Successful Pregnancies, Births, and Children Development Following Oocyte Cryostorage in Female Cancer Patients During 25 Years of Fertility Preservation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longhi, A.; Porcu, E.; Petracchi, S.; Versari, M.; Conticini, L.; Bacci, G. Reproductive functions in female patients treated with adjuvant and neoadjuvant chemotherapy for localized osteosarcoma of the extremity. Cancer Am. Cancer Soc. 2000, 89, 1961–1965. [Google Scholar]
- Donnez, J.; Dolmans, M.-M. Fertility preservation in women. N. Engl. J. Med. 2017, 377, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Loren, A.W.; Mangu, P.B.; Beck, L.N.; Brennan, L.; Magdalinski, A.J.; Partridge, A.H.; Quinn, G.; Wallace, W.H.; Oktay, K. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil. Steril. 2013, 100, 1214–1223. [Google Scholar] [CrossRef]
- Practice Committees of the American Society for Reproductive Medicine, Society for Reproductive Technologists. Mature oocyte cryopreservation: A guideline. Fertil. Steril. 2013, 99, 37–43. [Google Scholar] [CrossRef]
- Porcu, E.; Fabbri, R.; Damiano, G.; Giunchi, S.; Fratto, R.; Ciotti, P.; Venturoli, S.; Flamigni, C. Clinical experience and applications of oocyte cryopreservation. Mol. Cell. Endocrinol. 2000, 169, 33–37. [Google Scholar] [CrossRef]
- Porcu, E.; Fabbri, R.; Damiano, G.; Fratto, R.; Giunchi, S.; Venturoli, S. Oocytes cryopreservation in oncological patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113, S14–S16. [Google Scholar] [CrossRef]
- Porcu, E.; Venturoli, S. Progress with oocyte cryopreservation. Curr. Opin. Obstet. Gynecol. 2006, 18, 273–279. [Google Scholar] [CrossRef]
- Porcu, E.; Bazzocchi, A.; Notarangelo, L.; Paradisi, R.; Landolfo, C.; Venturoli, S. Human oocyte cryopreservation in infertility and oncology. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 529–535. [Google Scholar] [CrossRef]
- Noyes, N.; Labella, P.A.; Grifo, J.; Knopman, J.M. Oocyte cryopreservation: A feasible fertility preservation option for reproductive age cancer survivors. J. Assist. Reprod. Genet. 2010, 27, 495–499. [Google Scholar] [CrossRef]
- Druckenmiller, S.; Goldman, K.N.; Labella, P.A.; Fino, M.E.; Bazzocchi, A.; Noyes, N. Successful Oocyte Cryopreservation in Reproductive-Aged Cancer Survivors. Obstet. Gynecol. 2016, 127, 474–480. [Google Scholar] [CrossRef]
- Cipriani, L.; Porcu, E.; Damiano, G.; Sacilotto, F.; Cillo, G.; Roncarati, I.; Vergine, F. AMH, AFC and FSH long term follow up before and after chemotherapy in patients undergoing temporary ovarian suppression with GnRH analogs. Hum. Reprod. 2019, 34, 356–357. [Google Scholar]
- Chian, R.C.; Huang, J.Y.; Tan, S.L.; Lucena, E.; Saa, A.; Rojas, A.; Ruvalcaba Castellón, L.A.; García Amador, M.I.; Montoya Sarmiento, J.E. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod. Biomed. Online 2008, 16, 608–610. [Google Scholar] [CrossRef]
- Wennerholm, U.B.; Soderstrom-Anttila, V.; Bergh, C.; Aittomäki, K.; Hazekamp, J.; Nygren, K.G.; Selbing, A.; Loft, A. Children born after cryopreservation of embryos or oocytes: A systematic review of outcome data. Hum. Reprod. 2009, 24, 2158–2172. [Google Scholar] [CrossRef]
