Automated Global Longitudinal Strain Assessment in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
2.3. Echocardiography
2.4. Variability Analysis
2.5. Laboratory Tests
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
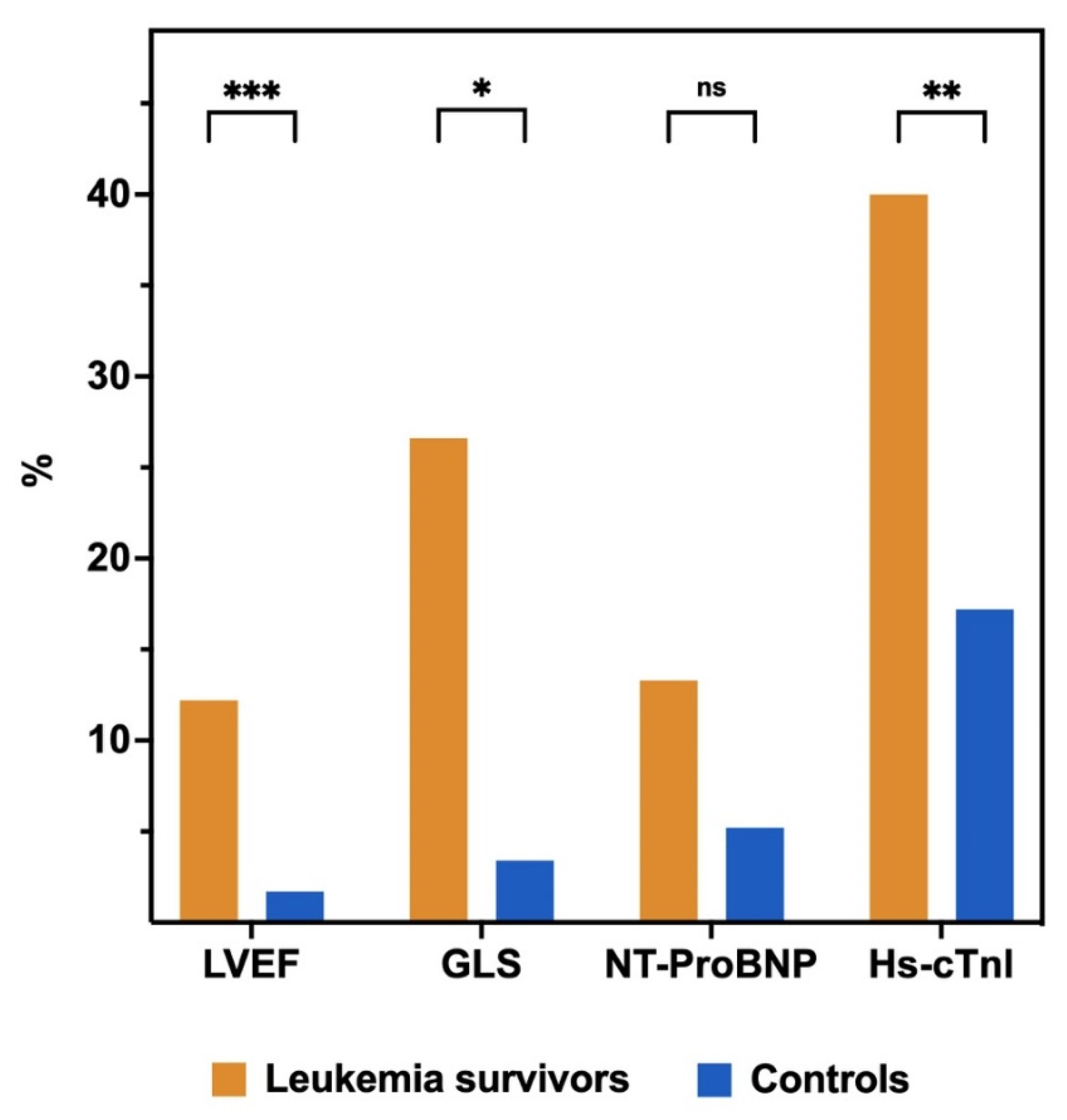
3.2. Prevalence of Left Ventricular Systolic Dysfunction and Biomarkers Abnormalities
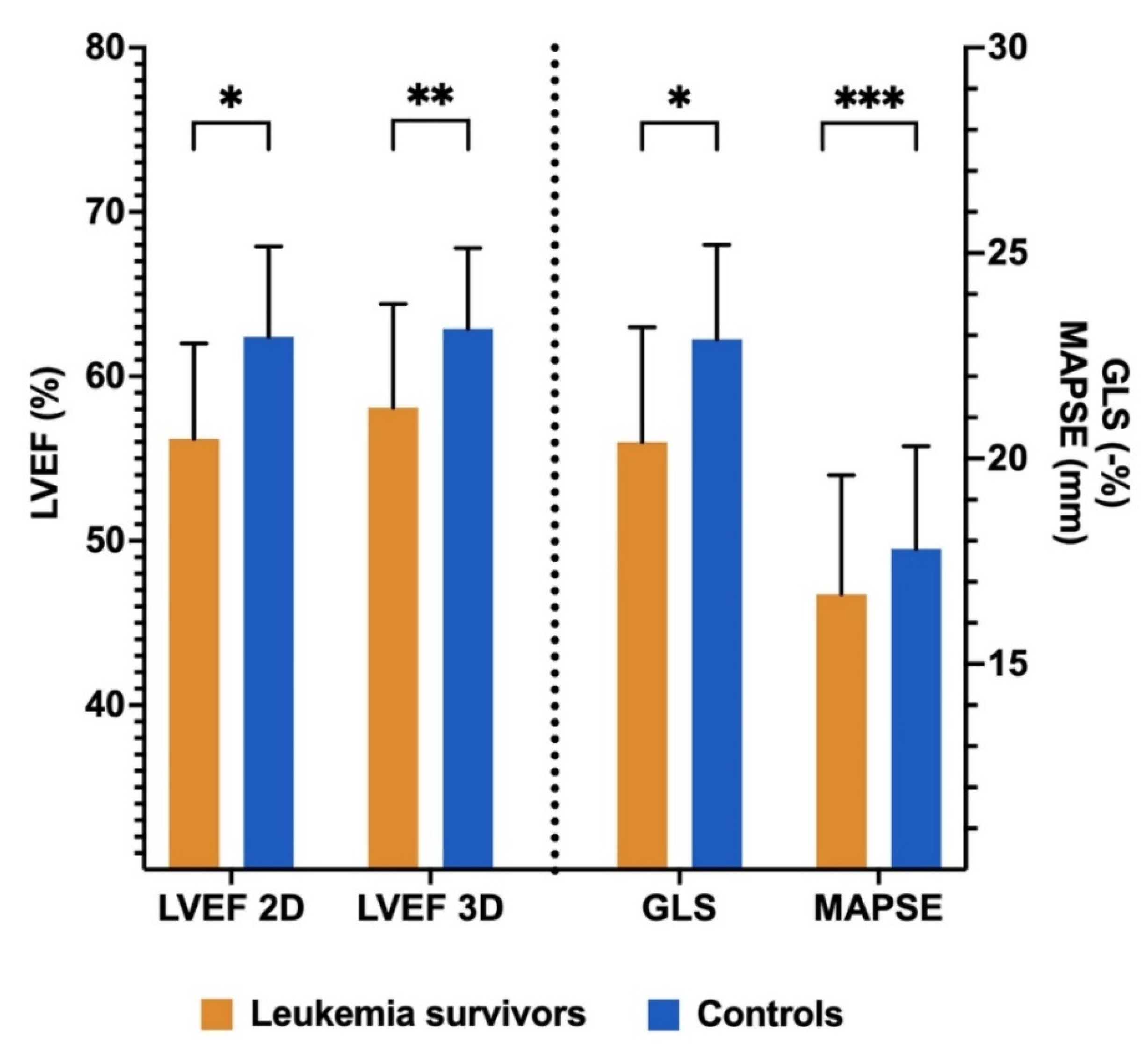
3.3. Comparison of Echocardiographic Parameters between Groups
3.4. Predictors of Left Ventricular Ejection Fraction in Leukemia Survivors
3.5. Predictors of Global Longitudinal Strain in Leukemia Survivors
3.6. Intraobserver and Interobserver Variability Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greaves, M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat. Cancer 2018, 18, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Mohty, M. Acute lymphoblastic leukaemia. Lancet 2020, 395, 1146–1162. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Yeazel, M.W.; Leisenring, W.M.; Kawashima, T.; Mertens, A.C.; Mitby, P.; Stovall, M.; Donaldson, S.S.; Green, D.M.; Sklar, C.A.; et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009, 339, b4606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmiegelow, K.; Levinsen, M.F.; Attarbaschi, A.; Baruchel, A.; Devidas, M.; Escherich, G.; Gibson, B.; Heydrich, C.; Horibe, K.; Ishida, Y.; et al. Second Malignant Neoplasms After Treatment of Childhood Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2013, 31, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Feijen, E.A.M.; Font-Gonzalez, A.; Van der Pal, H.J.H.; Kok, W.E.M.; Geskus, R.B.; Ronckers, C.M.; Bresters, D.; van Dalen, E.C.; Broeder, E.V.D.; Berg, M.H.V.D.; et al. Risk and Temporal Changes of Heart Failure among 5-Year Childhood Cancer Survivors: A DCOG-LATER Study. J. Am. Heart Assoc. 2019, 8, e009122. [Google Scholar] [CrossRef] [Green Version]
- Rathe, M.; Carlsen, N.L.T.; Oxhøj, H.; Nielsen, G. Long-term cardiac follow-up of children treated with anthracycline doses of 300 mg/m2 or less for acute lymphoblastic leukemia. Pediatr. Blood Cancer 2009, 54, 444–448. [Google Scholar] [CrossRef]
- Leerink, J.M.; van der Pal, H.J.; Kremer, L.C.; Feijen, E.A.; Meregalli, P.G.; Pourier, M.S.; Merkx, R.; Bellersen, L.; van Dalen, E.C.; Loonen, J.; et al. Refining the 10-Year Prediction of Left Ventricular Systolic Dysfunction in Long-Term Survivors of Childhood Cancer. JACC CardioOncol. 2021, 3, 62–72. [Google Scholar] [CrossRef]
