Impact of Regorafenib on Endothelial Transdifferentiation of Glioblastoma Stem-like Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Tumour Collection
2.2. Cell Culture and Treatments
2.3. Preparation of Regorafenib
2.4. Orthotopic Xenograft Generation
2.5. Immunohistochemistry
2.6. Neurosphere Formation Analysis
2.7. Cell Proliferation Analysis
2.8. Western Blotting
2.9. CD31 Cell Expression Quantification by Flow Cytometry Analysis
2.10. Pseudotube Formation Assay
2.11. Irradiation
2.12. In Vivo Matrigel™ Plug Assay
2.13. Statistical Analysis
3. Results
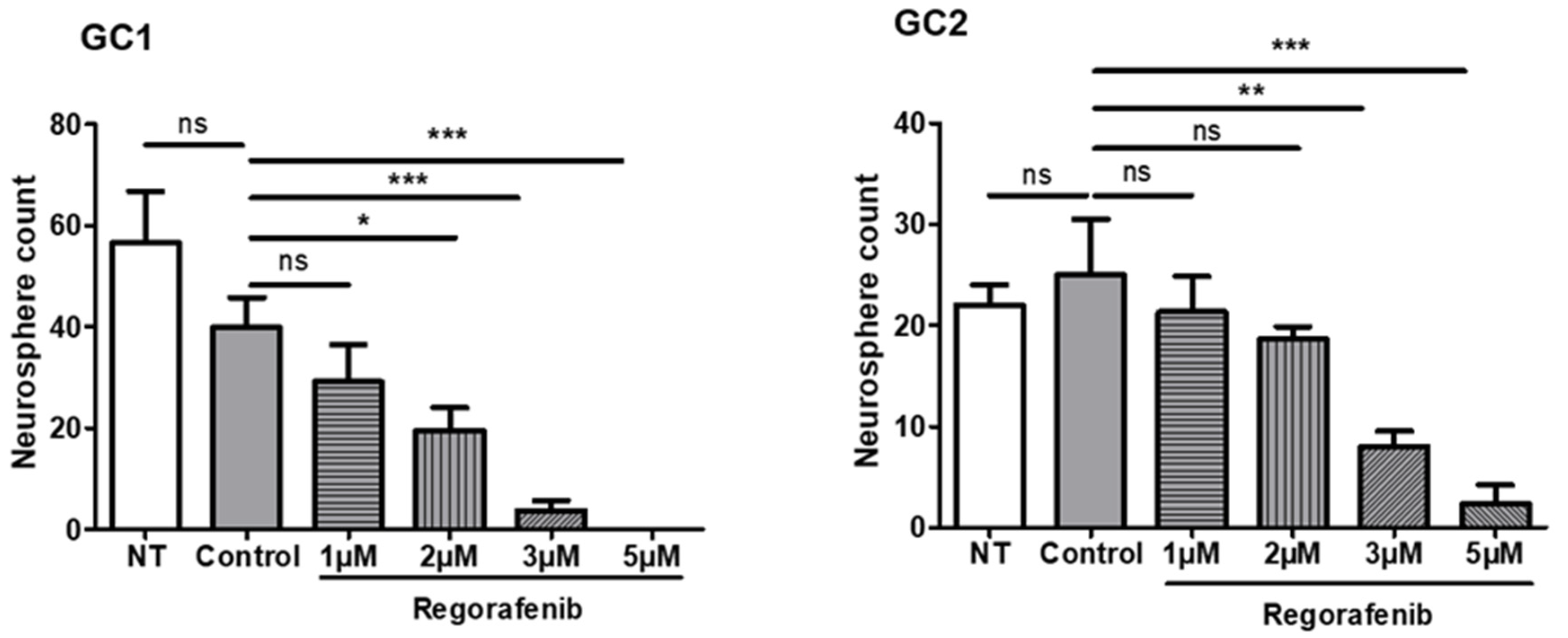
3.1. Regorafenib Inhibits the Tumourigenic Potential of GSC
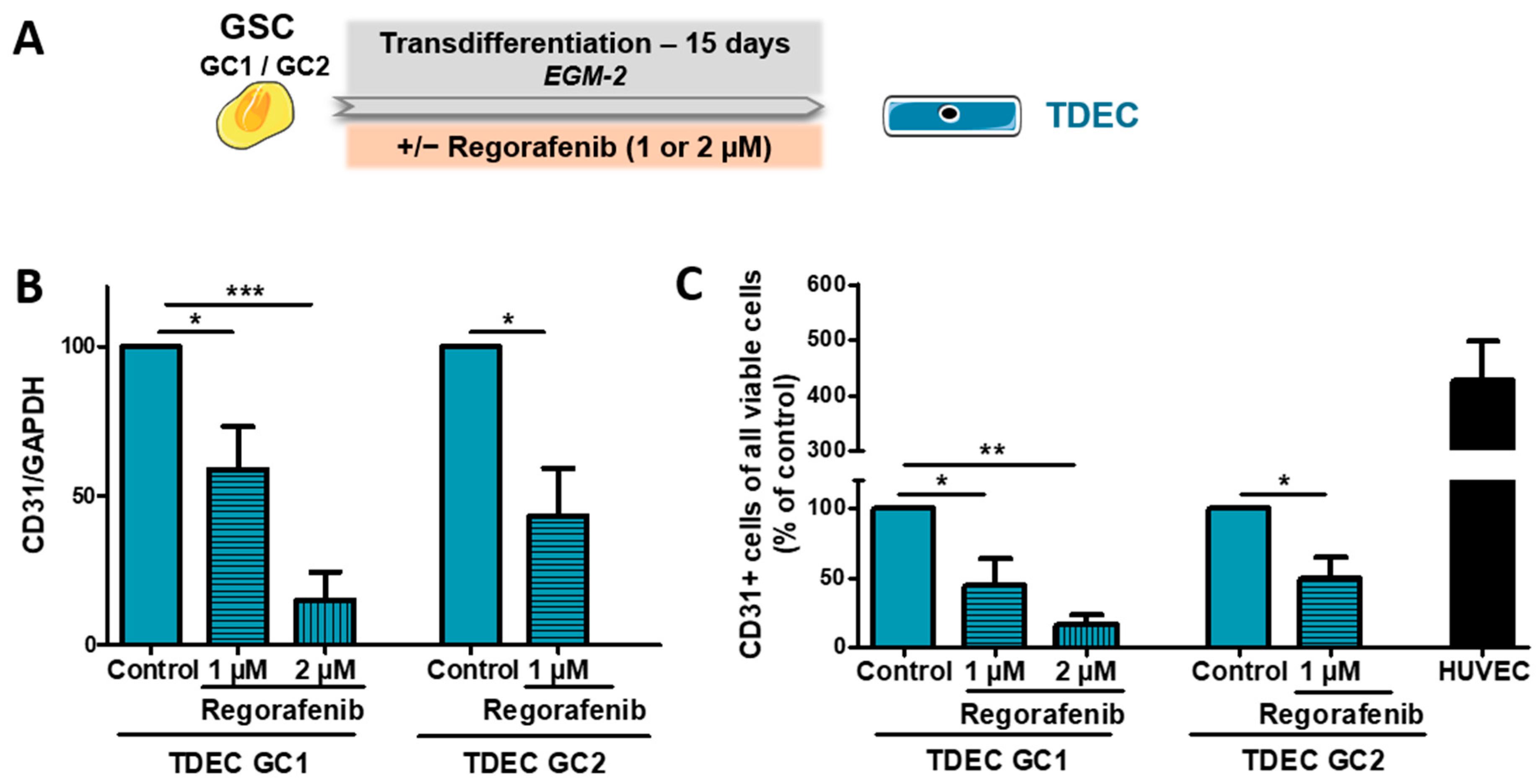
3.2. Regorafenib Inhibits Transdifferentiation of GSC In Vitro
