In Vivo and In Vitro Enhanced Tumoricidal Effects of Metformin, Active Vitamin D3, and 5-Fluorouracil Triple Therapy against Colon Cancer by Modulating the PI3K/Akt/PTEN/mTOR Network
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Animal Studies and Treatment Protocols
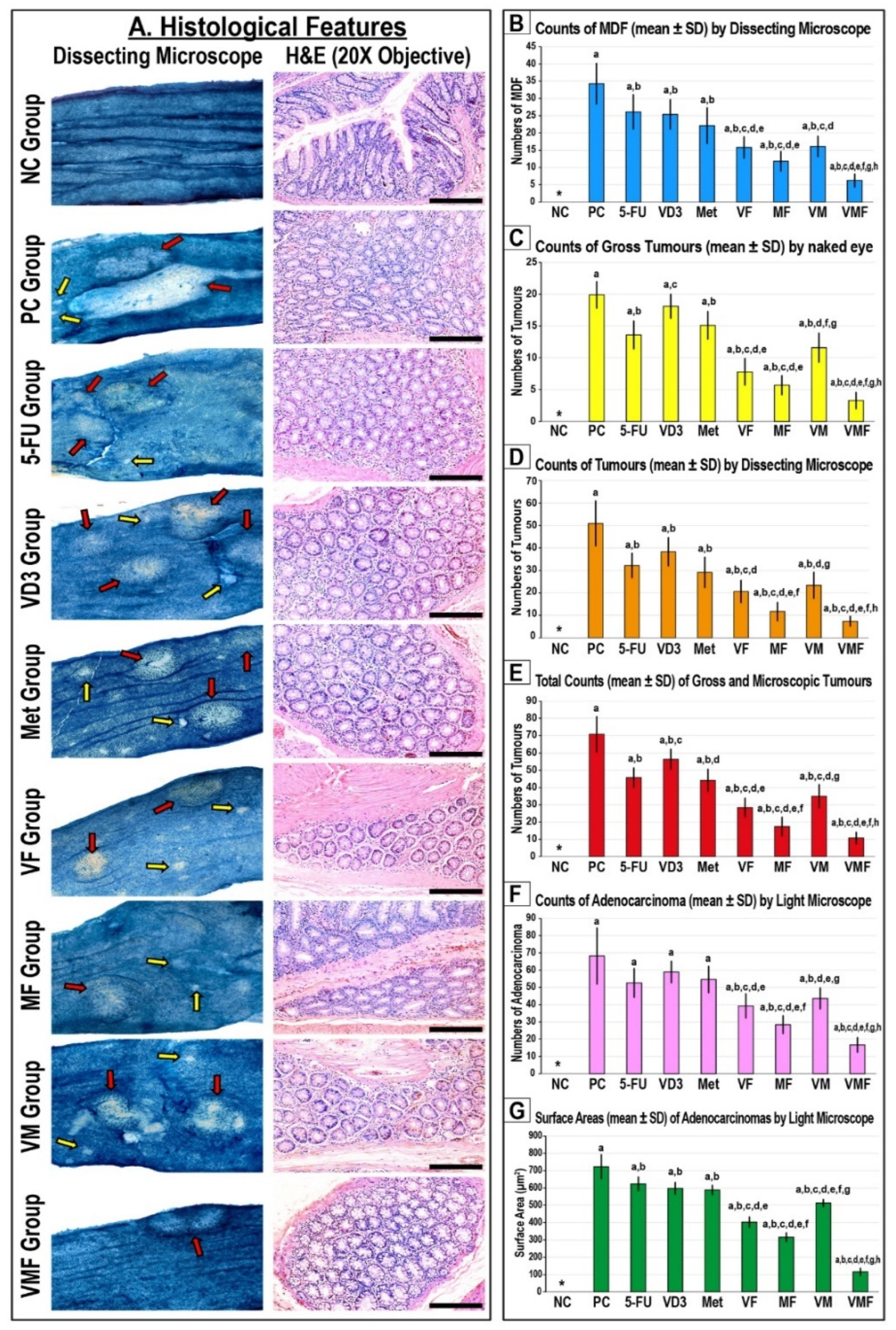
2.2.1. Gross Examination and Tumour Counting
2.2.2. Histopathological Studies of Colon Tissues
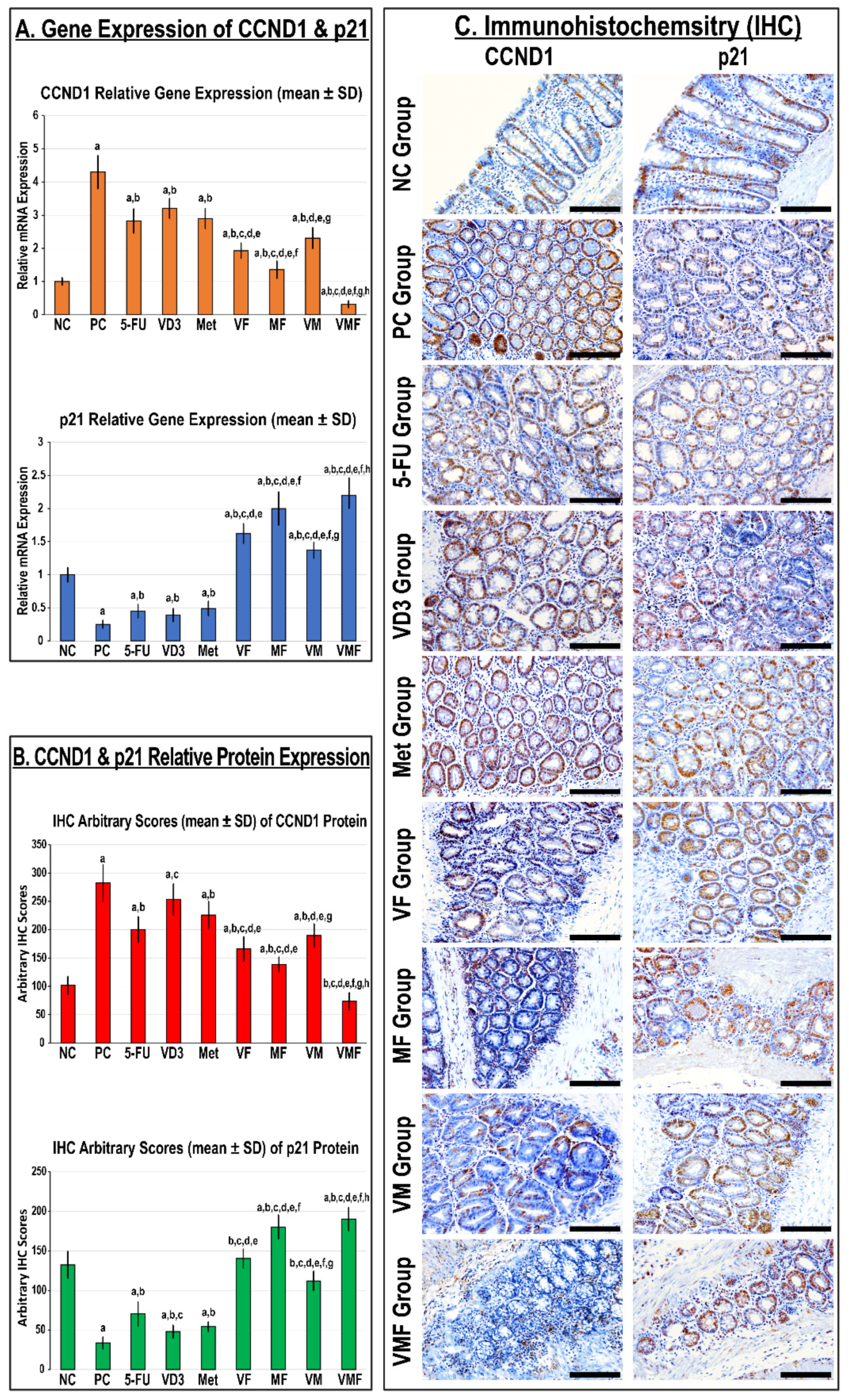
2.2.3. Immunohistochemistry (IHC)
2.2.4. Terminal Deoxynucleotidyl Transferase-dUTP Nick End Labelling (TUNEL) Assay
2.2.5. Liver and Renal Biochemical Profiles
2.2.6. ELISA
2.3. Cell Culture and Treatment Protocols
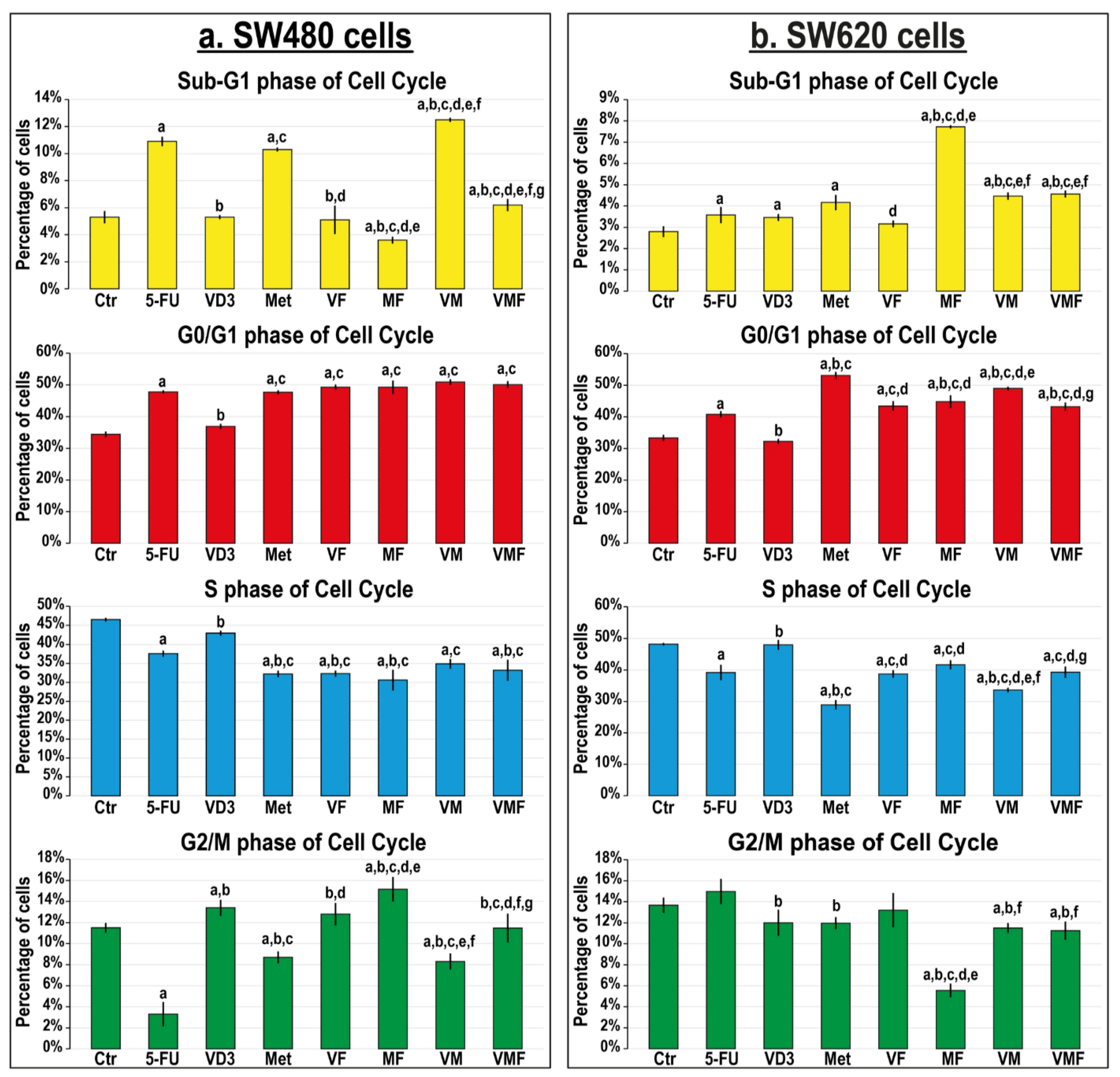
2.3.1. Cell Cycle Analysis
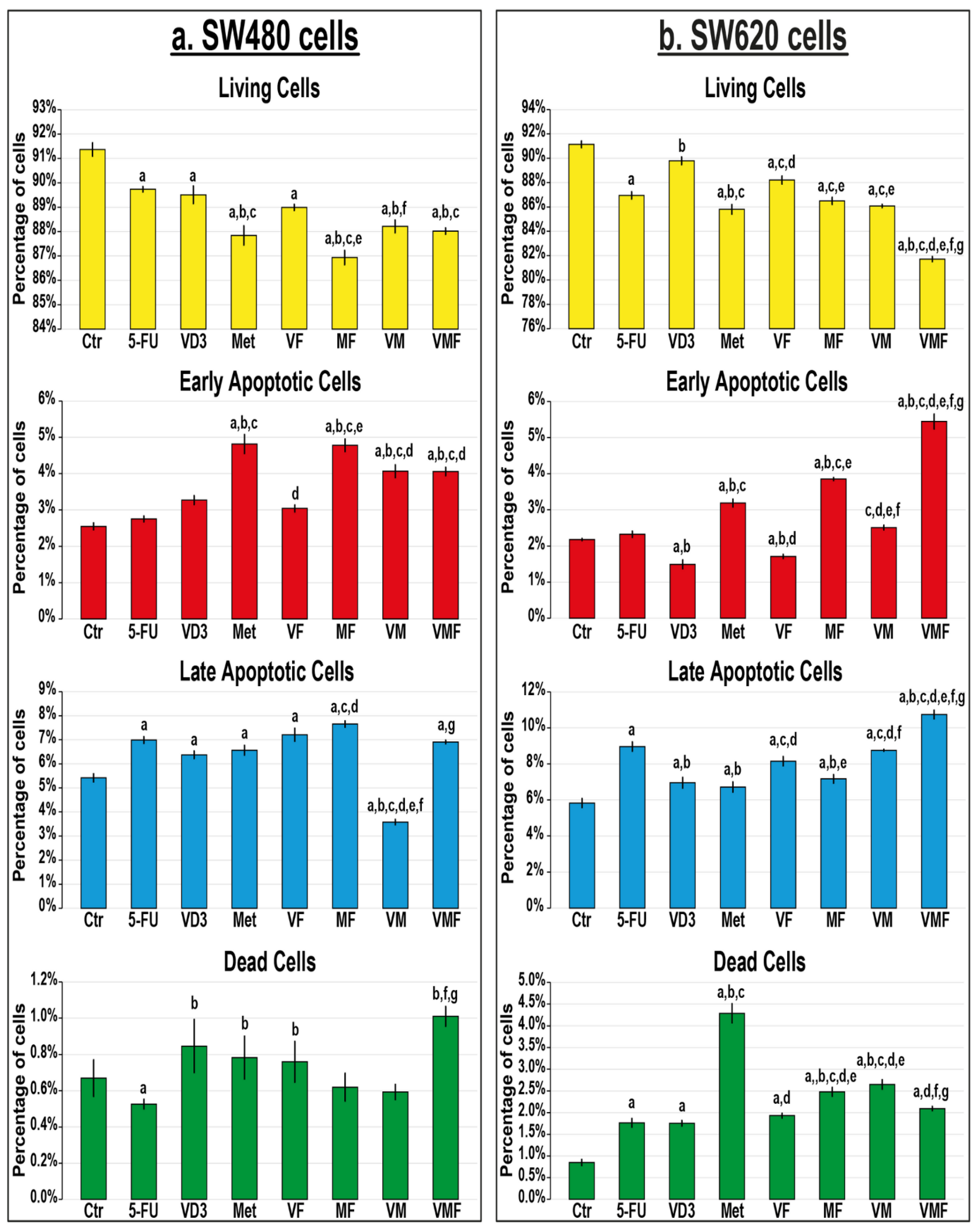
2.3.2. Apoptosis Assay
2.3.3. Quantitative Reverse-Transcription Polymerase Chain Reaction
2.3.4. Western Blot
2.4. Statistical Analysis
3. Results
3.1. Effects of Treatment Regimens on CRC Progression in Mice
3.2. Effects of the Different Treatment Protocols on Cell Cycle
3.2.1. In Vivo Expression of CCND1 and p21
3.2.2. In Vitro Cell Cycle Arrest and Expression of Cell Cycle Regulatory Molecules
3.3. Effects of the Different Treatment Protocols on Cell Death and Apoptosis Markers
3.3.1. Colonic Cell Apoptosis and Apoptosis Markers In Vivo
3.3.2. In Vitro Cell Apoptosis and Expression of Apoptosis Markers
3.4. Effects of the Different Treatment Protocols on the Expression of PI3K/Akt/mTOR Oncogenic Pathway
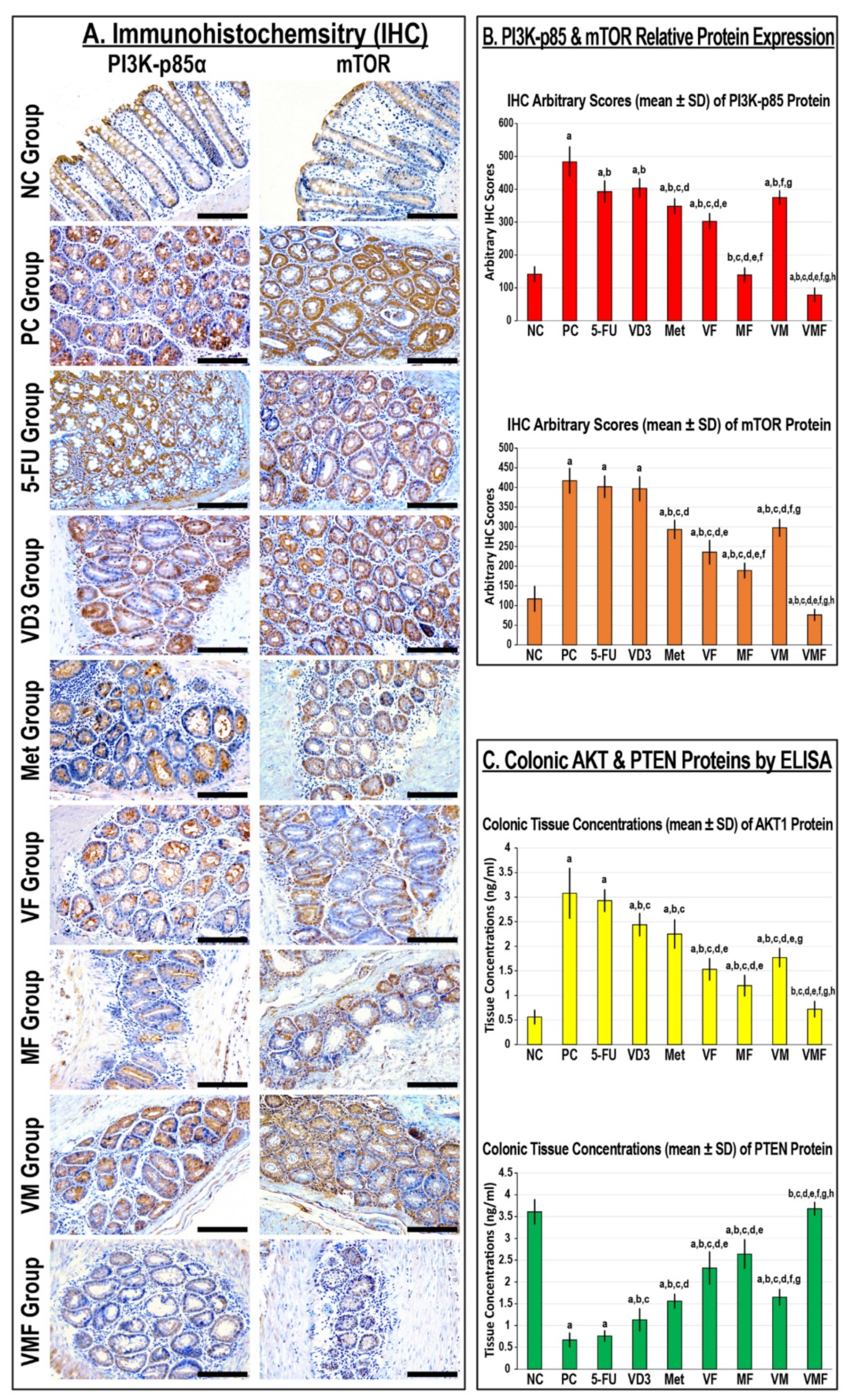
3.4.1. Protein Expression of PI3K/Akt/mTOR in Colonic Tissues
3.4.2. In Vitro Gene and Protein Expression of the PI3K/Akt/mTOR Oncogenic Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wei, J.; Xu, C.; Zhao, Z.; You, T. Prognostic significance of cyclin D1 expression in colorectal cancer: A meta-analysis of observational studies. PLoS ONE 2014, 9, e94508. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, K.; Pryczynicz, A.; Dymicka-Piekarska, V.; Famulski, W.; Guzińska-Ustymowicz, K. Immunohistochemical expression and serum level of survivin protein in colorectal cancer patients. Oncol. Lett. 2016, 12, 3591–3597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Maghrabi, J.; Al-Ahwal, M.; Buhmeida, A.; Syrjänen, K.; Sibyani, A.; Emam, E.; Ghanim, A.; Al-Qahtani, M. Expression of cell cycle regulators p21 and p27 as predictors of disease outcome in colorectal carcinoma. J. Gastrointest. Cancer 