Simple Summary
Our review discusses findings for the non-touch isolation technique in surgery, especially in surgery for non-small-cell lung cancer. This technique aims to prevent the release of circulating tumor cells (CTCs) from the tumor nest to the bloodstream during surgery, but its efficacy has not been clearly verified. We have summarized the history of CTC detection, relevance of CTCs to clinical practice, and evidence regarding this technique for lung cancer surgery.
Abstract
Circulating tumor cells (CTCs) are dislodged from the primary tumor into the bloodstream, travel within the bloodstream to distant organs, and finally extravasate and proliferate as epithelial metastatic deposits. The relationship between the existence of CTCs and tumor prognosis has been demonstrated by many researchers. In surgery for malignancies, the surgical manipulation of tumors and tissues around the tumor may lead to the release of CTCs into the bloodstream. The non-touch isolation technique (NTIT) has been advocated to prevent the release of CTCs during surgery. The concept of NTIT is the prevention of intraoperative increment of CTCs from the primary tumor by the early blockade of outflow vessels, and ‘pulmonary vein (PV)-first lobectomy’ during surgery for non-small-cell lung cancer (NSCLC) corresponds to this technique. The concept of PV-first lobectomy is well known among thoracic surgeons, but evidence of its efficacy for preventing the increase of intra- and postoperative CTCs and for improving postoperative prognosis is still uncertain. Our study summarizes evidence regarding the relationship between NTIT and CTCs in NSCLC and suggests the need for further research on CTCs and CTC-detecting modalities.
1. Introduction
Almost all patients with solid malignant tumors die from distant metastases. Recently, the outcomes and prognoses after treatment for localized malignant tumors have improved due to better diagnostic modalities that lead to the early detection of malignant tumors. However, the prognosis of patients with metastases remains poor. For example, the 5-year overall survival (OS) of patients with advanced lung cancer is reported to be approximately 1–2 years, even after treatment with molecular-targeted therapies [1]. “Distant metastasis” is thought to occur when tumor cells are dislodged from the primary cancer lesion, spread through the blood stream and/or lymphatic drainage system, affix to other organs, and proliferate. The detection of such tumor cells was first reported in 1869. Ashworth first observed circulating tumor cells (CTCs) in the blood of a man with metastatic cancer using a microscope [2]. Subsequently, some researchers reported the presence of CTCs in the blood stream [3,4,5,6,7,8,9,10], but research on the influence of CTCs on cancer prognosis did not progress even in the 2000s because of their rarity. Because CTCs exist only in low numbers in the blood stream (typically 1–10/mm3), they are difficult to detect and capture using conventional technologies such as microscopy. Recent progress in biomedical technology has led to the detection of CTCs in the bloodstream [11], and researchers have reported the prognostic influence of CTCs in colorectal [12], lung [13], breast [14], pancreatic [15], head and neck [16], and prostate [17] cancer.
The mechanism of epithelial cancer metastasis has been explained by the “epithelial-to-mesenchymal transition (EMT)” and “mesenchymal-to-epithelial transition (MET)” theory. In this theory, a series of sequential steps is involved. First, the EMT of individual cells occurs within the primary tumor, leading to their intravasation into the bloodstream, survival and travel as CTCs within the bloodstream, and finally, extravasation at distant sites, where MET culminates in their proliferation as epithelial metastatic deposits [18]. Yu et al. [19] demonstrated EMT in human breast cancer cells in the circulation. However, Yu et al. [19] also reported that CTCs that become resistant to chemotherapy had a very high appearance of markers representing the mesenchymal system and also showed an epithelium appearance. It is difficult to explain this phenomenon using only the EMT/MET theory in single-shot CTCs. To resolve this controversy, the “hybrid EMT/MET” theory in cluster CTCs has become noteworthy. Aceto et al. [20] showed the presence of CTC clusters in the blood stream and their important influence on patient prognosis. They showed that CTC clusters have a 23 to 50-fold increased metastatic potential compared to single CTCs. CTC clusters are composed of 2–50 cancer cells that occur as tumor-derived microemboli that may break off from primary tumors [20]. CTC clusters show various EMT/MET statuses, which consequently demonstrate adherence, migration, anti-anoikis, and tumor formation [21]. According to these results, CTC clusters may be the “distant metastases”. Such progress in research on CTCs as a prognostic factor has attracted the attention of many researchers.
