Outcome of Children and Adolescents with Recurrent Classical Hodgkin Lymphoma: The Italian Experience
Abstract
Simple Summary
Abstract
1. Introduction
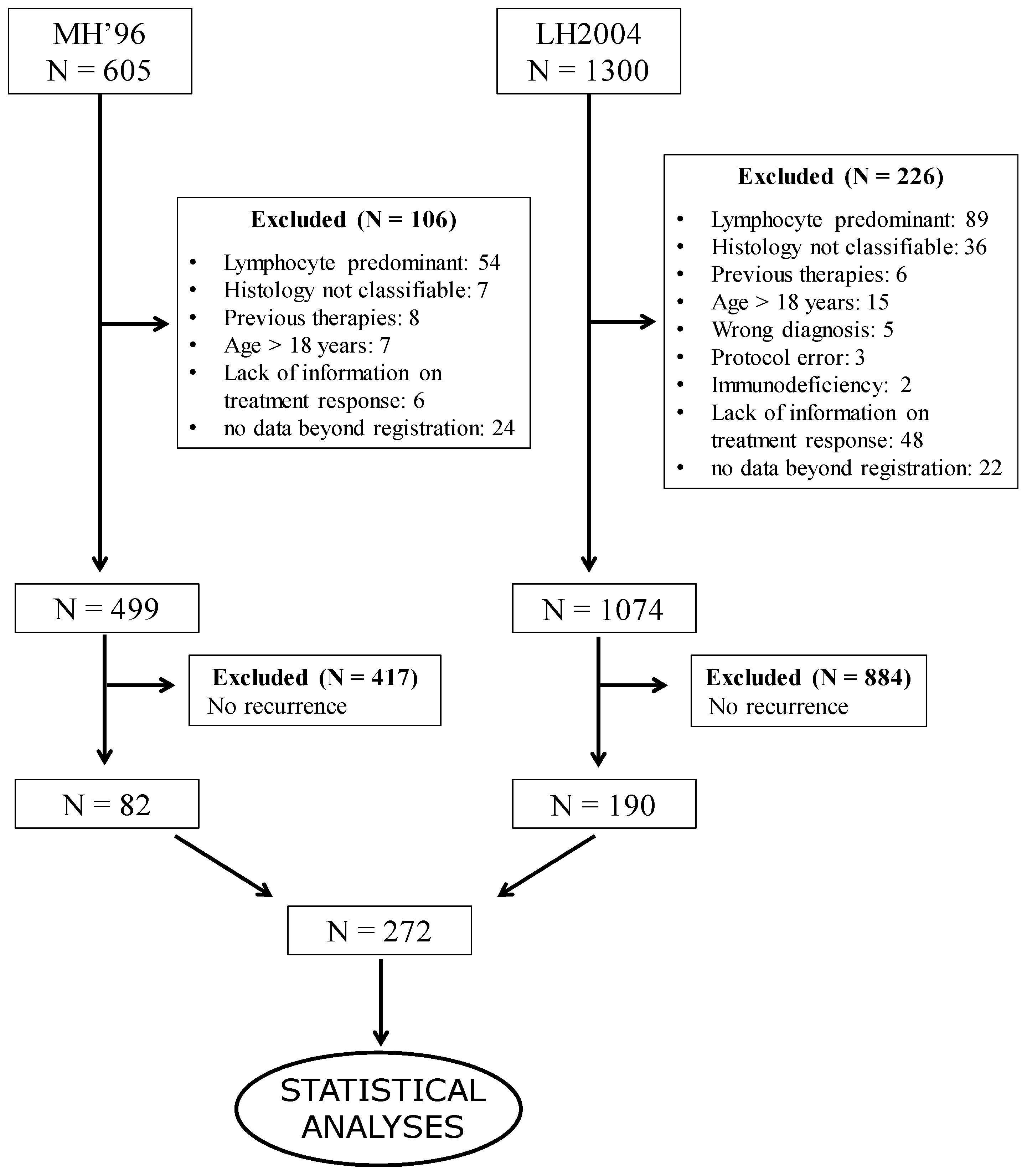
2. Patients and Methods
Statistical Methods
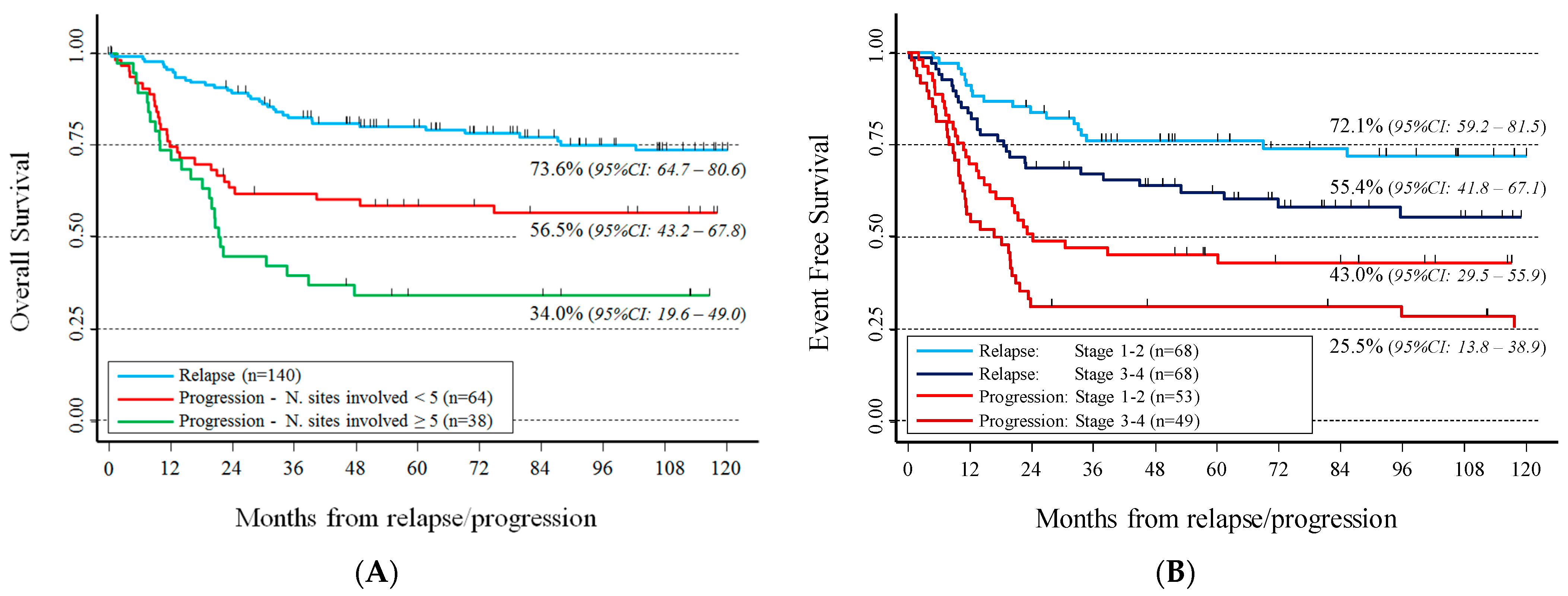
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voss, S.D.; Chen, L.; Constine, L.S.; Chauvenet, A.; Fitzgerald, T.J.; Kaste, S.C.; Slovis, T.; Schwartz, C.L. Surveillance Computed Tomography Imaging and Detecting of Relapse in Intermediate-and Advanced-Stage Pediatric Hodgkin’s Lymphoma: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 2635–2640. [Google Scholar] [CrossRef] [PubMed]
- Daw, S.; Hasenclever, D.; Mascarin, M.; Fernández-Teijeiro, A.; Balwierz, W.; Beishuizen, A.; Burnelli, R.; Cepelova, M.; Claviez, A.; Dieckmann, K.; et al. Risk and Response Adapted Treatment Guidelines for Managing First Relapsed and Refractory Classical Hodgkin Lymphoma in Children and Young People. Recommendations from the EuroNet Pediatric Hodgkin Lymphoma Group. HemaSphere 2020, 4, e329. [Google Scholar] [CrossRef] [PubMed]
- La Casce, A.S. Treating Hodgkin lymphoma in the new millennium: Relapsed and refractory disease. Hematol. Oncol. 2019, 37 (Suppl. 1), 87–91. [Google Scholar] [CrossRef] [PubMed]
- Warlick, E.D.; DeFor, T.E.; Bejanyan, N.; Holtan, S.; MacMillan, M.; Blazar, B.R.; Dusenbery, K.; Arora, M.; Bachanova, V.; Cooley, S.; et al. Reduced-Intensity Conditioning Followed by Related and Unrelated Allografts for Hematologic Malignancies: Expanded Analysis and Long-Term Follow-Up. Biol. Blood Marrow Transpl. 2019, 25, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, C.H.; Nademanee, A.; Masszi, T.; Agura, E.; Holowiecki, J.; Abidi, M.H.; Chen, A.I.; Stiff, P.; Gianni, A.M.; Carella, A.; et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 385, 1853–1862. [Google Scholar] [CrossRef]
- Bröckelmann, P.J.; Von Tresckow, B. Risk stratification and prognostic biomarkers in relapsed Hodgkin lymphoma. Br. J. Haematol. 2020, 190, 813–814. [Google Scholar] [CrossRef] [PubMed]