- Lenihan, D.J.; Fradley, M.G.; Dent, S.; Brezden-Masley, C.; Carver, J.; Filho, R.K.; Neilan, T.G.; Blaes, A.; Melloni, C.; Herrmann, J.; et al. Proceedings from the Global Cardio-Oncology Summit: The Top 10 Priorities to Actualize for CardioOncology. JACC CardioOncol. 2019, 1, 256–272. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Armenian, S.H.; Hudson, M.M.; Mulder, R.L.; Chen, M.H.; Constine, L.S.; Dwyer, M.; Nathan, P.C.; Tissing, W.J.; Shankar, S.; Sieswerda, E.; et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015, 16, e123–e136. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J. Am. Hear. Assoc. 2020, 9, e18403. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Banchs, J.; Mousavi, N.; Plana, J.C.; Scherrer-Crosbie, M.; Thavendiranathan, P.; Barac, A. Contemporary Role of Echocardiography for Clinical Decision Making in Patients during and After Cancer Therapy. JACC Cardiovasc. Imaging 2018, 11, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- McGregor, P.C.; Moura, F.A.; Banchs, J.; Aragam, J.R. Role of myocardial strain imaging in surveillance and management of cancer therapeutics-related cardiac dysfunction: A systematic review. Echocardiography 2021, 38, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.-F.; Hong, W.-J.; Chan, G.C.F.; Wong, S.J.; Ha, S.-Y. Left ventricular myocardial deformation and mechanical dyssynchrony in children with normal ventricular shortening fraction after anthracycline therapy. Heart 2010, 96, 1137–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, G.T.; Joshi, V.M.; Ness, K.K.; Marwick, T.H.; Zhang, N.; Srivastava, D.; Griffin, B.P.; Grimm, R.A.; Thomas, J.; Phelan, D.; et al. Comprehensive Echocardiographic Detection of Treatment-Related Cardiac Dysfunction in Adult Survivors of Childhood Cancer: Results from the St. Jude Lifetime Cohort Study. J. Am. Coll. Cardiol. 2015, 65, 2511–2522. [Google Scholar] [CrossRef] [Green Version]
- Niemelä, J.; Ylänen, K.; Suominen, A.; Pushparajah, K.; Mathur, S.; Sarkola, T.; Jahnukainen, K.; Eerola, A.; Poutanen, T.; Vettenranta, K.; et al. Cardiac Function after Cardiotoxic Treatments for Childhood Cancer—Left Ventricular Longitudinal Strain in Screening. Front. Cardiovasc. Med. 2021, 8, 1367. [Google Scholar] [CrossRef]