2. Non-Touch Isolation Technique in Surgery for Other Malignancies
As mentioned above, CTCs are released from the primary tumor into the bloodstream, especially in CTC clusters, which have a high metastatic potential and are released as tumorous microemboli. Theoretically, this release may be promoted by perioperative procedures, such as preoperative tumor biopsy and intraoperative manipulation. Some researchers have reported an increase in the number of CTCs after surgical manipulation in colorectal [22,23], hepatocellular [24], pancreatic [25], and lung [26,27] cancer. To avoid the release of CTCs by intraoperative surgical manipulation, a non-touch isolation technique (NTIT) has been devised for various cancer surgeries. The concept of NTIT is the prevention of the intraoperative increase in CTCs from primary tumors by the early blockade of outflow vessels.
NTIT was first described for colorectal cancer surgery in 1952. Barnes advocated the ligation of the vascular pedicles and division of the bowel prior to handling the cancer-bearing segment [28]. Thereafter, Turnbull et al. [29] reported the results of a retrospective study evaluating the long-term outcomes of patients who underwent colorectal cancer surgery with NTIT. Their study included 896 patients who underwent colorectal cancer surgery between 1950 and 1964 and showed better outcomes in patients with NTIT than in those without NTIT (5-year OS of 50.86% in patients with NTIT and 34.82% in patients without NTIT). To investigate the efficacy of NTIT, a randomized controlled trial (RCT) was conducted in the 1980s, but this trial could not show any statistical significance regarding NTIT due to the lack of statistical power [30]; therefore, NTIT is not yet a standard procedure for colorectal cancer. However, several studies have shown the efficacy of NTIT in colorectal cancer surgery [31]. An RCT with a large sample size that aims to evaluate the efficacy of NTIT for colorectal cancer surgery has been conducted in Japan [32]. The enrollment for this study has been completed, and the results will be released in the near future.
NTIT has also been adopted for surgery for pancreatic cancers and hepatocellular carcinoma. In the 1990s, Nakao et al. [33] first reported the use of NTIT for pancreatic head cancer using an antithrombogenic portal vein bypass catheter between the mesenteric and intrahepatic portal veins.
Kobayashi et al. proposed the use of NTIT for pancreatoduodenectomy without removing the portal vein for periampullary carcinoma [34]. Hirota et al. [35] proposed NTIT for pancreatoduodenectomy using a hanging-up and clamping technique. Hirota et al. [36] also reported that the 3-year OS rate after surgery for patients with NTIT was superior to that for patients without NTIT (75% and 14%, respectively). However, Gall et al. [37] showed no impact of NTIT on postoperative survival in pancreatic cancer, despite the significant decrease in the number of CTCs detected in the portal vein with NTIT. RCTs evaluating the efficacy of NTIT for pancreatic cancer have been conducted because of the rarity of surgery for pancreatic cancer, meaning that the efficacy of NTIT for pancreatic cancer is yet to be established.