- Schellong, G.; Dörffel, W.; Claviez, A.; Körholz, D.; Mann, G.; Scheel-Walter, H.G.; Bökkerink, J.P.; Riepenhausen, M.; Lüders, H.; Pötter, R.; et al. Salvage therapy of progressive and recurrent Hodgkin’s disease: Results from a multicenter study of the pediatric DAL/GPOH-HD study group. J. Clin. Oncol. 2005, 23, 6181–6189. [Google Scholar] [CrossRef] [PubMed]
- Burnelli, R.; Fiumana, G.; Rondelli, R.; Pillon, M.; Sala, A.; Garaventa, A.; D’Amore, E.S.G.; Sabattini, E.; Buffardi, S.; Bianchi, M.; et al. Comparison of Hodgkin’s Lymphoma in Children and Adolescents. A Twenty Year Experience with MH’96 and LH2004 AIEOP (Italian Association of Pediatric Hematology and Oncology) Protocols. Cancers 2020, 12, 1620. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Salar, A.; Martino, R.; Perea, G.; Ribera, J.M.; López-Guillermo, A.; Guardia, R.; Escoda, L.; Altés, A.; Sierra, J.; Montserrat, E. High-dose infusional ifosfamide, etoposide plus methylprednisolone followed by dexamethasone, high-dose ara-C and cisplatinum and autologous stem cell transplantation for refractory or relapsed aggressive non-Hodgkin’s lymphoma. Haematologica 2002, 87, 1028–1035. [Google Scholar] [PubMed]
- Cairo, M.S.; Krailo, M.D.; Morse, M.; Hutchinson, R.J.; Harris, R.E.; Kjeldsberg, C.R.; Kadin, M.E.; Radel, E.; Steinherz, L.J.; Morris, E.; et al. Long-term follow-up of short intensive multiagent chemotherapy without high-dose methotrexate (‘Orange’) in children with advanced non-lymphoblastic non-Hodgkin’s lymphoma: A children’s cancer group report. Leukemia 2002, 16, 594–600. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Argiris, A.; Seropian, S.; Cooper, D.L. High-dose BEAM chemotherapy with autologous peripheral blood progenitor-cell transplantation for unselected patients with primary refractory or relapsed Hodgkin’s disease. Ann. Oncol. 2000, 11, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S. Applied Survival Analysis—Regression Modelling of Time to Event Data; John Wiley & Sons, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Friedmann, A.M.; Wolfson, J.A.; Hudson, M.M.; Weinstein, H.J.; Link, M.P.; Billett, A.; Larsen, E.C.; Yock, T.; Donaldson, S.S.; Marcus, K.; et al. Relapse after treatment of pediatric Hodgkin lymphoma: Outcome and role of surveillance after end of therapy. Pediatric Blood Cancer 2013, 60, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Kingston, J.E.; Plowman, P.N.; Meller, S.; Pinkerton, R.; Barrett, A.; Sandland, R.; McElwain, T.J.; Malpas, J.S. Outcome of children with resistant and relapsed Hodgkin’s disease. Br. J. Cancer 1992, 66, 1155–1158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Metzger, M.L.; Hudson, M.M.; Krasin, M.J.; Wu, J.; Kaste, S.C.; Kun, L.E.; Sandlund, J.T.; Howard, S.C. Initial response to salvage therapy determines prognosis in relapsed pediatric Hodgkin lymphoma patients. Cancer 2010, 116, 4376–4384. [Google Scholar] [CrossRef] [PubMed]
- Camus, V.; Jardin, F. Cell-Free DNA for the Management of Classical Hodgkin Lymphoma. Pharmaceuticals 2021, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Primerano, S.; Burnelli, R.; Carraro, E.; Pillon, M.; Elia, C.; Farruggia, P.; Sala, A.; Vinti, L.; Buffardi, S.; Basso, G.; et al. Kinetics of Circulating Plasma Cell-Free DNA in Paediatric Classical Hodgkin Lymphoma. J. Cancer 2016, 7, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Buedts, L.; Wlodarska, I.; Finalet-Ferreiro, J.; Gheysens, O.; Dehaspe, L.; Tousseyn, T.; Fornecker, L.-M.; Lazarovici, J.; Casasnovas, R.-O.; Gac, A.-C.; et al. The landscape of copy number variations in classical Hodgkin lymphoma: A joint KU Leuven and LYSA study on cell-free DNA. Blood Adv. 2021, 5, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Mauz-Koerholz, C.; Neville, K.; Llort, A.; Beishuizen, A.; Daw, S.; Pillon, M.; Aladjidi, N.; Klingebiel, T.; Landman-Parker, J.; et al. Brentuximab vedotin for paediatric relapsed or refractory Hodgkin’s lymphoma and anaplastic large-cell lymphoma: A multicentre, open-label, phase 1/2 study. Lancet Haematol. 2018, 5, e450–e461. [Google Scholar] [CrossRef]
- Cole, P.D.; McCarten, K.M.; Pei, Q.; Spira, M.; Metzger, M.L.; Drachtman, R.A.; Horton, T.M.; Bush, R.; Blaney, S.M.; Weigel, B.J.; et al. Brentuximab-vedotin with gemcitabine for paediatric and young adult patients with relapsed or refractory Hodgkin’s lymphoma (AHOD1221): A Children’s Oncology Group, multicentre single-arm, phase 1–2 trial. Lancet Oncol. 2018, 19, 1229–1238. [Google Scholar] [CrossRef]

| Treatment Groups | MH’96 | LH2004 |
|---|---|---|