- Kawakami, H.; Wright, L.; Nolan, M.; Potter, E.L.; Yang, H.; Marwick, T.H. Feasibility, Reproducibility, and Clinical Implications of the Novel Fully Automated Assessment for Global Longitudinal Strain. J. Am. Soc. Echocardiogr. 2020, 34, 136–145.e2. [Google Scholar] [CrossRef]
- Feijen, E.A.M.; Leisenring, W.; Stratton, K.L.; Ness, K.K.; Van Der Pal, H.J.H.; Van Dalen, E.C.; Armstrong, G.T.; Aune, G.; Green, D.M.; Hudson, M.M.; et al. Derivation of Anthracycline and Anthraquinone Equivalence Ratios to Doxorubicin for Late-Onset Cardiotoxicity. JAMA Oncol. 2019, 5, 864–871. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narang, A.; Mor-Avi, V.; Prado, A.; Volpato, V.; Prater, D.; Tamborini, G.; Fusini, L.; Pepi, M.; Goyal, N.; Addetia, K.; et al. Machine learning based automated dynamic quantification of left heart chamber volumes. Eur. Heart J. Cardiovasc. Imaging 2018, 20, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Austin, P.C. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat. Med. 2016, 35, 5642–5655. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Saunderson, C.E.D.; Plein, S.; Manisty, C.H. Role of cardiovascular magnetic resonance imaging in cardio-oncology. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 383–396. [Google Scholar] [CrossRef]
- Liu, J.E.; Barac, A.; Thavendiranathan, P.; Scherrer-Crosbie, M. Strain Imaging in Cardio-Oncology. JACC CardioOncol. 2020, 2, 677–689. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Negishi, T.; Somerset, E.; Negishi, K.; Penicka, M.; Lemieux, J.; Aakhus, S.; Miyazaki, S.; Shirazi, M.; Galderisi, M.; et al. Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy. J. Am. Coll. Cardiol. 2020, 77, 392–401. [Google Scholar] [CrossRef]
- Aznar, E.G.C.; Casas, A.A.; Montañés, L.J.; Escribano, M.C.C.; Aizpún, J.I.L.; Villagrasa, P.S. Use of speckle tracking in the evaluation of late subclinical myocardial damage in survivors of childhood acute leukaemia. Int. J. Cardiovasc. Imaging 2018, 34, 1373–1381. [Google Scholar] [CrossRef]
- Christiansen, J.R.; Massey, R.; Dalen, H.; Kanellopoulos, A.; Hamre, H.; Fosså, S.D.; Ruud, E.; Kiserud, C.E.; Aakhus, S. Utility of Global Longitudinal Strain by Echocardiography to Detect Left Ventricular Dysfunction in Long-Term Adult Survivors of Childhood Lymphoma and Acute Lymphoblastic Leukemia. Am. J. Cardiol. 2016, 118, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Massey, R.J.; Diep, P.P.; Ruud, E.; Burman, M.M.; Kvaslerud, A.B.; Brinch, L.; Aakhus, S.; Gullestad, L.L.; Beitnes, J.O. Left Ventricular Systolic Function in Long-Term Survivors of Allogeneic Hematopoietic Stem Cell Transplantation. JACC CardioOncol. 2020, 2, 460–471. [Google Scholar] [CrossRef]