3. Non-Touch Isolation Technique in Surgery for Non-Small-Cell Lung Cancer
Regarding surgery for non-small-cell lung cancer (NSCLC), “pulmonary vein (PV)-first lobectomy” is regarded as NTIT. Anatomical lung resection, including lobectomy, which is the standard procedure for the treatment of NSCLC, requires the dissection of the lobar branch of the pulmonary artery (PA), PV, and bronchus. These structures have a complex three-dimensional location; thus, lung manipulation is required for anatomical lung resection. Regarding other types of cancers, thoracic surgeons and researchers, for many years, have thought that surgical manipulation results in the spillage of tumor cells from primary tumors, and many researchers have demonstrated an increase in the number of CTCs or surrogate substances in peripheral blood and the PV after surgical procedures for NSCLC (Table 1). PV-first lobectomy, which is characterized by early PV ligation, has been advocated since the 1950s [38], as it theoretically prevents the outflow of CTCs produced by surgical manipulation to the circulation. This is because the PV is the discharge canal for the central bloodstream; thus, PV-first lobectomy improves postoperative prognosis. To evaluate the efficacy of PV-first lobectomy, researchers recently attempted to demonstrate its effect on the behavior of perioperative CTCs and postoperative prognoses. For the analysis of perioperative changes in CTC after PV-first lobectomy, Kurusu et al. [39] conducted an RCT in a small cohort dividing patients into two groups (PA-first ligation and PV-first ligation) and showed that patients in the PV-first group had a lower positive conversion rate for peripheral arterial carcinoembryonic antigen (CEA) mRNA expression than patients in the PA-first group. Song et al. [40] showed in their RCT that the mRNA expression of CD44v6 and CK19 in the PA-first group increased after vessel ligation compared to before ligation, whereas the expressions in the PV-first group were similar before and after vessel ligation. Duan et al. [41] and Wei et al. [42] also reported the efficacy of the PV-first procedure in preventing CTC production. However, Ge et al. [43] conducted an RCT focused on the detection of CK19 and CEA mRNA in peripheral blood and reported no significant difference between the PV-first and PA-first groups in the trend of change of the perioperative values of CEA mRNA. Moreover, they detected circulating epithelial cells (supposed non-malignant bronchial epithelial cells entering into the bloodstream by surgical manipulation) in two patients in the control group who underwent surgery for non-malignant lesions, which suggests that CK19 mRNA might be detected even in the peripheral blood of patients without malignancies. Hashimoto et al. [44] also reported that the PV-first procedure had a significant influence on the intraoperative increase in CTCs, and the role of PV-first lobectomy in preventing the increment of CTCs is still uncertain (Table 2).

Table 1.
Summary of studies researching the relationship between surgical manipulation and change in CTCs detection in non-small-cell lung cancer.

Table 2.
Summary of studies investigating the relationship between the PV-first technique and change in postoperative CTCs detection in non-small-cell lung cancer.
To analyze the effect of PV-first lobectomy on postoperative prognosis, we systematically reviewed the literature. The selection of studies was based on the titles, abstracts, and full papers, with the following inclusion criteria: (1) comparative studies examining PV-first versus PA-first lobectomy, (2) RCTs or observational retrospective/prospective cohort and case-control studies, and (3) studies that reported postoperative prognostic outcomes, such as recurrence and survival rates. The literature search was conducted using PubMed with search terms: (“lung cancer” OR “lung carcinoma” OR “lung neoplasm”) AND (“vein-first” OR “artery-first” OR “vessel ligation” OR “vessel sequence”) AND (“surgery” OR “operat*” OR “postoperative”), and 46 studies were identified. Among them, six studies were included (Figure 1; Table 3). Refaely et al. [55] reported in their retrospective study that the sequence of vessel interruption (PV-first or PA-first) had no influence on disease recurrence. Kozak et al. [56] also reported the non-efficacy of the sequence of vessel interruption regarding postoperative survival in their RCT. However, their studies included patients who underwent lobectomy through open thoracotomy, which requires more aggressive manual manipulation than VATS lobectomy and may lead to the release of large amounts of CTCs in the early phase of surgery before PV ligation. Li et al. [57] reported the non-efficacy of PV-first lobectomy for postoperative survival in their study, which included only patients who underwent lobectomy by VATS, but their analyses were conducted with patient classification into three groups (PV-first, PA-first, and PA-PV-PA sequence), and no data comparing PV-first and PA-first alone were shown. However, recent studies that included only VATS lobectomy showed the efficacy of PV-first lobectomy for postoperative survival. Sumitomo et al. [58] reported in their retrospective cohort study that PV-first was an independent prognostic factor for better disease-free survival, and He et al. [59] reported in their retrospective study that PV-first lobectomy through the VATS approach was preferred for patients with squamous cell carcinoma, which seemed to metastasize through the bloodstream rather than the lymphatic stream. Moreover, Wei et al. [47] conducted a retrospective cohort study with propensity score matching to minimize selection bias and reported that the 5-year OS rate in the PV-first group was significantly better than that in the PA-first group (73.5% in PV-first vs. 57.6% in PA-first, p = 0.002). In addition, the 5-year disease-free survival and 5-year lung cancer-specific survival in the PV-first group were significantly better than those in the PA-first group. Huang et al. [60] conducted a meta-analysis of five studies, including those by Kozak et al. [56], Li et al. [57], Sumitomo et al. [58], He et al. [59], and Wei et al. [47], and concluded that PV-first ligation is recommended during lobectomy for patients with NSCLC whenever possible. Based on these results, we consider that PV-first lobectomy through the VATS approach has the potential to improve postoperative survival in patients with surgically resectable NSCLC. However, only a few studies have evaluated the relationship between the PV-first technique and postoperative survival. Large-scale, prospective, and multicenter RCTs are needed to clarify the efficacy of PV-first lobectomy.