| 1 IA, IIA supradiaphragmatic no bulky, no pulmonary hilum, <4 lymphatic sites, or IA, IIA infradiaphragmatic <4 lymphatic sites | 3× ABVD+:
| 3× ABVD+:
|
| 2 patients not included in groups 1 or 3 | 4× COPP/ABV+RT:
| 4× COPP/ABV+:
|
| 3 IIIB, IV; M/T ≥ 0.33 all stages | 6× COPP/ABV+RT:
| 4× COPP/ABV+:
|
| If RP ≤ 50% after 2° cycle: GR1: IEP/OPPA/COPP/IEP + RT; GR2 and 3: IEP/OPPA/IEP/OPPA/IEP + RT | ||
| ABVD Doxorubicin: 25 mg/m2 IV days 1 Bleomycin: 10 mg/m2 IV days 1 and 15 Vinblastine: 6 mg/m2 IV days 1 and 15 DTIC: 375 mg/m2 IV days 1 and 15 | COPP/ABV Cyclophosphamide: 600 mg/m2 IV day 1 Vincristine: 1.4 mg/m2 IV day 1 Prednisone: 40 mg/m2 orally days 1–14 Procarbazine: 100 mg/m2 orally days 1–7 Doxorubicin: 35 mg/m2 IV day 8 Bleomycin: 10 mg/m2 IV day 8 Vinblastine: 6 mg/m2 IV day 8 | IEP Ifosfamide: 2000 mg/m2 IV days 1–5 Etoposide: 120 mg/m2 IV days 1–5 Prednisone: 100 mg/m2 orally days 1–5 |
| OPPA Vincristine: 1.5 mg/m2 IV days 1 and 8 and 15 Procarbazine: 100 mg/m2 orally days 1–14 Prednisone: 60 mg/m2 IV days 1–14 Doxorubicin: 40 mg/m2 IV days 1 and 15 | COPP Cyclophosphamide: 500 mg/m2 IV days 1 and 8 Vincristine: 1.5 mg/m2 IV days 1 and 8 Procarbazine: 100 mg/m2 orally days 1–15 Prednisone: 40 mg/m2 orally days 1–15 | |
| Acronyms | Drugs | ||||
|---|---|---|---|---|---|
| IEP | High-dose Ifosfamide | Etoposide | Metil-Prednisolone | - | - |
| DHAP | Dexamethasone | High dose Cytosine-Arabinoside | Cisplatin | - | - |
| DECAL | Dexamethasone | Etoposide | Cisplatin | Cytosine-Arabinoside | L-asparaginase |
| BEAM | BCNU | Etoposide | Cytosine-Arabinoside | Melphalan | - |
| Patient Characteristics | n | % |
|---|---|---|
| Protocol | ||
| MH 96 | 82 | 30.2 |
| LH 2004 | 190 | 69.8 |
| Gender | ||
| Male | 158 | 58.1 |
| Female | 114 | 41.9 |
| Age (years) | ||
| <5 | 3 | 1.1 |
| 5–14 | 180 | 66.2 |
| ≥15 | 89 | 32.7 |
| Histology | ||
| Lymphocyte depleted | 5 | 1.8 |
| Mixed cellularity | 24 | 8.8 |
| Nodular sclerosis | 243 | 89.3 |
| Stage | ||
| 1 | 7 | 2.6 |
| 2 | 125 | 46.0 |
| 3 | 62 | 22.8 |
| 4 | 78 | 28.7 |
| Symptoms | ||
| A | 111 | 40.8 |
| B | 161 | 59.2 |
| Bulky | ||
| No | 97 | 35.7 |
| Yes | 175 | 64.3 |
| Number of involved sites | ||