- Noyes, N.; Porcu, E.; Borini, A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod. Biomed. Online 2009, 18, 769–776. [Google Scholar] [CrossRef]
- Goldman, K.N.; Noyes, N.L.; Knopman, J.M.; McCaffrey, C.; Grifo, J.A. Oocyte efficiency: Does live birth rate differ when analyzing cryopreserved and fresh oocytes on a per-oocyte basis? Fertil. Steril. 2013, 100, 712–717. [Google Scholar] [CrossRef]
- Porcu, E.; Fabbri, R.; Seracchioli, R.; Ciotti, P.M.; Magrini, O.; Flamigni, C. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil. Steril. 1997, 68, 724–726. [Google Scholar] [CrossRef]
- Fabbri, R.; Porcu, E.; Marsella, T.; Primavera, M.R.; Seracchioli, R.; Ciotti, P.M.; Magrini, O.; Venturoli, S.; Flamigni, C. Oocyte cryopreservation. Hum. Reprod. 1998, 13 (Suppl. 4), 98–108. [Google Scholar] [CrossRef]
- Porcu, E.; Fabbri, R.; Petracchi, S.; Seracchioli, R.; Ciotti, P.M.; Flamigni, C. Ongoing pregnancy after ICSI of epididymal spermatozoa into cryopreserved human oocytes. J. Assist. Reprod. Genet. 1999, 16, 283–285. [Google Scholar] [CrossRef]
- Porcu, E.; Fabbri, R.; Petracchi, S.; Ciotti, P.M.; Flamigni, C. Ongoing pregnancy after intracytoplasmic injection of testicular spermatozoa into cryopreserved human oocytes. Am. J. Obstet. Gynecol. 1999, 180, 1044–1045. [Google Scholar] [CrossRef]
- Porcu, E. Freezing Of Oocytes. Curr. Opin. Obstet. Gynecol. 1999, 11, 297–300. [Google Scholar] [CrossRef]
- Fabbri, R.; Porcu, E.; Marsella, T.; Rocchetta, G.; Venturoli, S.; Flamigni, C. Human oocyte cryopreservation: New perspectives regarding oocyte survival. Hum. Reprod. Mar. 2001, 16, 411–416. [Google Scholar] [CrossRef]
- Porcu, E. Oocyte freezing. Semin. Reprod. Med. 2001, 19, 221–230. [Google Scholar] [CrossRef]
- Porcu, E.; Contro, E.; Damiano, G.; Paradisi, R.; Venturoli, S. Pediatric outcome of childrens born from cryopreserved oocytes, embryos or semen | Niños concebidos mediante embriones, ovocitos o semen criopreservados. Cuad Med. Reprod. 2004, 10, 105–125. [Google Scholar]
- Ciotti, P.M.; Porcu, E.; Notarangelo, L.; Magrini, O.; Mazzocchi, A.; Venturoli, S. Meiotic spindle recovery is faster in vitrification of human oocytes compared to slow freezing. Fertil. Steril. 2009, 91, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Borini, A.; Setti, P.E.L.; Anserini, P.; De Luca, R.; De Santis, L.; Porcu, E.; La Sala, G.B.; Ferraretti, A.; Bartolotti, T.; Coticchio, G.; et al. Multicenter observational study on slow-cooling oocyte cryopreservation: Clinical outcome. Fertil. Steril. 2010, 94, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Setti, P.E.L.; Porcu, E.; Patrizio, P.; Vigiliano, V.; de Luca, R.; D’Aloja, P.; Spoletini, R.; Scaravelli, G. Human oocyte cryopreservation with slow freezing versus vitrification. Results from the National Italian Registry data, 2007–2011. Fertil. Steril. 2014, 102, 90–95.e2. [Google Scholar] [CrossRef] [PubMed]
- Porcu, E.; Tranquillo, M.L.; Notarangelo, L.; Ciotti, P.M.; Calza, N.; Zuffa, S.; Mori, L.; Nardi, E.; Dirodi, M.; Cipriani, L.; et al. High-security closed devices are efficient and safe to protect human oocytes from potential risk of viral contamination during vitrification and storage especially in the COVID-19 pandemic. J. Assist. Reprod. Genet. 2021, 38, 681–688. [Google Scholar] [CrossRef]