2012, 43, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.L.; Cawthorne, C.; Zhou, C.; Hodgkinson, C.L.; Walker, M.J.; Trapani, F.; Kadirvel, M.; Brown, G.; Dawson, M.J.; MacFarlane, M.; et al. A caspase-3 ‘death-switch’ in colorectal cancer cells for induced and synchronous tumor apoptosis in vitro and in vivo facilitates the development of minimally invasive cell death biomarkers. Cell Death Dis. 2013, 4, e613. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Bae, J.M.; Wen, X.; Jung, S.; Kim, Y.; Kim, K.J.; Cho, N.Y.; Kim, J.H.; Han, S.W.; Kim, T.Y.; et al. p53 expression status is associated with cancer-specific survival in stage III and high-risk stage II colorectal cancer patients treated with oxaliplatin-based adjuvant chemotherapy. Br. J. Cancer 2019, 120, 797–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Can, G.; Akpinar, B.; Baran, Y.; Zhivotovsky, B.; Olsson, M. 5-Fluorouracil signaling through a calcium-calmodulin-dependent pathway is required for p53 activation and apoptosis in colon carcinoma cells. Oncogene 2013, 32, 4529–4538. [Google Scholar] [CrossRef] [Green Version]
- Akpinar, B.; Bracht, E.V.; Reijnders, D.; Safarikova, B.; Jelinkova, I.; Grandien, A.; Vaculova, A.H.; Zhivotovsky, B.; Olsson, M. 5-Fluorouracil-induced RNA stress engages a TRAIL-DISC-dependent apoptosis axis facilitated by p53. Oncotarget 2015, 6, 43679–43697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satapathy, S.R.; Sjölander, A. Cysteinyl leukotriene receptor 1 promotes 5-fluorouracil resistance and resistance-derived stemness in colon cancer cells. Cancer Lett. 2020, 488, 50–62. [Google Scholar] [CrossRef]
- Koulis, C.; Yap, R.; Engel, R.; Jardé, T.; Wilkins, S.; Solon, G.; Shapiro, J.D.; Abud, H.; McMurrick, P. Personalized Medicine-Current and Emerging Predictive and Prognostic Biomarkers in Colorectal Cancer. Cancers 2020, 12, 812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S.M.; Gulhati, P.; Rampy, B.A.; Han, Y.; Rychahou, P.G.; Doan, H.Q.; Weiss, H.L.; Evers, B.M. Novel expression patterns of PI3K/Akt/mTOR signaling pathway components in colorectal cancer. J. Am. Coll. Surg. 2010, 210, 767–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, C.M.; Winnay, J.; Kondo, T.; Bronson, R.T.; Guimaraes, A.R.; Alemán, J.O.; Luo, J.; Stephanopoulos, G.; Weissleder, R.; Cantley, L.C.; et al. The phosphoinositide 3-kinase regulatory subunit p85alpha can exert tumor suppressor properties