| 1–3 | 64 | 23.5 |
| 4–7 | 116 | 42.7 |
| ≥8 | 92 | 33.8 |
| Extra-nodal site involvement | ||
| No | 178 | 65.4 |
| Yes | 94 | 34.6 |
| Treatment group | ||
| 1 | 12 | 4.4 |
| 2 | 35 | 12.9 |
| 3 | 225 | 82.7 |
| Radiotherapy | ||
| No according to protocol | 6 | 2.2 |
| No for disease progression | 68 | 25.0 |
| Yes | 195 | 71.7 |
| Missing | 3 | 1.1 |
| Patient Characteristics | n | % |
|---|---|---|
| Type of recurrence | ||
| Progression | 117 | 43.0 |
| Relapse | 155 | 57.0 |
| Early relapse (3–12 months from OT) | 79 | 51.0 |
| Late relapse (≥12 months from OT) | 76 | 49.0 |
| Age (years) | ||
| <5 | 3 | 1.1 |
| 5–14 | 121 | 44.5 |
| ≥5 | 148 | 54.4 |
| Stage | ||
| 1 | 30 | 12.6 |
| 2 | 91 | 38.2 |
| 3 | 36 | 15.1 |
| 4 | 81 | 34.0 |
| Missing | 34 | 12.5 |
| Number of involved sites * | ||
| 1 | 72 | 29.8 |
| 2–4 | 102 | 42.5 |
| ≥5 | 68 | 28.1 |
| Missing | 30 | 11.0 |
| Extra-nodal site involvement ** | ||
| No | 156 | 64.7 |
| Yes | 85 | 35.3 |
| Missing | 31 | 11.4 |
| Recurrence at the same site | ||
| No | 41 | 16.9 |
| Yes | 201 | 83.1 |
| Missing | 30 | 11.0 |
| Recurrence after Radiotherapy | ||
| Recurrence in non-irradiated sites | 30 | 16.7 |
| Recurrence in the irradiated site | 150 | 83.3 |
| Missing | 15 | 7.7 |
| Patient Characteristics | N/D | OS | 95% CI | p |
|---|---|---|---|---|
| Whole cohort | 272/89 | 65.3 | 59.0–70.9 | |
| Protocol | 0.042 | |||
| MH96 | 82/36 | 56.5 | 45.0–66.5 | |
| LH2004 | 190/53 | 69.5 | 61.7–76.0 | |
| Gender | 0.687 | |||
| Male | 158/53 | 62.3 | 53.2–70.2 | |
| Female | 114/36 | 66.9 | 56.7–75.3 | |
| Age (years) | 0.729 | |||
| 0–14 | 183/62 | 64.5 | 56.8–71.2 | |
| ≥15 | 89/27 | 66.6 | 53.9–76.5 | |
| Histology | 0.356 | |||
| Lymphocyte depleted | 5/2 | 50.0 | 5.8–84.5 | |
| Mixed cellularity | 24/10 | 57.4 | 35.2–74.4 | |
| Nodular sclerosis | 243/77 | 66.4 | 59.7–72.3 | |
| Stage | 0.885 | |||
| 1–2 | 132/44 | 65.5 | 56.2–73.2 | |
| 3–4 | 140/45 | 65.3 | 56.2–73.0 | |
| Symptoms | 0.052 | |||
| A | 111/29 | 72.0 | 61.8–80.0 | |
| B | 161/60 | 60.8 | 52.5–68.2 | |
| Bulky disease | 0.141 | |||
| No | 97/26 | 71.2 | 60.0–79.8 | |
| Yes | 175/63 | 62.3 | 54.4–69.2 | |
| Number of involved sites * | 0.667 * | |||