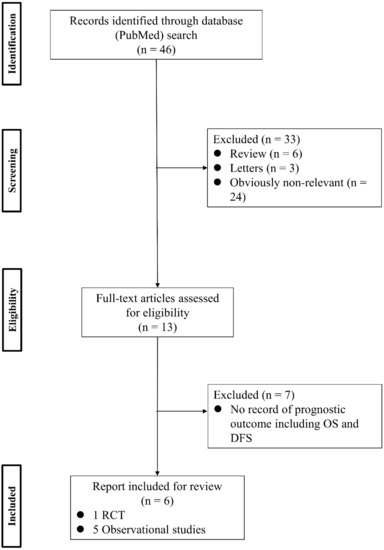
Figure 1.
Flowchart of the retrieval of relevant studies. DFS: disease-free survival, OS: overall survival, RCT: randomized controlled trial.

Table 3.
Summary of studies researching relation between PV-first technique and postoperative prognosis in non-small-cell lung cancer.
4. Conclusions
Surgical manipulation has the potential to induce increments in intra- and postoperative CTCs. NTIT, which is considered a PV-first technique in surgery for NSCLC, theoretically prevents the release of these CTCs by early blockade of outflow vessels. However, evidence of the efficacy of NTIT in the prevention of CTC release and postoperative prognosis is still unsatisfactory. This may be partially due to insufficient knowledge regarding the biological behavior of CTCs and the lack of technologies to detect CTCs. We believe that increased research into the biology of CTCs and the technologies for detecting CTCs will accelerate the interest of clinical physicians, which will lead to other clinical studies and contribute to improved patient prognosis.
Author Contributions
Conceptualization, H.A. and N.S.; methodology, H.A.; software, H.A.; validation, H.A., H.I. and N.S.; formal analysis, H.A.; investigation, H.A.; resources, H.A.; data curation, H.A.; writing—original draft preparation, H.A.; writing—review and editing, H.A.; visualization, H.A.; supervision, H.A. and H.I.; project administration, H.A.; funding acquisition, None. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, D.H.; Tsao, M.S.; Kambartel, K.O.; Isobe, H.; Huang, M.S.; Barrios, C.H.; Khattak, A.; de Marinis, F.; Kothari, S.; Arunachalam, A.; et al. Molecular Testing and Treatment Patterns for Patients with Advanced Non-Small Cell Lung Cancer: PIvOTAL Observational Study. PLoS ONE 2018, 13, e0202865. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, T.R. A Case of Cancer in Which Cells Similar to Those in the Tumors Were Seen in the Blood After Death. Aust. Med. J. 1869, 14, 146–147. [Google Scholar]
- Colombo, C.; Rolfo, F.; Maggi, G. Further Research on the Isolation of Tumor Cells from Circulating Blood. Minerva Med. 1959, 50, 2217–2223. [Google Scholar] [PubMed]
- Wilson, J.K. The Detection of Tumor Cells in Circulating Blood. Bull. Tulane Univ. Med. Fac. 1959, 18, 171–182. [Google Scholar]
- Romsdahl, M.M.; Potter, J.F.; Malmgren, R.A.; Chu, E.W.; Brindley, C.O.; Smith, R.R. A Clinical Study of Circulating Tumor Cells in Malignant Melanoma. Surg. Gynecol. Obstet. 1960, 111, 675–681. [Google Scholar]
- Soost, H.J. On the Incidence of Tumor Cells in the Circulating Blood. Dtsch. Med. Wochenschr. 1960, 85, 893–899. [Google Scholar] [CrossRef]
- Graeber, F. The Methodology of Cytological Cancer Diagnosis, Especially the Demonstration of Tumor Cells in the Circulating Blood in Man. Verh. Dtsch. Ges. Pathol. 1961, 45, 264–266. [Google Scholar]
- Romsdahl, M.D.; Chu, E.W.; Hume, R.; Smith, R.R. The Time of Metastasis and Release of Circulating Tumor Cells as Determined in an Experimental System. Cancer 1961, 14, 883–888. [Google Scholar] [CrossRef]
- Saito, H. Studies on Cancer Cells in the Circulating Blood. II. Experimental Study on the Fate of Intraportally Injected Tumor Cells and Metastasis Formation. Acta Med. Biol. 1961, 9, 151–173. [Google Scholar]