through negative regulation of growth factor signaling. Cancer Res. 2010, 70, 5305–5315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francipane, M.G.; Lagasse, E. mTOR pathway in colorectal cancer: An update. Oncotarget 2014, 5, 49–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reita, D.; Bour, C.; Benbrika, R.; Groh, A.; Pencreach, E.; Guérin, E.; Guenot, D. Synergistic Anti-Tumor Effect of mTOR Inhibitors with Irinotecan on Colon Cancer Cells. Cancers 2019, 11, 1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanankutty, A. PI3K/ Akt/ mTOR Pathway as a Therapeutic Target for Colorectal Cancer: A Review of Preclinical and Clinical Evidence. Curr. Drug Targets 2019, 20, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Zoltick, E.S.; Weinstein, S.J.; Fedirko, V.; Wang, M.; Cook, N.R.; Eliassen, A.H.; Zeleniuch-Jacquotte, A.; Agnoli, C.; Albanes, D.; et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J. Natl. Cancer Inst. 2019, 111, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jiang, L.; Chi, X.; Hochwald, S.; Qiu, F.; Luo, Y.; Lu, Q.; Yang, X.; Huang, H.; Xu, J. The association of serum vitamin D-binding protein and 25-hydroxyvitamin D in pre-operative and post-operative colorectal cancer. J. Clin. Lab. Anal. 2020, 34, e23154. [Google Scholar] [CrossRef]
- Liaudat, A.C.; Bohl, L.P.; Tolosa de Talamoni, N.G.; Maletto, B.; Pistoresi-Palencia, M.C.; Picotto, G. Oxidative stress, cell cycle arrest and differentiation contribute toward the antiproliferative action of BSO and calcitriol on Caco-2 cells. Anti-Cancer Drugs 2014, 25, 810–818. [Google Scholar] [CrossRef]
- Aslam, A.; Ahmad, J.; Baghdadi, M.A.; Idris, S.; Almaimani, R.; Alsaegh, A.; Alhadrami, M.; Refaat, B. Chemopreventive effects of vitamin D(3) and its analogue, paricalcitol, in combination with 5-fluorouracil against colorectal cancer: The role of calcium signalling molecules. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166040. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Wu, L.; Wang, Y.; Yuan, X. Long Non-coding RNA MEG3 Activated by Vitamin D Suppresses Glycolysis in Colorectal Cancer via Promoting c-Myc Degradation. Front. Oncol. 2020, 10, 274. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.Y.; Weng, Y.T.; Li, P.C.; Hsieh, N.T.; Li, C.I.; Liu, H.S.; Lee, M.F. Calcitriol Suppresses Warburg Effect and Cell Growth in Human Colorectal Cancer Cells. Life 2021, 11, 963. [Google Scholar] [CrossRef]