| 1–3 | 64/22 | 65.6 | 52.2–76.1 | |
| 4–7 | 116/39 | 64.1 | 54.1–72.5 | |
| ≥8 | 92/28 | 66.4 | 54.8–75.7 | |
| Extra-nodal site involvement ** | 0.220 | |||
| No | 178/62 | 63.7 | 55.8–70.5 | |
| Yes | 94/27 | 68.6 | 57.4–77.5 | |
| Treatment Group | 0.220 | |||
| 1 | 12/3 | 83.3 | 48.2–95.6 | |
| 2 | 35/7 | 76.9 | 56.9–88.5 | |
| 3 | 225/79 | 62.6 | 55.5–68.8 | |
| Radiotherapy (post-chemotherapy) | 0.197 | |||
| No according to protocol | 6/0 | 100 | ||
| No for disease progression | 68/25 | 62.3 | 49.5–72.8 | |
| Yes | 195/63 | 65.2 | 57.5–71.9 |
| Patient Characteristics | N/D | OS | 95% CI | p |
|---|---|---|---|---|
| Type of recurrence | <0.001 | |||
| Progression | 117/54 | 52.1 | 42.5–61.0 | |
| Relapse | 155/35 | 75.3 | 66.9–81.8 | |
| Early relapse | 79/18 | 73.7 | 60.9–82.9 | 0.991 |
| Late relapse | 76/17 | 76.5 | 64.3–85.0 | |
| Age (years) | 0.252 | |||
| 0–14 | 124/46 | 61.5 | 52.0–69.6 | |
| ≥15 | 148/43 | 68.7 | 59.7–76.1 | |
| Stage | 0.029 | |||
| 1–2 | 121/36 | 69.0 | 59.6–76.7 | |
| 3–4 | 117/48 | 57.2 | 47.1–66.1 | |
| Number of involved sites * | 0.023 * | |||
| 1 | 72/22 | 68.4 | 56.0–78.0 | |
| 2–4 | 102/32 | 65.8 | 55.0–74.7 | |
| ≥5 | 68/32 | 52.5 | 39.3–64.2 | |
| Extra-nodal site involvement ** | 0.087 | |||
| No | 156/51 | 66.9 | 58.7–73.8 | |
| Yes | 85/35 | 54.9 | 42.7–65.5 | |
| Recurrence at the same site | 0.412 | |||
| No | 41/13 | 66.4 | 49.0–79.0 | |
| Yes | 201/73 | 62.3 | 54.9–68.9 | |
| Recurrence after Radiotherapy | 0.110 | |||
| Recurrence in a non-irradiated site | 30/7 | 75.7 | 55.5–87.6 | |
| Recurrence in the same irradiated site | 150/54 | 61.8 | 52.8–69.5 |
| Patient Characteristics | N/E | EFS | 95% CI | p |
|---|---|---|---|---|
| Type of recurrence | <0.001 | |||
| Progression | 117/69 | 38.0 | 28.6–47.4 | |
| Relapse | 155/52 | 64.6 | 56.0–71.9 | |
| Early relapse | 79/30 | 59.7 | 47.3–70.0 | 0.244 |
| Late relapse | 76/22 | 69.7 | 57.2–79.3 | |
| Age (years) | 0.571 | |||
| 0–14 | 124/59 | 51.6 | 42.2–60.3 | |
| ≥15 | 148/62 | 54.3 | 45.1–62.7 | |
| Stage | 0.011 | |||
| 1–2 | 121/48 | 59.3 | 49.8–67.6 | |
| 3–4 | 117/64 | 42.3 | 32.4–51.8 | |
| Number of involved sites * | 0.079 * | |||
| 1 | 72/31 | 56.4 | 44.0–67.0 | |
| 2–4 | 102/45 | 52.4 | 41.2–62.4 | |