- Slieker, M.G.; Fackoury, C.; Slorach, C.; Hui, W.; Friedberg, M.; Fan, C.-P.S.; Manlhiot, C.; Dillenburg, R.; Kantor, P.; Mital, S.; et al. Echocardiographic Assessment of Cardiac Function in Pediatric Survivors of Anthracycline-Treated Childhood Cancer. Circ. Cardiovasc. Imaging 2019, 12, e008869. [Google Scholar] [CrossRef] [PubMed]
- Mavinkurve-Groothuis, A.M.; Groot-Loonen, J.; Marcus, K.A.; Bellersen, L.; Feuth, T.; Bökkerink, J.P.; Hoogerbrugge, P.M.; de Korte, C.; Kapusta, L. Myocardial Strain and Strain Rate in Monitoring Subclinical Heart Failure in Asymptomatic Long-Term Survivors of Childhood Cancer. Ultrasound Med. Biol. 2010, 36, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.R.; Hamre, H.; Massey, R.; Dalen, H.; Beitnes, J.O.; Fosså, S.D.; Kiserud, C.E.; Aakhus, S. Left Ventricular Function in Long-Term Survivors of Childhood Lymphoma. Am. J. Cardiol. 2014, 114, 483–490. [Google Scholar] [CrossRef]
- Sorensen, K.; Levitt, G.A.; Bull, C.; Dorup, I.; Sullivan, I.D. Late anthracycline cardiotoxicity after childhood cancer: A prospective longitudinal study. Cancer 2003, 97, 1991–1998. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Lipsitz, S.R.; Sallan, S.E.; Dalton, V.M.; Mone, S.M.; Gelber, R.D.; Colan, S.D. Chronic Progressive Cardiac Dysfunction Years After Doxorubicin Therapy for Childhood Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2005, 23, 2629–2636. [Google Scholar] [CrossRef]
- Van der Pal, H.J.; van Dalen, E.C.; Hauptmann, M.; Kok, W.E.; Caron, H.N.; Bos, C.V.D.; Oldenburger, F.; Koning, C.C.; van Leeuwen, F.E.; Kremer, L.C. Cardiac Function in 5-Year Survivors of Childhood Cancer: A long-term follow-up study. Arch. Intern. Med. 2010, 170, 1247–1255. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Plana, J.C.; Zhang, N.; Srivastava, D.; Green, D.M.; Ness, K.K.; Donovan, F.D.; Metzger, M.L.; Arevalo, A.; Durand, J.-B.; et al. Screening Adult Survivors of Childhood Cancer for Cardiomyopathy: Comparison of Echocardiography and Cardiac Magnetic Resonance Imaging. J. Clin. Oncol. 2012, 30, 2876–2884. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Adams, M.J.; Colan, S.D.; Constine, L.S.; Herman, E.H.; Hsu, D.T.; Hudson, M.M.; Kremer, L.C.; Landy, D.C.; Miller, T.L.; et al. Long-term Cardiovascular Toxicity in Children, Adolescents, and Young Adults Who Receive Cancer Therapy: Pathophysiology, Course, Monitoring, Management, Prevention, and Research Directions: A scientific statement from the American Heart Association. Circulation 2013, 128, 1927–1995. [Google Scholar] [CrossRef] [Green Version]
- Khanna, A.; Pequeno, P.; Gupta, S.; Thavendiranathan, P.; Lee, D.S.; Abdel-Qadir, H.; Nathan, P.C. Increased Risk of All Cardiovascular Disease Subtypes among Childhood Cancer Survivors: Population-Based Matched Cohort Study. Circulation 2019, 140, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chow, E.J.; Oeffinger, K.C.; Border, W.L.; Leisenring, W.M.; Meacham, L.R.; Mulrooney, D.; Sklar, C.; Stovall, M.; Robison, L.L.; et al. Traditional Cardiovascular Risk Factors and Individual Prediction of Cardiovascular Events in Childhood Cancer Survivors. JNCI J. Natl. Cancer Inst. 2020, 112, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.A.; Postma, A.; Vonk, J.M.; Zwart, N.; Berg, M.P.V.D.; Bink-Boelkens, M.T.; Dolsma, W.V.; Smit, A.J.; de Vries, E.G.; Tissing, W.; et al. Systolic and diastolic dysfunction in long-term adult survivors of childhood cancer. Eur. J. Cancer 2011, 47, 2453–2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leerink, J.M.; Verkleij, S.J.; Feijen, E.A.M.; Mavinkurve-Groothuis, A.M.C.; Pourier, M.S.; Ylänen, K.; Tissing, W.J.; Louwerens, M.; Heuvel, M.M.V.D.; Broeder, E.V.D.-D.; et al. Biomarkers to diagnose ventricular dysfunction in childhood cancer survivors: A systematic review. Heart 2019, 105, 210–216. [Google Scholar] [CrossRef]
- Welsh, P.; Preiss, D.; Hayward, C.; Shah, A.; McAllister, D.; Briggs, A.; Boachie, C.; McConnachie, A.; Padmanabhan, S.; Welsh, C.; et al. Cardiac Troponin T and Troponin I in the General Population. Circulation 2019, 139, 2754–2764. [Google Scholar] [CrossRef]

| CLSs (n = 90) | Control Group (n = 58) | p-Value | |
|---|---|---|---|
| Age at diagnosis (years) | 4 (3–7) | - | - |
| Age at exam (years) | 24.6 ± 9.7 | 23.6 ± 10.8 | 0.593 |
| Time since diagnosis (years) | 18 (11–26) | - | - |
| Sex (% female) | 34 (37.8%) | 34 (58.6%) | 0.018 |
| Weight (kg) | 64.8 ± 18.3 | 61.9 ± 17.2 | 0.333 |