- Amable, G.; Martínez-León, E.; Picco, M.E.; Di Siervi, N.; Davio, C.; Rozengurt, E.; Rey, O. Metformin inhibits β-catenin phosphorylation on Ser-552 through an AMPK/PI3K/Akt pathway in colorectal cancer cells. Int. J. Biochem. Cell Biol. 2019, 112, 88–94. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Duan, H.; Yuan, L. Metformin Suppresses the Proliferation and Promotes the Apoptosis of Colon Cancer Cells Through Inhibiting the Expression of Long Noncoding RNA-UCA1. OncoTargets Ther. 2020, 13, 4169–4181. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Q.; Yan, A.; Chang, H.; Ding, Y.; Tao, J.; Qiao, C. Metformin revert insulin-induced oxaliplatin resistance by activating mitochondrial apoptosis pathway in human colon cancer HCT116 cells. Cancer Med. 2020, 9, 3875–3884. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, F.; Hosseini, S.M.; Omidi, M.; Hosseini, S.F.; Rezaei, M. Cytotoxicity of metformin against HT29 colon cancer cells contributes to mitochondrial Sirt3 upregulation. J. Biochem. Mol. Toxicol. 2021, 35, e22662. [Google Scholar] [CrossRef] [PubMed]
- Marciano, O.; Mehazri, L.; Shpungin, S.; Varvak, A.; Zacksenhaus, E.; Nir, U. Fer and FerT Govern Mitochondrial Susceptibility to Metformin and Hypoxic Stress in Colon and Lung Carcinoma Cells. Cells 2021, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Refaat, B.; El-Shemi, A.G.; Kensara, O.A.; Mohamed, A.M.; Idris, S.; Ahmad, J.; Khojah, A. Vitamin D3 enhances the tumouricidal effects of 5-Fluorouracil through multipathway mechanisms in azoxymethane rat model of colon cancer. J. Exp. Clin. Cancer Res. CR 2015, 34, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, J.; Tang, R.; Yang, M.; Sun, Q. Metformin Inhibited Proliferation and Metastasis of Colorectal Cancer and presented a Synergistic Effect on 5-FU. BioMed. Res. Int. 2020, 2020, 9312149. [Google Scholar] [CrossRef]
- Fernandes, J.M.; Jandrey, E.H.F.; Koyama, F.C.; Leite, K.R.M.; Camargo, A.A.; Costa, É.T.; Perez, R.O.; Asprino, P.F. Metformin as an Alternative Radiosensitizing Agent to 5-Fluorouracil During Neoadjuvant Treatment for Rectal Cancer. Dis. Colon Rectum 2020, 63, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Abu El Maaty, M.A.; Strassburger, W.; Qaiser, T.; Dabiri, Y.; Wölfl, S. Differences in p53 status significantly influence the cellular response and cell survival to 1,25-dihydroxyvitamin D3-metformin cotreatment in colorectal cancer cells. Mol. Carcinog. 