| ≥5 | 68/38 | 43.6 | 31.6–55.0 | |
| Extra-nodal site involvement ** | 0.019 | |||
| No | 156/67 | 56.5 | 48.1–64.0 | |
| Yes | 85/47 | 40.0 | 28.4–51.3 | |
| Recurrence at the same site | 0.331 | |||
| No | 41/18 | 56.8 | 39.8–70.7 | |
| Yes | 201/96 | 49.8 | 42.3–56.9 | |
| Recurrence after radiotherapy | 0.067 | |||
| Recurrence in a non-irradiated site | 30/10 | 69.4 | 49.4–82.8 | |
| Recurrence in the same irradiated site | 150/70 | 50.0 | 41.0–58.3 |
| Univariable Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Patient Characteristics | N/D | HR | 95% CI | p | HR | 95% CI | p |
| Characteristics at diagnosis | |||||||
| Protocol | 0.042 | <0.001 | |||||
| MH96 | 82/36 | 1 (ref) | - | 1 (ref) | - | ||
| LH2004 | 190/53 | 0.65 | 0.42–0.99 | 0.43 | 0.27–0.70 | ||
| Symptoms at diagnosis | 0.054 | 0.184 | |||||
| A | 111/29 | 1 (ref) | - | 1 (ref) | - | ||
| B | 161/60 | 1.5 | 0.99–2.4 | 1.4 | 0.86–2.2 | ||
| Characteristics at recurrence | |||||||
| Type of recurrence | <0.001 | <0.001 | |||||
| Progression | 117/54 | 1 (ref) | - | 1 (ref) | - | ||
| Relapse | 155/35 | 0.38 | 0.25–0.58 | 0.33 | 0.21–0.52 | ||
| Stage | 0.029 | 0.147 | |||||
| 1–2 | 121/36 | 1 (ref) | - | 1 (ref) | - | ||
| 3–4 | 117/48 | 1.6 | 1.0–2.5 | 1.4 | 0.88–2.3 | ||
| Extra-nodal site involvement * | 0.093 | 0.409 | |||||
| No | 156/51 | 1 (ref) | - | 1 (ref) | - | ||
| Yes | 85/35 | 1.5 | 0.95–2.2 | 1.2 | 0.76–2.0 | ||
| Number of involved sites ** | 0.054 | 0.009 | |||||
| 1 | 72/22 | 1 (ref) | - | 1 (ref) | - | ||
| 2–4 | 102/32 | 1.1 | 0.66–1.9 | 1.5 | 0.85–2.5 | ||
| ≥5 | 68/32 | 1.9 | 1.1–3.2 | 2.5 | 1.4–4.4 | ||
| Continuous variable | 242/86 | 1.1 | 1.04–1.2 | 0.003 | 1.1 | 1.1–1.2 | 0.001 |
| Univariable Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Patient Characteristics | N/E | HR | 95% CI | p | HR | 95% CI | p |
| Characteristics at diagnosis | |||||||
| Protocol | 0.099 | 0.006 | |||||
| MH96 | 82/45 | 1 (ref) | - | 1 (ref) | - | ||
| LH2004 | 190/76 | 0.73 | 0.50–1.1 | 0.56 | 0.38–0.84 | ||
| Symptoms | 0.067 | 0.200 | |||||
| A | 111/42 | 1 (ref) | - | 1 (ref) | - | ||
| B | 161/79 | 1.4 | 0.98–2.1 | 1.3 | 0.87–1.9 | ||
| Characteristics at recurrence | |||||||