- Wuest, G.; Birk, G. Demonstration and Incidence of Tumor Cells in Circulating Human Blood. Med. Welt 1962, 17, 922–928. [Google Scholar]
- Panchapakesan, B.; Caprara, R.; Velasco, V.; Loomis, J.; King, B.; Xu, P.; Burkhead, T.; Sethu, P.; Stallons, L.J.; McGregor, W.G.; et al. Micro- and Nanotechnology Approaches for Capturing Circulating Tumor Cells. Cancer Nanotechnol. 2010, 1, 3–11. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Aigner, M.; Thorlund, K.; Mollberg, N.; Motschall, E.; Jensen, K.; Diener, M.K.; Büchler, M.W.; Koch, M.; Weitz, J. Meta-Analysis Shows That Detection of Circulating Tumor Cells Indicates Poor Prognosis in Patients with Colorectal Cancer. Gastroenterology 2010, 138, 1714–1726. [Google Scholar] [CrossRef]
- Hou, J.M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical Significance and Molecular Characteristics of Circulating Tumor Cells and Circulating Tumor Microemboli in Patients with Small-Cell Lung Cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Tjensvoll, K.; Nordgård, O.; Smaaland, R. Circulating Tumor Cells in Pancreatic Cancer Patients: Methods of Detection and Clinical Implications. Int. J. Cancer 2014, 134, 1–8. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Perry, C.; Jovanovic, L.; Nelson, C.; Punyadeera, C. Circulating Tumour Cells in Metastatic Head and Neck Cancers. Int. J. Cancer 2015, 136, 2515–2523. [Google Scholar] [CrossRef]
- Danila, D.C.; Fleisher, M.; Scher, H.I. Circulating Tumor Cells as Biomarkers in Prostate Cancer. Clin. Cancer Res. 2011, 17, 3903–3912. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating Tumor Cell Clusters Are Oligoclonal Precursors of Breast Cancer Metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Sawabata, N. Circulating Tumor Cells in Lung Cancer: Cluster Circulating Tumor Cells as Hybrid Epithelial-Mesenchymal Transition/Mesenchymal-Epithelial Transition (E/M). J. Thorac. Dis. 2017, 9, 3547–3550. [Google Scholar] [CrossRef]
- Cole, W.H.; Packard, D.; Southwick, H.W. Carcinoma of the Colon with Special Reference to Prevention of Recurrence. JAMA 1954, 155, 1549–1553. [Google Scholar] [CrossRef]
- Weitz, J.; Kienle, P.; Lacroix, J.; Willeke, F.; Benner, A.; Lehnert, T.; Herfarth, C.; von Knebel Doeberitz, M. Dissemination of Tumor Cells in Patients Undergoing Surgery for Colorectal Cancer. Clin. Cancer Res. 1998, 4, 343–348. [Google Scholar]
- Louha, M.; Nicolet, J.; Zylberberg, H.; Sabile, A.; Vons, C.; Vona, G.; Poussin, K.; Tournebize, M.; Capron, F.; Pol, S.; et al. Liver Resection and Needle Liver Biopsy Cause Hematogenous Dissemination of Liver Cells. Hepatology 1999, 29, 879–882. [Google Scholar] [CrossRef]
- Sergeant, G.; Roskams, T.; van Pelt, J.; Houtmeyers, F.; Aerts, R.; Topal, B. Perioperative Cancer Cell Dissemination Detected with a Real-Time RT-PCR Assay for EpCAM Is Not Associated with Worse Prognosis in Pancreatic Ductal Adenocarcinoma. BMC Cancer 2011, 11, 47. [Google Scholar] [CrossRef]
- Yao, X.; Williamson, C.; Adalsteinsson, V.A.; D’Agostino, R.S.; Fitton, T.; Smaroff, G.G.; William, R.T.; Wittrup, K.D.; Love, J.C. Tumor Cells Are Dislodged into the Pulmonary Vein During Lobectomy. J. Thorac. Cardiovasc. Surg. 2014, 148, 3224–3231. [Google Scholar] [CrossRef]