2017, 56, 2486–2498. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Q.L.; Liu, X.; Dong, S.H.; Li, H.X.; Li, C.Y.; Guo, L.S.; Gao, J.M.; Berger, N.A.; Li, L.; et al. Combined use of vitamin D3 and metformin exhibits synergistic chemopreventive effects on colorectal neoplasia in rats and mice. Cancer Prev. Res. 2015, 8, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taheri, S.; Asim, M.; Al Malki, H.; Fituri, O.; Suthanthiran, M.; August, P. Intervention using vitamin D for elevated urinary albumin in type 2 diabetes mellitus (IDEAL-2 Study): Study protocol for a randomised controlled trial. Trials 2018, 19, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, R.; Boukichou Abdelkader, N.; Acosta, T.; Gilis-Januszewska, A.; Gómez-Huelgas, R.; Makrilakis, K.; Kamenov, Z.; Paulweber, B.; Satman, I.; Djordjevic, P.; et al. Early prevention of diabetes microvascular complications in people with hyperglycaemia in Europe. ePREDICE randomized trial. Study protocol, recruitment and selected baseline data. PLoS ONE 2020, 15, e0231196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refaat, B.; El-Shemi, A.G.; Mohamed, A.M.; Kensara, O.A.; Ahmad, J.; Idris, S. Activins and their related proteins in colon carcinogenesis: Insights from early and advanced azoxymethane rat models of colon cancer. BMC Cancer 2016, 16, 879. [Google Scholar] [CrossRef] [Green Version]
- Refaat, B.; Zekri, J.; Aslam, A.; Ahmad, J.; Baghdadi, M.A.; Meliti, A.; Idris, S.; Sultan, S.; Alardati, H.; Saimeh, H.A.; et al. Profiling Activins and Follistatin in Colorectal Cancer According to Clinical Stage, Tumour Sidedness and Smad4 Status. Pathol. Oncol. Res. 2021, 27, 1610032. [Google Scholar] [CrossRef]
- Refaat, B.; Abdelghany, A.H.; BaSalamah, M.A.; El-Boshy, M.; Ahmad, J.; Idris, S. Acute and Chronic Iron Overloading Differentially Modulates the Expression of Cellular Iron-homeostatic Molecules in Normal Rat Kidney. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2018, 66, 825–839. [Google Scholar] [CrossRef] [Green Version]
- El-Boshy, M.; Alsaegh, A.; Qasem, A.H.; Sindi, R.A.; Abdelghany, A.H.; Gadalla, H.; Reda, D.; Azzeh, F.; Idris, S.; Ahmad, J.; et al. Enhanced renoprotective actions of Paricalcitol and omega-3 fatty acids co-therapy against diabetic nephropathy in rat. J. Adv. Res. 2021; in press. [Google Scholar] [CrossRef]