- Revelli, A.; Porcu, E.; Levi Setti, P.E.; Delle Piane, L.; Merlo, D.F.; Anserini, P. Is letrozole needed for controlled ovarian stimulation in patients with estrogen receptor-positive breast cancer? Gynecol. Endocrinol. 2013, 29, 993–996. [Google Scholar] [CrossRef]
- Porcu, E.; Cillo, G.M.; Cipriani, L.; Sacilotto, F.; Notarangelo, L.; Damiano, G.; Dirodi, M. RONCARATII Impact of BRCA1 and BRCA2 mutations on ovarian reserve and fertility preservation outcomes in young women with breast cancer. J. Assist. Reprod. Genet. 2019, 10, 1007–1108. [Google Scholar]
- Porcu, E.; Venturoli, S.; Damiano, G.; Ciotti, P.; Notarangelo, L.; Paradisi, R.; Moscarini, M.; Ambrosini, G. Healthy twins delivered after oocyte cryopreservation and bilateral ovariectomy for ovarian cancer. Reprod. Biomed. Online 2008, 17, 265–267. [Google Scholar] [CrossRef]
- Kuwayama, M. Highly efficient vitrification for cryopreservation of human oocytes and embryos. Cryotop Method 2007, 67, 73–80. [Google Scholar]
- Massarotti, C.; Scaruffi, P.; Lambertini, M.; Remorgida, V.; Del Mastro, L.; Anserini, P. State of the art on oocyte cryopreservation in female cancer patients: A critical review of the literature. Cancer Treat. Rev. 2017, 57, 50–57. [Google Scholar] [CrossRef]
- Andrei, F.; Salvatori, P.; Cipriani, L.; Damiano, G.; Dirodi, M.; Trombini, E.; Rossi, N.; Porcu, E. Self-efficacy, coping strategies and quality of life in women and men requiring assisted reproductive technology treatments for anatomical or non-anatomical infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 241–246. [Google Scholar] [CrossRef]
- Lanzoni, M.; Morris, J.; Garne, E.; Loane, M.; Kinsner-Ovaskainen, A. European Monitoring of Congenital Anomalies: JRC-EUROCAT Report on Statistical Monitoring of Congenital Anomalies (2006–2015); EUR 29010 EN.; Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-79-77305-1. [Google Scholar] [CrossRef]
- Hoekman, E.J.; Broeders, E.A.B.J.; Louwe, L.A.; Nout, R.A.; Jansen, F.W.; de Kroon, C.D. Ovarian function after ovarian transposition and additional pelvic radiotherapy: A systematic review. Eur. J. Surg. Oncol. 2019, 45, 1328–1340. [Google Scholar] [CrossRef]
- Chen, C. Pregnancy after Human Oocyte Cryopreservation. Lancet 1986, 1, 884–886. [Google Scholar] [CrossRef]
- Yang, D.; Brown, S.E.; Nguyen, K.; Reddy, V.; Brubaker, C.; Winslow, K.L. Live birth after the transfer of human embryos developed from cryopreserved oocytes harvested before cancer treatment. Fertil. Steril. 2007, 87, 1469.e1–1469.e4. [Google Scholar] [CrossRef]
- Sánchez-Serrano, M.; Crespo, J.; Mirabet, V.; Cobo, A.C.; Escribá, M.-J.; Simón, C.; Pellicer, A. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil. Steril. 2010, 93, 268.e11–268.e13. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, D.R.; Han, J.E.; Kim, Y.S.; Lee, W.S.; Won, H.J.; Kim, J.W.; Yoon, T.K. Live birth with vitrified-warmed oocytes of a chronic myeloid leukemia patient nine years after allogenic bone marrow transplantation. J. Assist. Reprod. Gen. 2011, 28, 1167–1170. [Google Scholar] [CrossRef]