- Matsutani, N.; Sawabata, N.; Yamaguchi, M.; Woo, T.; Kudo, Y.; Kawase, A.; Shiono, S.; Iinuma, H.; Morita, S.; Kawamura, M. Does Lung Cancer Surgery Cause Circulating Tumor Cells?—A Multicenter, Prospective Study. J. Thorac. Dis. 2017, 9, 2419–2426. [Google Scholar] [CrossRef]
- Barnes, J.P. Physiologic Resection of the Right Colon. Surg. Gynecol. Obstet. 1952, 94, 722–726. [Google Scholar]
- Turnbull, R.B., Jr.; Kyle, K.; Watson, F.R.; Spratt, J. Cancer of the Colon: The Influence of the No-Touch Isolation Technic on Survival Rates. Ann. Surg. 1967, 166, 420–427. [Google Scholar] [CrossRef]
- Wiggers, T.; Jeekel, J.; Arends, J.W.; Brinkhorst, A.P.; Kluck, H.M.; Luyk, C.I.; Munting, J.D.; Povel, J.A.; Rutten, A.P.; Volovics, A. No-Touch Isolation Technique in Colon Cancer: A Controlled Prospective Trial. Br. J. Surg. 1988, 75, 409–415. [Google Scholar] [CrossRef]
- Fujita, J.; Uyama, I.; Sugioka, A.; Komori, Y.; Matsui, H.; Hasumi, A. Laparoscopic Right Hemicolectomy with Radical Lymph Node Dissection Using the No-Touch Isolation Technique for Advanced Colon Cancer. Surg. Today 2001, 31, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Takii, Y.; Shimada, Y.; Moriya, Y.; Nakamura, K.; Katayama, H.; Kimura, A.; Shibata, T.; Fukuda, H. A Randomized Controlled Trial of the Conventional Technique Versus the No-Touch Isolation Technique for Primary Tumor Resection in Patients with Colorectal Cancer: Japan Clinical Oncology Group Study JCOG1006. Jpn. J. Clin. Oncol. 2014, 44, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Takagi, H. Isolated Pancreatectomy for Pancreatic Head Carcinoma Using Catheter Bypass of the Portal Vein. Hepato-Gastroenterology 1993, 40, 426–429. [Google Scholar] [PubMed]
- Kobayashi, S.; Asano, T.; Ochiai, T. A Proposal of No-Touch Isolation Technique in Pancreatoduodenectomy for Periampullary Carcinomas. Hepato-Gastroenterology 2001, 48, 372–374. [Google Scholar]
- Hirota, M.; Kanemitsu, K.; Takamori, H.; Chikamoto, A.; Tanaka, H.; Sugita, H.; Sand, J.; Nordback, I.; Baba, H. Pancreatoduodenectomy Using a No-Touch Isolation Technique. Am. J. Surg. 2010, 199, e65–e68. [Google Scholar] [CrossRef]
- Hirota, M.; Shimada, S.; Yamamoto, K.; Tanaka, E.; Sugita, H.; Egami, H.; Ogawa, M. Pancreatectomy Using the No-Touch Isolation Technique Followed by Extensive Intraoperative Peritoneal Lavage to Prevent Cancer Cell Dissemination: A Pilot Study. JOP 2005, 6, 143–151. [Google Scholar]
- Gall, T.M.H.; Jacob, J.; Frampton, A.E.; Krell, J.; Kyriakides, C.; Castellano, L.; Stebbing, J.; Jiao, L.R. Reduced Dissemination of Circulating Tumor Cells with No-Touch Isolation Surgical Technique in Patients with Pancreatic Cancer. JAMA Surg. 2014, 149, 482–485. [Google Scholar] [CrossRef]
- Aylwin, J.A. Avoidable Vascular Spread in Resection for Bronchial Carcinoma. Thorax 1951, 6, 250–267. [Google Scholar] [CrossRef][Green Version]
- Kurusu, Y.; Yamashita, J.; Hayashi, N.; Mita, S.; Fujino, N.; Ogawa, M. The Sequence of Vessel Ligation Affects Tumor Release into the Circulation. J. Thorac. Cardiovasc. Surg. 1998, 116, 107–113. [Google Scholar] [CrossRef]