- Refaat, B.; Abdelghany, A.H.; Ahmad, J.; Abdalla, O.M.; Elshopakey, G.E.; Idris, S.; El-Boshy, M. Vitamin D3 enhances the effects of omega-3 oils against metabolic dysfunction-associated fatty liver disease in rat. BioFactors, 2021; preprint. [Google Scholar] [CrossRef]
- Patntirapong, S.; Chanruangvanit, C.; Lavanrattanakul, K.; Satravaha, Y. Assessment of bisphosphonate treated-osteoblast behaviors by conventional assays and a simple digital image analysis. Acta Histochem. 2021, 123, 151659. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Almaimani, R.A.; Almasmoum, H.; Ghaith, M.M.; El-Boshy, M.; Idris, S.; Ahmad, J.; Abdelghany, A.H.; BaSalamah, M.A.; Mahbub, A.; Refaat, B. Enhanced remedial effects for vitamin D3 and calcium co-supplementation against pre-existing lead nephrotoxicity in mice: The roles of renal calcium homeostatic molecules. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Matuo, R.; Sousa, F.G.; Escargueil, A.E.; Grivicich, I.; Garcia-Santos, D.; Chies, J.A.; Saffi, J.; Larsen, A.K.; Henriques, J.A. 5-Fluorouracil and its active metabolite FdUMP cause DNA damage in human SW620 colon adenocarcinoma cell line. J. Appl. Toxicol. 2009, 29, 308–316. [Google Scholar] [CrossRef]
- Kang, Y.H.; Lee, J.S.; Lee, N.H.; Kim, S.H.; Seo, C.S.; Son, C.G. Coptidis Rhizoma Extract Reverses 5-Fluorouracil Resistance in HCT116 Human Colorectal Cancer Cells via Modulation of Thymidylate Synthase. Molecules 2021, 26, 1856. [Google Scholar] [CrossRef]
- Kim, E.J.; Kang, G.J.; Kang, J.I.; Boo, H.J.; Hyun, J.W.; Koh, Y.S.; Chang, W.Y.; Kim, Y.R.; Kwon, J.M.; Maeng, Y.H.; et al. Over-activation of AKT signaling leading to 5-Fluorouracil resistance in SNU-C5/5-FU cells. Oncotarget 2018, 9, 19911–19928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Jin, Y.; Fan, Z. The Mechanism of Warburg Effect-Induced Chemoresistance in Cancer. Front. Oncol. 2021, 11, 698023. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef]
- Meng, F.; Song, L.; Wang, W. Metformin Improves Overall Survival of Colorectal Cancer Patients with Diabetes: A Meta-Analysis. J. Diabetes Res. 2017, 2017, 5063239. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, Y.; Zhou, C.; Shen, L.; Tu, F.; Xu, J.; Liu, C. For colorectal cancer patients with type II diabetes, could metformin improve the survival rate? A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Lu, R.; Wu, S.; Chatterjee, I.; Zhou, D.; Xia, Y.; Sun, J. Vitamin D Receptor Protects Against Dysbiosis and Tumorigenesis via the JAK/STAT Pathway in Intestine. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 729–746. [Google Scholar] [CrossRef] [PubMed]