- Garcia-Velasco, J.A.; Domingo, J.; Cobo, A.; Martinez, M.; Carmona, L.; Pellicer, A. Five years’ experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil. Steril. 2013, 99, 1994–1999. [Google Scholar] [CrossRef]
- Martinez, M.; Rabadan, S.; Domingo, J.; Cobo, A.; Pellicer, A.; Garcia-Velasco, J.A. Obstetric outcome after oocyte vitrification and warming for fertility preservation in women with cancer. Reprod. Biomed. Online 2014, 29, 722–728. [Google Scholar] [CrossRef]
- Da Motta, E.L.A.; Bonavita, M.; Alegretti, J.R.; Chehin, M.; Serafini, P. Live birth after 6 years of oocyte vitrification in a survivor with breast cancer. J. Assist. Reprod. Genet. 2014, 31, 1397–1400. [Google Scholar] [CrossRef][Green Version]
- Alvarez, M.; Solé, M.; Devesa, M.; Fábregas, R.; Boada, M.; Tur, R.; Coroleu, B.; Veiga, A.; Barri, P.N. Live birth using vitrifiedwarmed oocytes in invasive ovarian cancer: Case report and literature review. Reprod. Biomed. Online 2014, 28, 663–668. [Google Scholar] [CrossRef]
- Doyle, J.O.; Richter, K.S.; Lim, J.; Stillman, R.J.; Graham, J.R.; Tucker, M.J. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil. Steril. 2016, 105, 459–466.e2. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference: Methods and Development; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Sauer, M.V.; Paulson, R.J.; Ary, B.A.; Lobo, R.A. Three hundred cycles of oocyte donation at the University of Southern California: Assessing the effect of age and infertility diagnosis on pregnancy and implantation rates. J. Assist. Reprod. Genet. 1994, 11, 92–96. [Google Scholar] [CrossRef]
- Chow, E.J.; Stratton, K.L.; Leisenring, W.; Oeffinger, K.C.; Sklar, C.A.; Donaldson, S.S.; Ginsberg, J.P.; Kenney, L.B.; Levine, J.M.; Robison, L.L.; et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2016, 17, 567–576. [Google Scholar] [CrossRef]
- Cobo, A.; García-Velasco, J.; Domingo, J.; Pellicer, A.; Remohí, J. Elective and Onco-fertility preservation: Factors related to IVF outcomes. Hum. Reprod. 2018, 33, 2222–2231. [Google Scholar] [CrossRef]
- Specchia, C.; Baggiani, A.; Immediata, V.; Ronchetti, C.; Cesana, A.; Smeraldi, A.; Scaravelli, G.; Levi-Setti, P.E. Oocyte Cryopreservation in Oncological Patients: Eighteen Years Experience of a Tertiary Care Referral Center. Front. Endocrinol. 2019, 10, 600. [Google Scholar] [CrossRef]
- Waimey, K.E.; Smith, B.M.; Confino, R.; Jeruss, J.S.; Pavone, M.E. Understanding Fertility in Young Female Cancer Patients. J. Women’s Health 2015, 24, 812–818. [Google Scholar] [CrossRef]
- Perrin, J.; Saias-Magnan, J.; Broussais, F.; Bouabdallah, R.; D’Ercole, C.; Courbiere, B. First French live-birth after oocyte vitrification performed before chemotherapy for fertility preservation. J. Assist. Reprod. Genet. 2016, 33, 663–666. [Google Scholar] [CrossRef]