- Song, P.P.; Zhang, W.; Zhang, B.; Liu, Q.; Du, J. Effects of Different Sequences of Pulmonary Artery and Vein Ligations During Pulmonary Lobectomy on Blood Micrometastasis of Non-Small Cell Lung Cancer. Oncol. Lett. 2013, 5, 463–468. [Google Scholar] [CrossRef]
- Duan, X.; Zhu, Y.; Cui, Y.; Yang, Z.; Zhou, S.; Han, Y.; Yu, D.; Xiao, N.; Cao, X.; Li, Y.; et al. Circulating Tumor Cells in the Pulmonary Vein Increase Significantly After Lobectomy: A Prospective Observational Study. Thorac. Cancer 2019, 10, 163–169. [Google Scholar] [CrossRef]
- Wei, S.; Guo, C.; He, J.; Tan, Q.; Mei, J.; Yang, Z.; Liu, C.; Pu, Q.; Ma, L.; Yuan, Y.; et al. Effect of Vein-First vs Artery-First Surgical Technique on Circulating Tumor Cells and Survival in Patients with Non-Small Cell Lung Cancer: A Randomized Clinical Trial and Registry-Based Propensity Score Matching Analysis. JAMA Surg. 2019, 154, e190972. [Google Scholar] [CrossRef]
- Ge, M.J.; Shi, D.; Wu, Q.C.; Wang, M.; Li, L.B. Observation of Circulating Tumour Cells in Patients with Non-Small Cell Lung Cancer by Real-Time Fluorescent Quantitative Reverse Transcriptase-Polymerase Chain Reaction in Peroperative Period. J. Cancer Res. Clin. Oncol. 2006, 132, 248–256. [Google Scholar] [CrossRef]
- Hashimoto, M.; Tanaka, F.; Yoneda, K.; Takuwa, T.; Matsumoto, S.; Okumura, Y.; Kondo, N.; Tsubota, N.; Tsujimura, T.; Tabata, C.; et al. Significant Increase in Circulating Tumour Cells in Pulmonary Venous Blood During Surgical Manipulation in Patients with Primary Lung Cancer. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 775–783. [Google Scholar] [CrossRef]
- Yamashita, J.I.; Kurusu, Y.; Fujino, N.; Saisyoji, T.; Ogawa, M. Detection of Circulating Tumor Cells in Patients with Non-Small Cell Lung Cancer Undergoing Lobectomy by Video-Assisted Thoracic Surgery: A Potential Hazard for Intraoperative Hematogenous Tumor Cell Dissemination. J. Thorac. Cardiovasc. Surg. 2000, 119, 899–905. [Google Scholar] [CrossRef]
- Sawabata, N.; Okumura, M.; Utsumi, T.; Inoue, M.; Shiono, H.; Minami, M.; Nishida, T.; Sawa, Y. Circulating Tumor Cells in Peripheral Blood Caused by Surgical Manipulation of Non-Small-Cell Lung Cancer: Pilot Study Using an Immunocytology Method. Gen. Thorac. Cardiovasc. Surg. 2007, 55, 189–192. [Google Scholar] [CrossRef]
- Sawabata, N.; Funaki, S.; Hyakutake, T.; Shintani, Y.; Fujiwara, A.; Okumura, M. Perioperative Circulating Tumor Cells in Surgical Patients with Non-Small Cell Lung Cancer: Does Surgical Manipulation Dislodge Cancer Cells Thus Allowing Them to Pass into the Peripheral Blood? Surg. Today 2016, 46, 1402–1409. [Google Scholar] [CrossRef]
- Huang, H.B.; Ge, M.J. The Effects of Different Surgical Approaches on the Perioperative Level of Circulating Tumor Cells in Patients with Non-Small Cell Lung Cancer. Thorac. Cardiovasc. Surg. 2016, 64, 515–519. [Google Scholar] [CrossRef]
- Reddy, R.M.; Murlidhar, V.; Zhao, L.; Grabauskiene, S.; Zhang, Z.; Ramnath, N.; Lin, J.; Chang, A.C.; Carrott, P.; Lynch, W.; et al. Pulmonary Venous Blood Sampling Significantly Increases the Yield of Circulating Tumor Cells in Early-Stage Lung Cancer. J. Thorac. Cardiovasc. Surg. 2016, 151, 852–858. [Google Scholar] [CrossRef]
