| Estrogen receptor (ER)+ | 8 | 7 | |
| Progesterone receptor (PR)+ | 7 | 7 | |
| HER2 positive | 1 | 0 | |
| Ferucarbotran dose in volume | 1 mL | 0.5 mL | p Value |
| Ferucarbotran dose in mg a | 44.6 | 22.3 | |
| Number of participants | 9 | 9 | |
| Time from administration to surgery (median, range) | 20 h (5–24) | 20 h (20–24) | |
| Number of isolated lymph nodes (case average) | 20 (2.2) | 19 (2.1) | |
| Iron content (µg) | |||
| Average (range; percentagec) iron content in 1st SLN (µg) | 42.8 (1.3–95.0, 0.15%) | 21.9 (1.1–71.0, 0.16%) | 0.131 |
| Average iron content per case b (percentage c) (µg) | 52.4 (0.17–94.9) (0.18%) | 24.6 (0.7–71) (0.17%) | 0.073 |
| Iron content of first and second SLN (percentage d) (µg) | 42.8(1.32–94.9) vs. 10.0 (4.02–27.5) (19%) | 21.9 (1.1–71) vs. 4.1 (0.7–11.2) (11.8%) | 0.001/0.001 e |
| Iron content of macrometastatic LN and non-metastatic first SLN(µg)(range) | 45.2 (13.2–77.0)-vs. 50.4 (1.32–94.9) | 0.95 | |
| Average (range) ion magnetic field in 1st SLN (µT) | 7.3 (1.0–19.0) | 6.0 (0.5–18.0) | 0.918 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taruno, K.; Kuwahata, A.; Sekino, M.; Nakagawa, T.; Kurita, T.; Enokido, K.; Nakamura, S.; Takei, H.; Kusakabe, M. Exploratory Study of Superparamagnetic Iron Oxide Dose Optimization in Breast Cancer Sentinel Lymph Node Identification Using a Handheld Magnetic Probe and Iron Quantitation. Cancers 2022, 14, 1409. https://doi.org/10.3390/cancers14061409
Taruno K, Kuwahata A, Sekino M, Nakagawa T, Kurita T, Enokido K, Nakamura S, Takei H, Kusakabe M. Exploratory Study of Superparamagnetic Iron Oxide Dose Optimization in Breast Cancer Sentinel Lymph Node Identification Using a Handheld Magnetic Probe and Iron Quantitation. Cancers. 2022; 14(6):1409. https://doi.org/10.3390/cancers14061409
Chicago/Turabian StyleTaruno, Kanae, Akihiko Kuwahata, Masaki Sekino, Takayuki Nakagawa, Tomoko Kurita, Katsutoshi Enokido, Seigo Nakamura, Hiroyuki Takei, and Moriaki Kusakabe. 2022. "Exploratory Study of Superparamagnetic Iron Oxide Dose Optimization in Breast Cancer Sentinel Lymph Node Identification Using a Handheld Magnetic Probe and Iron Quantitation" Cancers 14, no. 6: 1409. https://doi.org/10.3390/cancers14061409
APA StyleTaruno, K., Kuwahata, A., Sekino, M., Nakagawa, T., Kurita, T., Enokido, K., Nakamura, S., Takei, H., & Kusakabe, M. (2022). Exploratory Study of Superparamagnetic Iron Oxide Dose Optimization in Breast Cancer Sentinel Lymph Node Identification Using a Handheld Magnetic Probe and Iron Quantitation. Cancers, 14(6), 1409. https://doi.org/10.3390/cancers14061409