- Alvarez, R.M.; Ramanathan, P. Fertility preservation in female oncology patients: The influence of the type of cancer on ovarian stimulation response. Hum. Reprod. 2018, 33, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Mirallie, S.; Leperlier, F.; Reignier, A.; Barriere, P.; Freour, T. Ovarian reserve and response to stimulation in women undergoing fertility preservation according to malignancy type. Reprod. Biomed. Online 2018, 37, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Azim, A.A.; Costantini-Ferrando, M.; Oktay, K. Safety of fertility preservation by ovarian stimulation with letrozole and Gonadotropins in patients with breast cancer: A prospective controlled study. J. Clin. Oncol. 2008, 26, 2630–2635. [Google Scholar] [CrossRef] [PubMed]
- Turan, V.; Bedoschi, G.; Moy, F.; Oktay, K. Safety and feasibility of performing two consecutive ovarian stimulation cycles with the use of letrozole-gonadotropin protocol for fertility preservation in breast cancer patients. Fertil. Steril. 2013, 100, 1681. [Google Scholar] [CrossRef]
- Cobo, A.; Garcia-Velasco, J.A.; Domingo, J.; Remohí, J.; Pellicer, A. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients? Fertil. Steril. 2013, 99, 1485–1495. [Google Scholar] [CrossRef]
- Cil, A.P.; Bang, H.; Oktay, K. Age-specific probability of live birth with oocyte cryopreservation: An individual patient data meta-analysis. Fertil. Steril. 2013, 100, 492–499.e3. [Google Scholar] [CrossRef]
- Berg, M.V.D.; Van Dijk, M.; Byrne, J.; Campbell, H.; Berger, C.; Borgmann-Staudt, A.; Calaminus, G.; Dirksen, U.; Winther, J.F.; Fossa, S.D.; et al. Fertility Among Female Survivors of Childhood, Adolescent, and Young Adult Cancer: Protocol for Two Pan-European Studies (PanCareLIFE). JMIR Res. Protoc. 2018, 7, e10824. [Google Scholar] [CrossRef]
- Dittrich, R.; Kliesch, S.; Schüring, A.; Balcerek, M.; Baston-Büst, D.M.; Beck, R.; Beckmann, M.W.; Behringer, K.; Borgmann-Staudt, A.; Cremer, W.; et al. Fertility Preservation for Patients with Malignant Disease. Guideline of the DGGG, DGU and DGRM (S2k-Level, AWMF Registry No. 015/082, November 2017)—Recommendations and Statements for Girls and Women. Geburtshilfe Frauenheilkd. 2018, 78, 567–584. [Google Scholar] [CrossRef]
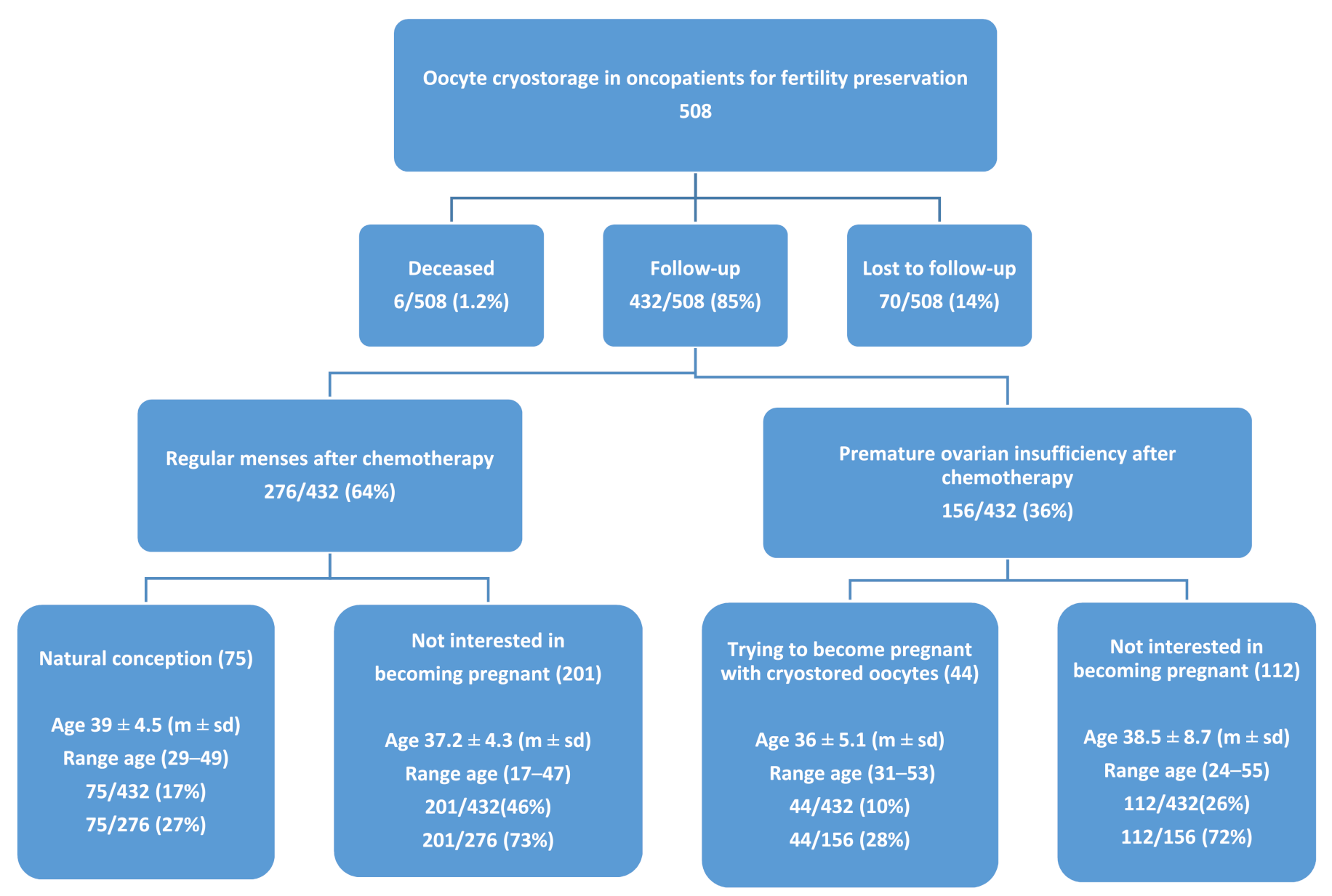
| Cryostorage (t0) | Follow-Up (t1) | p t0 vs. t1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients (%) | AGE | AFC | AMH | FSH | AGE | AFC | AMH | FSH | |||
| Total Patients | 508 | 29.4 ± 4.0 | 7.1 ± 5.0 | 1.6 ± 0.8 | 13.1 ± 8.1 | 37.6 ± 5.6 | 2.8 ± 1.6 | 0.7 ± 0.3 | 34.5 ± 17.4 | Age t0–t1 0.001 AFC t0–t1 0.001 AMH t0–t1 0.001 FSH t0–t1 0.001 | |
| Regular menses after chemotherapy 276/432 (64%) (a) | 276 | 28.5 ± 5.2 | 8.7 ± 2.2 | 1.6 ± 0.6 | 7.8 ± 4.5 | 38.1 ± 4.2 | 4.3 ± 2.8 | 1.3 ± 0.3 | 6.8 ± 2.9 | at t0 Age a vs. b 0.001 AFC a vs. b 0.001 AMH a vs. b 0.001 FSH a vs. b 0.001 | |
| Natural conception (a1) | 75/276 (27%) | 29 ± 4.8 | 9.3 ± 3.8 | 1.9 ± 0.6 | 7.4 ± 3.2 | 39 ± 4.5 | 5.3 ± 3.2 | 1.5 ± 0.4 | 6.7 ± 4.5 | Age t0–t1 0.001 AFC t0–t1 0.001 AMH t0–t1 0.001 FSH t0–t1 0.274 | |
| Not interested in becoming pregnant (a2) | 201/276 (73%) | 28.6 ± 6.7 | 8.2 ± 4.7 | 1.5 ± 0.9 | 9.5 ± 6.6 | 37.2 ± 4.3 | 4.5 ± 2.1 | 1.2 ± 0.5 | 7.2 ± 3.3 | Age t0–t1 0.001 AFC t0–t1 0.001 AMH t0–t1 0.001 FSH t0–t1 0.001 | |
| POI after chemotherapy 156/432 (36%) (b) | 156 | 31.5 ± 4.8 | 7.2 ± 1.7 | 1.1 ± 0.7 | 9.1 ± 3.2 | 37.2 ± 4.9 | 0.4 ± 0.3 | 0.2 ± 0.1 | 63.2 ± 17.0 | at t1 Age a vs. b 0.044 AFC a vs. b 0.001 AMH a vs. b 0.001 FSH a vs. b 0.001 | |