| Type | <0.001 | <0.001 | |||||
| Progression | 117/69 | 1 (ref) | - | 1 (ref) | - | ||
| Relapse | 155/52 | 0.43 | 0.30–0.61 | 0.34 | 0.23–0.51 | ||
| Stage | 0.012 | 0.005 | |||||
| 1–2 | 121/48 | 1 (ref) | - | 1 (ref) | - | ||
| 3–4 | 117/64 | 1.6 | 1.1–2.4 | 1.7 | 1.2–2.5 | ||
| Number of involved sites * | 0.072 | 0.492 | |||||
| Continuous variable | 242/114 | 1.06 | 1.0–1.12 | 1.02 | 0.96–1.10 | ||
| Extra-nodal site involvement ** | 0.022 | 0.783 | |||||
| No | 156/67 | 1 (ref) | - | 1 (ref) | - | ||
| Yes | 85/47 | 1.6 | 1.1–2.3 | 1.1 | 0.62–1.9 | ||
| Recurrence after radiotherapy *** | 0.052 | 0.031 | |||||
| Non-irradiated site | 30/10 | 1 (ref.) | - | 1 (ref.) | - | ||
| Same irradiated site | 150/70 | 1.8 | 0.95–3.6 | 2.0 | 1.0–4.1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garaventa, A.; Parodi, S.; Guerrini, G.; Farruggia, P.; Sala, A.; Pillon, M.; Buffardi, S.; Rossi, F.; Bianchi, M.; Zecca, M.; et al. Outcome of Children and Adolescents with Recurrent Classical Hodgkin Lymphoma: The Italian Experience. Cancers 2022, 14, 1471. https://doi.org/10.3390/cancers14061471
Garaventa A, Parodi S, Guerrini G, Farruggia P, Sala A, Pillon M, Buffardi S, Rossi F, Bianchi M, Zecca M, et al. Outcome of Children and Adolescents with Recurrent Classical Hodgkin Lymphoma: The Italian Experience. Cancers. 2022; 14(6):1471. https://doi.org/10.3390/cancers14061471
Chicago/Turabian StyleGaraventa, Alberto, Stefano Parodi, Giulia Guerrini, Piero Farruggia, Alessandra Sala, Marta Pillon, Salvatore Buffardi, Francesca Rossi, Maurizio Bianchi, Marco Zecca, and et al. 2022. "Outcome of Children and Adolescents with Recurrent Classical Hodgkin Lymphoma: The Italian Experience" Cancers 14, no. 6: 1471. https://doi.org/10.3390/cancers14061471
APA StyleGaraventa, A., Parodi, S., Guerrini, G., Farruggia, P., Sala, A., Pillon, M., Buffardi, S., Rossi, F., Bianchi, M., Zecca, M., Vinti, L., Facchini, E., Casini, T., Bernasconi, S., Amoroso, L., D’Amico, S., Provenzi, M., De Santis, R., Sau, A., ... Burnelli, R. (2022). Outcome of Children and Adolescents with Recurrent Classical Hodgkin Lymphoma: The Italian Experience. Cancers, 14(6), 1471. https://doi.org/10.3390/cancers14061471