- Kotlarz, A.; Przybyszewska, M.; Swoboda, P.; Neska, J.; Miłoszewska, J.; Grygorowicz, M.A.; Kutner, A.; Markowicz, S. Imatinib inhibits the regrowth of human colon cancer cells after treatment with 5-FU and cooperates with vitamin D analogue PRI-2191 in the downregulation of expression of stemness-related genes in 5-FU refractory cells. J. Steroid Biochem. Mol. Biol. 2019, 189, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Omura, Y.; Lu, D.; Jones, M.K.; Nihrane, A.; Duvvi, H.; Yapor, D.; Shimotsuura, Y.; Ohki, M. Optimal Dose of Vitamin D3 400 I.U. for Average Adults has A Significant Anti-Cancer Effect, While Widely Used 2000 I.U. or Higher Promotes Cancer: Marked Reduction of Taurine & 1α, 25(OH)2D3 Was Found In Various Cancer Tissues and Oral Intake of Optimal Dose of Taurine 175mg for Average Adults, Rather Than 500mg, Was Found to Be A New Potentially Safe and More Effective Method of Cancer Treatment. Acupunct. Electro-Ther. Res. 2016, 41, 39–60. [Google Scholar] [CrossRef]
- Hernández-Alonso, P.; Canudas, S.; Boughanem, H.; Toledo, E.; Sorlí, J.V.; Estruch, R.; Castañer, O.; Lapetra, J.; Alonso-Gómez, A.M.; Gutiérrez-Bedmar, M.; et al. Dietary vitamin D intake and colorectal cancer risk: A longitudinal approach within the PREDIMED study. Eur. J. Nutr. 2021, 60, 4367–4378. [Google Scholar] [CrossRef] [PubMed]
- Provenzani, A.; Fronza, R.; Loreni, F.; Pascale, A.; Amadio, M.; Quattrone, A. Global alterations in mRNA polysomal recruitment in a cell model of colorectal cancer progression to metastasis. Carcinogenesis 2006, 27, 1323–1333. [Google Scholar] [CrossRef] [Green Version]
- Maamer-Azzabi, A.; Ndozangue-Touriguine, O.; Bréard, J. Metastatic SW620 colon cancer cells are primed for death when detached and can be sensitized to anoikis by the BH3-mimetic ABT-737. Cell Death Dis. 2013, 4, e801. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Washington, M.K.; Powell, A.E.; Sullivan, R.; Sundberg, J.P.; Wright, N.; Coffey, R.J.; Dove, W.F. Pathology of rodent models of intestinal cancer: Progress report and recommendations. Gastroenterology 2013, 144, 705–717. [Google Scholar] [CrossRef] [Green Version]