| Not interested in becoming pregnant (b1) | 112/156 (72%) | 32 ± 6.7 | 7.2 ± 1.8 | 1.3 ± 0.9 | 9.02 ± 5.4 | 38.5 ± 8.7 | 0.7 ± 0.5 | 0.03 ± 0.01 | 72.0 ± 43.0 | Age t0–t1 0.001 AFC t0–t1 0.001 AMH t0–t1 0.001 FSH t0–t1 0.001 | |
| Trying to become pregnant with cryostored oocytes (b2) | 44/156 (28%) | 29.4 ± 4.0 | 7.3 ± 2.1 | 1.4 ± 0.8 | 9.3 ± 2.8 | 36.0 ± 5.1 | 0.5 ± 0.3 | 0.23 ± 0.11 | 56.0 ± 23.0 | Age t0–t1 0.001 AFC t0–t1 0.001 AMH t0–t1 0.001 FSH t0–t1 0.001 | |
| Lost to follow-up (70) | 70/508 (14%) | 29.7 ± 3.5 | 8.6 ± 5.1 | 1.8 ± 0.7 | 7.7 ± 3.2 | - | - | - | - | ||
| Deceased (6) | 6/508 (1.2%) | 29.3 ± 2.1 | 5.1 ± 1.8 | 0.9 ± 0.3 | 10.1 ± 2.3 | - | - | - | - | ||
| Oncological Patients | Nononcological Patients | p | |
|---|---|---|---|
| Patients (n) | 508 | 1042 | |
| Age (years) (m ± sd) | 29.4 ± 4.0 | 30.0 ± 6.8 | 0.066 |
| FSH (IU/L) (m ± sd) | 13.1 ± 8.1 | 12.4 ± 7.7 | 0.099 |
| AMH (ng/mL) (m ± sd) | 1.6± 0.8 | 1.6 ± 0.9 | 1.000 |
| AFC n (m ± sd) | 7.1 ± 5.0 | 7.3 ± 2.0 | 0.263 |
| Length of storage (years) (range) (m ± sd) | 1–25 (5.6 ± 3.2) | 1–25 (4.8 ± 3.7) | 0.210 |
| FSH administrated (IU) (m ± sd) | 2630 ± 1402 | 2750 ± 1305 | 0.098 |
| Follicles > 16 mm n (m ± sd) | 8.0 ± 3.0 | 7.7 ± 3.4 | 0.091 |
| E2 max (pg/mL) (m ± sd) | 1280 ± 645 | 1345 ± 1070 | 0.207 |
| Oocytes retrieved n (m ± sd) | 3604 (8.8 ± 6.9) | 7644 (8.5 ± 6.8) | 0.067 |
| Oocytes cryopreserved n (m ± sd) | 2966 (6.1 ± 4.2) | 5046 (5.9 ± 4.8) | 0.060 |
| Oncological Patients | Nononcological Patients | p | |
|---|---|---|---|
| Patients (n) | 44 | 870 | |
| Age at cryopreservation years (m ± sd) | 29.4 ± 4.0 | 30.0 ± 6.8 | 0.562 |
| Age at oocyte thawing/warming years (m ± sd) | 36.0 ± 5.1 | 37.1 ± 4.2 | 0.094 |
| Thawing/warming cycles (n) | 64 | 1315 | |
| Length of storage years (range) (m ± sd) | 2–15 (5.0 ± 3.8) | 2–15 (4.8 ± 3.7) | 0.146 |
| Thawed/warmed oocytes n (m ± sd) | 194 (3.7 ±1.9) | 4208 (3.5 ± 1.8) | 0.131 |
| Oocytes survived (%) | 157 (80.9) | 3172 (75.4) | 0.094 |
| Oocytes fertilized (%) | 101/138 (73.2) | 2172/2793 (77.8) | 0.249 |
| Embryo transfers (%) | 57/64 (89.1) | 1165/1294 (90.0) | 0.969 |
| Embryo transferred n (m ± sd) | 100 (1.7 ± 0.7) | 2044 (1.8 ± 0.6) | 0.107 |
| Pregnancies n | 18 | 361 | 0.958 |
| Births n | 13 | 283 | |
| Newborns n | 15 | 302 | 0.772 |
| Miscarriages n (%) | 4 (22) | 78/361 (21.6) | 0.817 |
| Pregnancy per patient (%) | 18/44 (41.0) | 361/870 (41.4) | 0.936 |
| Pregnancy per cycle (%) | 18/64 (28.1) | 361/1315 (27.4) | 0.980 |
| Pregnancy per transfer (%) | 18/57 (31.5) | 361/1165 (31.0) | 0.958 |
| Births per patient (%) | 13/44 (29.9) | 283/870 (32.5) | 0.805 |
| Births per cycle (%) | 13/64 (20.3) | 283/1315 (21.5) | 0.941 |
| Births per transfer (%) | 13/57 (22.8) | 283/1165 (24.2) | 0.866 |
| Newborns per patient (%) | 15/44 (34.1) | 302/870 (34.7) | 0.938 |
| Newborns per cycle (%) | 15/64 (23.4) | 302/1315 (23.9) | 0.949 |
| Newborns per transfer (%) | 15/57 (26.3) | 302/1165 (26.0) | 0.999 |
| Oncological Patients | Nononcological Patients | p | |
|---|---|---|---|
| Oocytes cryopreserved n (m ± sd) | 2966 (6.1 ± 4.2) | 5046 (5.9 ± 4.8) | 0.060 |