| Height (cm) | 165.6 ± 13.3 | 164.2 ± 13.8 | 0.539 |
| Body mass index (kg/m2) | 23.3 ± 5.1 | 22.6 ± 4.4 | 0.346 |
| Body surface area (m2) | 1.7 ± 0.3 | 1.7 ± 0.3 | 0.366 |
| SBP (mmHg) | 116.2 ± 11.3 | 115.6 ± 11.1 | 0.768 |
| DBP (mmHg) | 69.6 ± 7.9 | 69.4 ± 7.9 | 0.908 |
| Heart rate (bpm) | 72.5 ± 11.1 | 71.7 ± 11.5 | 0.646 |
| Current smoker (%) | 14 (15.6%) | 1 (1.7%) | 0.005 |
| Hypertension (%) | 3 (3.3%) | 0 (0.0%) | 0.280 |
| Hypercholesterolemia (%) | 12 (13.3%) | 4 (6.9%) | 0.283 |
| Total cholesterol (mg/dL) | 175.6 ± 32.6 | 174.1 ± 33.8 | 0.796 |
| HDL (mg/dL) | 54.5 ± 15.2 | 61.4 ± 11.8 | 0.006 |
| LDL (mg/dL) | 95.6 ± 26.6 | 95.4 ± 27.5 | 0.971 |
| Triglycerides (mg/dL) | 101.9 ± 48.8 | 86.9 ± 34.2 | 0.045 |
| HbA1c (%) | 5.3 (5.1–5.5) | 5.3 (5.1–5.5) | 0.690 |
| Diabetes mellitus (%) | 4 (4.4%) | 1 (1.7%) | 0.649 |
| Obesity | 9 (10.0%) | 7 (12.1%) | 0.901 |
| Sedentarism (%) | 37 (41.1%) | 20 (34.5%) | 0.525 |
| Hypothyroidism (%) | 7 (7.8%) | 0 (0.0%) | 0.043 |
| Anthracycline dose (mg/m2) | 138 (72–192) | - | - |
| Radiotherapy (%) | 3 (3.3%) | - | - |
| HSCT (%) | 17 (18.9%) | - | - |
| Hs-cTnI (ng/L) | 2.5 (2.5–4.5) | 2.5 (2.5–2.5) | 0.032 |
| NT-ProBNP (pg/mL) | 35.0 (35.0–66.5) | 35.0 (35.0–49.0) | 0.175 |
| CLSs (n = 90) | Control Group (n = 58) | p-Value | IPW Beta (RSE), p-Value | |
|---|---|---|---|---|
| LVDD (mm) | 45.6 ± 6.8 | 44.9 ± 6.1 | 0.595 | 0.09 (1.14), 0.935 |
| LVSD (mm) | 28.9 ± 6.0 | 26.2 ± 4.6 | 0.005 | 2.27 (0.92), 0.015 |
| IVS (mm) | 7.6 ± 1.5 | 7.7 ± 1.3 | 0.824 | 0.17 (0.23), 0.457 |
| LVEDV (mL) | 87.4 ± 29.2 | 87.1 ± 31.4 | 0.950 | 3.19 (5.42), 0.557 |
| LVESV (mL) | 39.1 ± 13.9 | 34.2 ± 15.2 | 0.046 | 2.57 (2.65), 0.334 |
| LA volume (mL) | 35.8 ± 15.6 | 36.3 ± 13.8 | 0.868 | 2.51 (3.02), 0.407 |
| LVEF Teichholz (%) | 66.8 ± 6.4 | 72.2 ± 7.6 | <0.001 | 5.12 (1.25), <0.001 |
| 2D-LVEF (%) | 56.2 ± 5.8 | 62.4 ± 5.5 | <0.001 | 5.45 (0.95), <0.001 |
| 3D-LVEF (%) | 58.1 ± 6.3 | 62.9 ± 4.9 | <0.001 | 4.14 (1.33), 0.003 |
| GLS (-%) | 20.4 ± 2.8 | 22.9 ± 2.3 | <0.001 | 2.28 (0.45), <0.001 |
| MAPSE (mm) | 16.7 ± 2.9 | 17.8 ± 2.5 | 0.016 | 1.14 (0.48), 0.019 |
| Peak E velocity (cm/s) | 94.7 ± 18.4 | 96.3 ± 15.9 | 0.579 | 0.99 (2.87), 0.729 |
| Peak A velocity (cm/s) | 57.1 ± 15.6 | 58.1 ± 17.6 | 0.714 | 2.02 (2.85), 0.479 |
| Mitral E/A ratio | 1.8 ± 0.7 | 1.8 ± 0.7 | 0.785 | 0.03 (0.11), 0.795 |