- Sun, A.; Li, C.; Chen, R.; Huang, Y.; Chen, Q.; Cui, X.; Liu, H.; Thrasher, J.B.; Li, B. GSK-3β controls autophagy by modulating LKB1-AMPK pathway in prostate cancer cells. Prostate 2016, 76, 172–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häggblad Sahlberg, S.; Mortensen, A.C.; Haglöf, J.; Engskog, M.K.; Arvidsson, T.; Pettersson, C.; Glimelius, B.; Stenerlöw, B.; Nestor, M. Different functions of AKT1 and AKT2 in molecular pathways, cell migration and metabolism in colon cancer cells. Int. J. Oncol. 2017, 50, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trafalis, D.T.; Sagredou, S.; Dalezis, P.; Voura, M.; Fountoulaki, S.; Nikoleousakos, N.; Almpanakis, K.; Deligiorgi, M.V.; Sarli, V. Anticancer Activity of Triazolo-Thiadiazole Derivatives and Inhibition of AKT1 and AKT2 Activation. Pharmaceutics 2021, 13, 493. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almaimani, R.A.; Aslam, A.; Ahmad, J.; El-Readi, M.Z.; El-Boshy, M.E.; Abdelghany, A.H.; Idris, S.; Alhadrami, M.; Althubiti, M.; Almasmoum, H.A.; et al. In Vivo and In Vitro Enhanced Tumoricidal Effects of Metformin, Active Vitamin D3, and 5-Fluorouracil Triple Therapy against Colon Cancer by Modulating the PI3K/Akt/PTEN/mTOR Network. Cancers 2022, 14, 1538. https://doi.org/10.3390/cancers14061538
Almaimani RA, Aslam A, Ahmad J, El-Readi MZ, El-Boshy ME, Abdelghany AH, Idris S, Alhadrami M, Althubiti M, Almasmoum HA, et al. In Vivo and In Vitro Enhanced Tumoricidal Effects of Metformin, Active Vitamin D3, and 5-Fluorouracil Triple Therapy against Colon Cancer by Modulating the PI3K/Akt/PTEN/mTOR Network. Cancers. 2022; 14(6):1538. https://doi.org/10.3390/cancers14061538
Chicago/Turabian StyleAlmaimani, Riyad Adnan, Akhmed Aslam, Jawwad Ahmad, Mahmoud Zaki El-Readi, Mohamed E. El-Boshy, Abdelghany H. Abdelghany, Shakir Idris, Mai Alhadrami, Mohammad Althubiti, Hussain A. Almasmoum, and et al. 2022. "In Vivo and In Vitro Enhanced Tumoricidal Effects of Metformin, Active Vitamin D3, and 5-Fluorouracil Triple Therapy against Colon Cancer by Modulating the PI3K/Akt/PTEN/mTOR Network" Cancers 14, no. 6: 1538. https://doi.org/10.3390/cancers14061538
APA StyleAlmaimani, R. A., Aslam, A., Ahmad, J., El-Readi, M. Z., El-Boshy, M. E., Abdelghany, A. H., Idris, S., Alhadrami, M., Althubiti, M., Almasmoum, H. A., Ghaith, M. M., Elzubeir, M. E., Eid, S. Y., & Refaat, B. (2022). In Vivo and In Vitro Enhanced Tumoricidal Effects of Metformin, Active Vitamin D3, and 5-Fluorouracil Triple Therapy against Colon Cancer by Modulating the PI3K/Akt/PTEN/mTOR Network. Cancers, 14(6), 1538. https://doi.org/10.3390/cancers14061538