| Thawed/warmed oocytes n (m ± sd) | 194 (3.7 ± 1.9) | 4208 (3.5 ± 1.8) | 0.131 |
| Oocyte still in storage n (m ± sd) | 2772 (2.5 ± 1.1) | 838 (2.4 ± 1.4) | 0.031 |
| Sex | Current Age (y/mths) | Mother’s Cancer Type | Slow-Freezing/Vitrification | Length of Storage (y) | Length of Gestation (Weeks) | Delivery | Twins | Apgar Score 1 min | Apgar Score 5 min | Weight (g) | Length (cm) | Head Circumference (cm) | Malformations | Current Height (cm) | Current Weight (kg) | Teething (mths) | Walking (mths) | Language (mths) | Puberty (y) | Educational Stage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 7 y 6 mths | Breast cancer | Slow | 3 | 39 | CS | 10 | 10 | 3320 | 52 | 32 | - | 134 | 29 | 8 | 12 | 24 | - | 1° grade | |
| F | 7 y 4 mths | Breast cancer | Vitri | 2 | 37 | CS | Yes | 9 | 10 | 2050 | 47 | 32 | - | 132 | 25 | 9 | 10 | 20 | - | 1° grade |
| F | 7 y 4 mths | Breast cancer | Vitri | 2 | 37 | CS | Yes | 9 | 10 | 2045 | 48 | 33 | - | 130 | 26 | 9 | 11 | 18 | - | 1° grade |
| F | 10 mths | Breast cancer | Vitri | 3 | 39 | CS | 8 | 9 | 3670 | 48 | 34 | - | 70 | 12 | ||||||
| F | 6 y 5 mths | BOT | Vitri | 3 | 38 | CS | 9 | 10 | 3900 | 50 | 34 | - | 115 | 21 | 10 | 10 | 13 | - | 1° grade | |
| M | 4 y 1 mths | BOT | Vitri | 2 | 39 | CS | 10 | 10 | 3250 | 48 | 33 | - | 99 | 15 | 6 | 14 | 20 | - | Kindergarten | |
| F | 10 y | BOT | Slow | 5 | 38 | CS | 9 | 10 | 2980 | 48 | 32 | Labiopalatoschisis | 140 | 32 | 8 | 12 | 18 | - | 1° grade | |
| F | 13 y 5 mths | BOT | Slow | 4 | 34 | CS | Yes | 9 | 10 | 2120 | 48 | 33 | - | 152 | 48 | 8 | 14 | 16 | 11 | Middle school |
| F | 13 y 5 mths | BOT | Slow | 4 | 34 | CS | Yes | 9 | 10 | 2090 | 48 | 32 | - | 148 | 50 | 8 | 13 | 16 | 12 | Middle school |
| M | 7 y | Endometrial cancer | Slow | 4 | 40 | CS | 10 | 10 | 3850 | 50 | 34 | - | 133 | 30 | 12 | 9 | 14 | - | 1° grade | |
| M | 1 y 10 mths | NHL | Slow | 6 | 39 | CS | 9 | 10 | 3130 | 49 | 33 | - | 104 | 17 | 12 | 12 | Few words | - | Kindergarten | |
| M | 2 y | HL | Vitri | 7 | 38 | SD | 9 | 10 | 2575 | 48 | 32 | - | 85 | 16 | 10 | 12 | 12 | - | - | |
| M | 2 y 1 mths | HL | Vitri | 5 | 39 | SD | 10 | 10 | 3125 | 49 | 32 | - | 87 | 14 | 11 | 11 | 14 | - | - | |
| F | 2 y 4 mths | HL | Vitri | 4 | 40 | CS | 10 | 10 | 3320 | 55 | 34 | - | 88 | 13 | 9 | 14 | 14 | - | - | |
| M | 1 mths | BOT | Vitri | 3 | 39 | SD | 9 | 10 | 3150 | 50 | 33 | - | 87 | 6 |
| Authors | Malignancy | Age at Cryopreservation | Cryopreservation Technique | Age at Thawing/Warming | N MII Oocytes Cryopreserved | No. of Embryos Transferred | Pregnancies | Delivery | No. of Live Births | Sex |
|---|---|---|---|---|---|---|---|---|---|---|
| Yang et al., 2007 [36] | Hodgkin lymphoma | 27 | Slow freezing | 33 | 13 | 3 + 3 + 3 (gestational carrier) | Single | - | 1 | M |
| Porcu et al., 2008 [31] | Borderline ovarian tumor | 27 | Slow freezing | 31 | 7 | 3 | Twins | CS | 2 | F, F |
| Sanchez-Serrano et al., 2010 [37] | Breast cancer | 36 | Vitrification of oocytes after stimulation of ovarian tissue transplanted | 36 | 9 | 2 | Twins | CS | 2 | M, M |
| Kim et al., 2011 [38] | Chronic myeloid leukemia | 22 | Vitrification | 31 | 7 | 2 | Single | CS | 1 | M |