| Peak e’ lat velocity (cm/s) | 19.4 ± 4.6 | 20.0 ± 4.4 | 0.439 | 0.14 (0.74), 0.847 |
| E/e’ lat ratio | 5.0 ± 1.3 | 5.0 ± 1.3 | 0.849 | 0.05 (0.21), 0.788 |
| Peak e’ med velocity (cm/s) | 13.9 ± 4.0 | 14.9 ± 3.6 | 0.131 | 0.68 (0.64), 0.288 |
| E/e’ med ratio | 7.3 ± 2.3 | 6.8 ± 2.0 | 0.175 | 0.39 (0.36), 0.281 |
| E/e’ average ratio | 6.2 ± 1.7 | 5.9 ± 1.5 | 0.279 | 0.19 (0.27), 0.465 |
| Gradient RV-RA (mmHg) | 19.2 ± 4.9 | 16.1 ± 3.9 | 0.024 | 3.15 (1.39), 0.027 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | p-Value | Beta | 95% CI | p-Value | |
| Sex (female) | 2.42 | −0.04 to 4.88 | 0.054 | 2.07 | −0.05 to 4.19 | 0.056 |
| Age at diagnosis (years) | −0.02 | −0.29 to 0.25 | 0.866 | |||
| Age at exam (years) | −0.12 | −0.24 to 0.01 | 0.056 | |||
| Time since diagnosis (years) | −0.15 | −0.29 to −0.01 | 0.034 | −0.09 | −0.21 to 0.025 | 0.122 |
| BMI (kg/m2) | −0.11 | −0.35 to 0.13 | 0.383 | |||
| HR (bpm) | 0.07 | −0.03 to 0.18 | 0.180 | |||
| SBP (mmHg) | 0.06 | −0.05 to 0.16 | 0.303 | |||
| DBP (mmHg) | −0.19 | −0.39 to 0.18 | 0.403 | |||
| Hypertension | −8.02 | −14.59 to −1.44 | 0.017 | |||
| Hypercholesterolemia | −2.94 | −6.47 to 0.59 | 0.102 | |||
| Diabetes mellitus | −11.64 | −17.02 to −6.26 | 0.001 | −8.15 | −13.31 to −2.99 | 0.002 |
| Obesity | −2.41 | −6.44 to 1.62 | 0.238 | |||
| Sedentarism | −0.85 | −3.33 to 1.62 | 0.494 | |||
| Current smoker | −1.79 | −5.13 to 1.55 | 0.290 | |||
| Hypothyroidism | −3.51 | −8.00 to 0.98 | 0.124 | |||
| Anthracycline dose | −0.02 | −0.04, −0.01 | 0.004 | |||
| Anthracycline dose >250 mg/m2 | −8.45 | −12.99 to −3.89 | 0.001 | −5.88 | −10.07 to −1.69 | 0.006 |
| Radiotherapy | −10.43 | −16.85 to −4.01 | 0.002 | −6.49 | −12.48 to −0.51 | 0.034 |
| HSCT | −4.15 | −7.14 to −1.16 | 0.007 | |||
| Hs-cTnI (ng/L) | −3.68 | −0.68 to −0.05 | 0.021 | |||
| Hs-cTnI > 2.5 ng/L | −0.11 | −2.62 to 2.41 | 0.933 | |||
| NT-ProBNP (pg/mL) | −0.19 | −0.04 to 0.01 | 0.066 | |||
| NT-ProBNP > 125 pg/mL | −1.86 | −5.45 to 1.72 | 0.305 | |||
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | p-Value | Beta | 95% CI | p-Value | |
| Sex (female) | 1.03 | −0.19 to 2.24 | 0.096 | 1.11 | −0.04 to 2.27 | 0.058 |
| Age at diagnosis (years) | −0.11 | −0.24 to 0.02 | 0.097 | |||
| Age at exam (years) | −0.06 | −0.12 to −0.01 | 0.043 | −0.02 | −0.08 to 0.04 | 0.465 |
| Time since diagnosis (years) | −0.05 | −0.12 to 0.02 | 0.169 | |||