- Murlidhar, V.; Reddy, R.M.; Fouladdel, S.; Zhao, L.; Ishikawa, M.K.; Grabauskiene, S.; Zhang, Z.; Lin, J.; Chang, A.C.; Carrott, P.; et al. Poor Prognosis Indicated by Venous Circulating Tumor Cell Clusters in Early-Stage Lung Cancers. Cancer Res. 2017, 77, 5194–5206. [Google Scholar] [CrossRef]
- Hu, W.; Yang, Y.; Zhang, L.; Yin, J.; Huang, J.; Huang, L.; Gu, H.; Jiang, G.; Fang, J. Post Surgery Circulating Free Tumor DNA Is a Predictive Biomarker for Relapse of Lung Cancer. Cancer Med. 2017, 6, 962–974. [Google Scholar] [CrossRef]
- Tamminga, M.; de Wit, S.; van de Wauwer, C.; van den Bos, H.; Swennenhuis, J.F.; Klinkenberg, T.J.; Hiltermann, T.J.N.; Andree, K.C.; Spierings, D.C.J.; Lansdorp, P.M.; et al. Analysis of Released Circulating Tumor Cells During Surgery for Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 1656–1666. [Google Scholar] [CrossRef]
- Sawabata, N.; Nakamura, T.; Kawaguchi, T.; Watanabe, T.; Ouji, N.S.; Ito, T.; Taniguchi, S. Circulating Tumor Cells Detected Only After Surgery for Non-Small Cell Lung Cancer: Is It a Predictor of Recurrence? J. Thorac. Dis. 2020, 12, 4623–4632. [Google Scholar] [CrossRef]
- Katopodis, P.; Anikin, V.; Kishore, U.; Carter, T.; Hall, M.; Asadi, N.; Polychronis, A.; Karteris, E. Circulating Tumour Cells and Circulating Cell-Free DNA in Patients with Lung Cancer: A Comparison Between Thoracotomy and Video-Assisted Thoracoscopic Surgery. BMJ Open Respir. Res. 2021, 8, e000917. [Google Scholar] [CrossRef]
- Refaely, Y.; Sadetzki, S.; Chetrit, A.; Simansky, D.A.; Paley, M.; Modan, B.; Yellin, A. The Sequence of Vessel Interruption During Lobectomy for Non-Small Cell Lung Cancer: Is It Indeed Important? J. Thorac. Cardiovasc. Surg. 2003, 125, 1313–1320. [Google Scholar] [CrossRef]
- Kozak, A.; Alchimowicz, J.; Safranow, K.; Wójcik, J.; Kochanowski, L.; Kubisa, B.; Pieróg, J.; Grodzki, T. The Impact of the Sequence of Pulmonary Vessel Ligation during Anatomic Resection for Lung Cancer on Long-Term Survival—A Prospective Randomized Trial. Adv. Med. Sci. 2013, 58, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jiang, G.; Chen, Y.; Wang, J. Curative Effects of Different Sequences of Vessel Interruption During the Completely Thoracoscopic Lobectomy on Early Stage Non-Small Cell Lung Cancer. Ann. Thorac. Cardiovasc. Surg. 2015, 21, 536–543. [Google Scholar] [CrossRef][Green Version]
- Sumitomo, R.; Fukui, T.; Marumo, S.; Otake, Y.; Huang, C.L. Effects of Vessel Interruption Sequence During Thoracoscopic Lobectomy for Non-Small Cell Lung Cancer. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 464–470. [Google Scholar] [CrossRef]
- He, H.H.; He, J.X.; Hao, Z.X.; Wang, W.; He, J.X. Association Between Different Sequences of Vessel Ligation During Video-Assisted Thoracoscopic Lobectomy and Survival in Patients with Non-Small Cell Lung Cancer. J. Thorac. Dis. 2019, 11, 686–693. [Google Scholar] [CrossRef]
- Huang, K.L.; Deng, H.Y.; Fan, M.; Zheng, Q.; Lin, S.; Zhu, D.; Zhou, Q. The Sequence of Pulmonary Vessels Ligation During Lobectomy for Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2021, 47, 1535–1540. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).