| Garcia Velasco et al., 2013 [39] | Hodgkin lymphoma | 33 | Vitrification | 35 | 4 | 2 | Single | VD | 1 | M |
| Martinez et al., 2014 [40] | Breast cancer | 30 | Vitrification | 33 | 5 | 2 | Single | CS | 1 | M |
| Martinez et al., 2014 [40] | Breast cancer | 33 | Vitrification | 38 | 3 | 2 | Single | VD | 1 | F |
| Martinez et al., 2014 [40] | Breast cancer | 37 | Vitrification | 40 | 8 | 2 | Single | CS | 1 | M |
| Martinez et al., 2014 [40] | Hodgkin lymphoma | 33 | Vitrification | 35 | 4 | 2 | Single | VD | 1 | F |
| Alvez Da Motta et al., 2014 [41] | Breast cancer | 36 | Vitrification | 41 | 28 | 3 + 3 | Single | CS | 1 | - |
| Alvarez et al., 2014 [42] | Invasive ovarian cancer | 28 | Vitrification | 29 | 14 | 2 | Heterotopic | CS | 1 | M |
| Druckenmiller et al., 2016 [11] | Gynecological cancer | 28 | Vitrification | - | 8 | 2 (gestational carrier) | Twins | - | 2 | - |
| Druckenmiller et al., 2016 [11] | Breast cancer | 33 | Slow freezing | - | 8 | 2 (gestational carrier) | Single | - | 1 | - |
| Druckenmiller et al., 2016 [11] | Breast cancer | 39 | Slow freezing | - | 8 | 2 | Single | - | 1 | - |
| Druckenmiller et al., 2016 [11] | Breast cancer | 40 | Slow freezing | - | 8 | 2 | Single | - | 1 | - |
| Perrin et al., 2016 [43] | Hodgkin lymphoma | 29 | Vitrification | 31 | 5 | 2 | Single | VD | 1 | F |
| Doyle et al., 2016 [44] | - | Vitrification | - | - | - | Single | - | 1 | - | |
| Specchia et al., 2019 [45] | Breast cancer | 35 | Vitrification | 40 | 9 | 3 + 1 | Single | - | 1 | - |
| Specchia et al., 2019 [45] | Breast cancer | 36 | Vitrification | 40 | 13 | 2 | Single | - | 1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcu, E.; Cipriani, L.; Dirodi, M.; De Iaco, P.; Perrone, A.M.; Zinzani, P.L.; Taffurelli, M.; Zamagni, C.; Ciotti, P.M.; Notarangelo, L.; et al. Successful Pregnancies, Births, and Children Development Following Oocyte Cryostorage in Female Cancer Patients During 25 Years of Fertility Preservation. Cancers 2022, 14, 1429. https://doi.org/10.3390/cancers14061429
Porcu E, Cipriani L, Dirodi M, De Iaco P, Perrone AM, Zinzani PL, Taffurelli M, Zamagni C, Ciotti PM, Notarangelo L, et al. Successful Pregnancies, Births, and Children Development Following Oocyte Cryostorage in Female Cancer Patients During 25 Years of Fertility Preservation. Cancers. 2022; 14(6):1429. https://doi.org/10.3390/cancers14061429
Chicago/Turabian StylePorcu, Eleonora, Linda Cipriani, Maria Dirodi, Pierandrea De Iaco, Anna Myriam Perrone, Pier Luigi Zinzani, Mario Taffurelli, Claudio Zamagni, Patrizia Maria Ciotti, Leonardo Notarangelo, and et al. 2022. "Successful Pregnancies, Births, and Children Development Following Oocyte Cryostorage in Female Cancer Patients During 25 Years of Fertility Preservation" Cancers 14, no. 6: 1429. https://doi.org/10.3390/cancers14061429
APA StylePorcu, E., Cipriani, L., Dirodi, M., De Iaco, P., Perrone, A. M., Zinzani, P. L., Taffurelli, M., Zamagni, C., Ciotti, P. M., Notarangelo, L., Calza, N., & Damiano, G. (2022). Successful Pregnancies, Births, and Children Development Following Oocyte Cryostorage in Female Cancer Patients During 25 Years of Fertility Preservation. Cancers, 14(6), 1429. https://doi.org/10.3390/cancers14061429