| BMI (kg/m2) | −0.09 | −0.22 to 0.03 | 0.131 | |||
| HR (bpm) | −0.04 | −0.09 to 0.01 | 0.114 | |||
| SBP (mmHg) | 0.03 | −0.02 to 0.09 | 0.210 | |||
| DBP (mmHg) | 0.01 | −0.061 to 0.08 | 0.711 | |||
| Hypertension | −0.63 | −3.90 to 2.64 | 0.703 | |||
| Hypercholesterolemia | −0.82 | −2.68 to 1.05 | 0.385 | |||
| Diabetes mellitus | −2.56 | −5.79 to 0.67 | 0.118 | |||
| Obesity | −1.46 | −3.64 to 0.71 | 0.184 | |||
| Sedentarism | −0.79 | −2.01 to 0.41 | 0.191 | |||
| Current smoker | −2.57 | −4.10 to −1.05 | 0.001 | −2.29 | −3.84 to −0.74 | 0.004 |
| Hypothyroidism | −0.89 | −3.08 to 1.30 | 0.421 | |||
| Anthracycline dose | −0.01 | −0.01 to 0.01 | 0.892 | 0.01 | −0.01 to 0.01 | 0.410 |
| Anthracycline dose >250 mg/m2 | −1.21 | −3.55 to 1.14 | 0.308 | |||
| Radiotherapy | 0.09 | −3.18 to 3.37 | 0.954 | |||
| HSCT | −1.49 | −3.00 to 0.02 | 0.052 | −1.63 | −3.21 to −0.06 | 0.042 |
| Hs-cTnI (ng/L) | −0.03 | −0.16 to −0.15 | 0.970 | |||
| Hs-cTnI > 2.5 ng/L | 0.320 | −0.91 to 1.55 | 0.606 | |||
| NT-ProBNP (pg/mL) | 0.001 | −0.01 to 0.01 | 0.972 | |||
| NT-ProBNP > 125 pg/mL | 0.442 | −1.30 to 2.18 | 0.615 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Manzanares, R.; Castillo, J.C.; Molina, J.R.; Ruiz-Ortiz, M.; Mesa, D.; Ojeda, S.; Anguita, M.; Pan, M. Automated Global Longitudinal Strain Assessment in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Cancers 2022, 14, 1513. https://doi.org/10.3390/cancers14061513
Gonzalez-Manzanares R, Castillo JC, Molina JR, Ruiz-Ortiz M, Mesa D, Ojeda S, Anguita M, Pan M. Automated Global Longitudinal Strain Assessment in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Cancers. 2022; 14(6):1513. https://doi.org/10.3390/cancers14061513
Chicago/Turabian StyleGonzalez-Manzanares, Rafael, Juan C. Castillo, Jose R. Molina, Martin Ruiz-Ortiz, Dolores Mesa, Soledad Ojeda, Manuel Anguita, and Manuel Pan. 2022. "Automated Global Longitudinal Strain Assessment in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia" Cancers 14, no. 6: 1513. https://doi.org/10.3390/cancers14061513
APA StyleGonzalez-Manzanares, R., Castillo, J. C., Molina, J. R., Ruiz-Ortiz, M., Mesa, D., Ojeda, S., Anguita, M., & Pan, M. (2022). Automated Global Longitudinal Strain Assessment in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Cancers, 14(6), 1513. https://doi.org/10.3390/cancers14061513