3.3. Regorafenib Inhibits Irradiation (IR)-Induced Transdifferentiation of GSC In Vitro
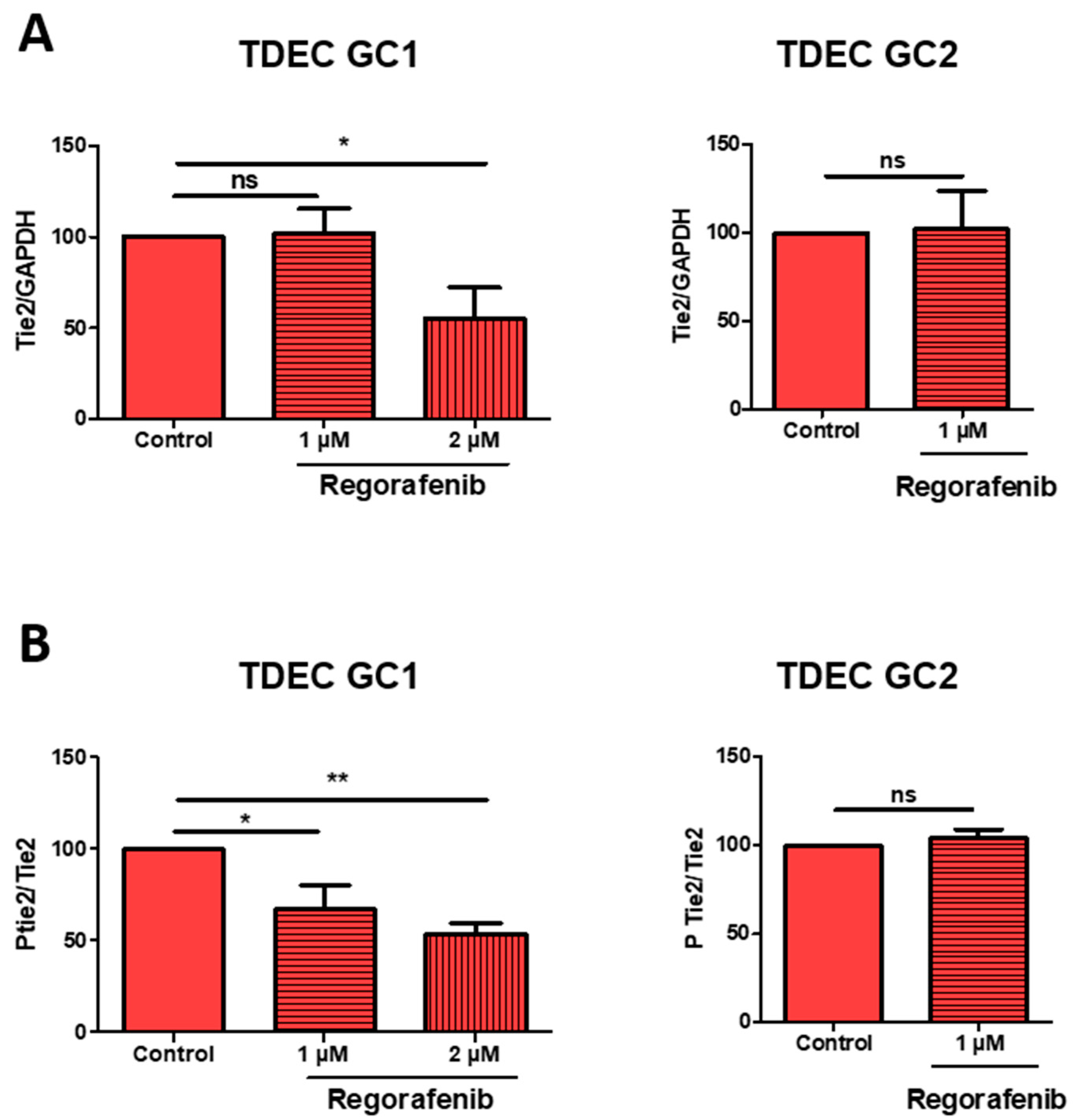
3.4. High-Dose Regorafenib Inhibits the Tie2 Signalling Pathway in TDEC IR+ In Vitro
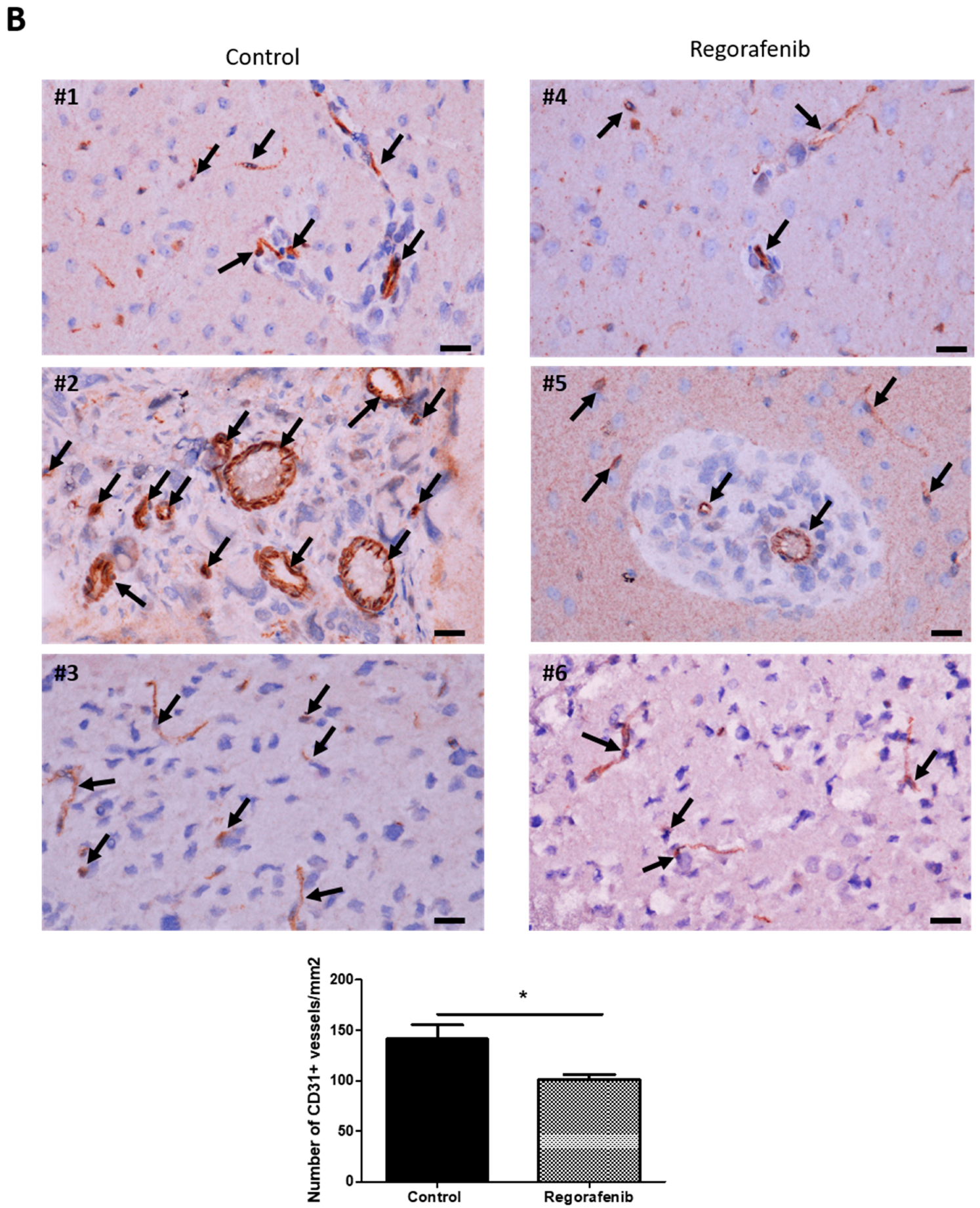
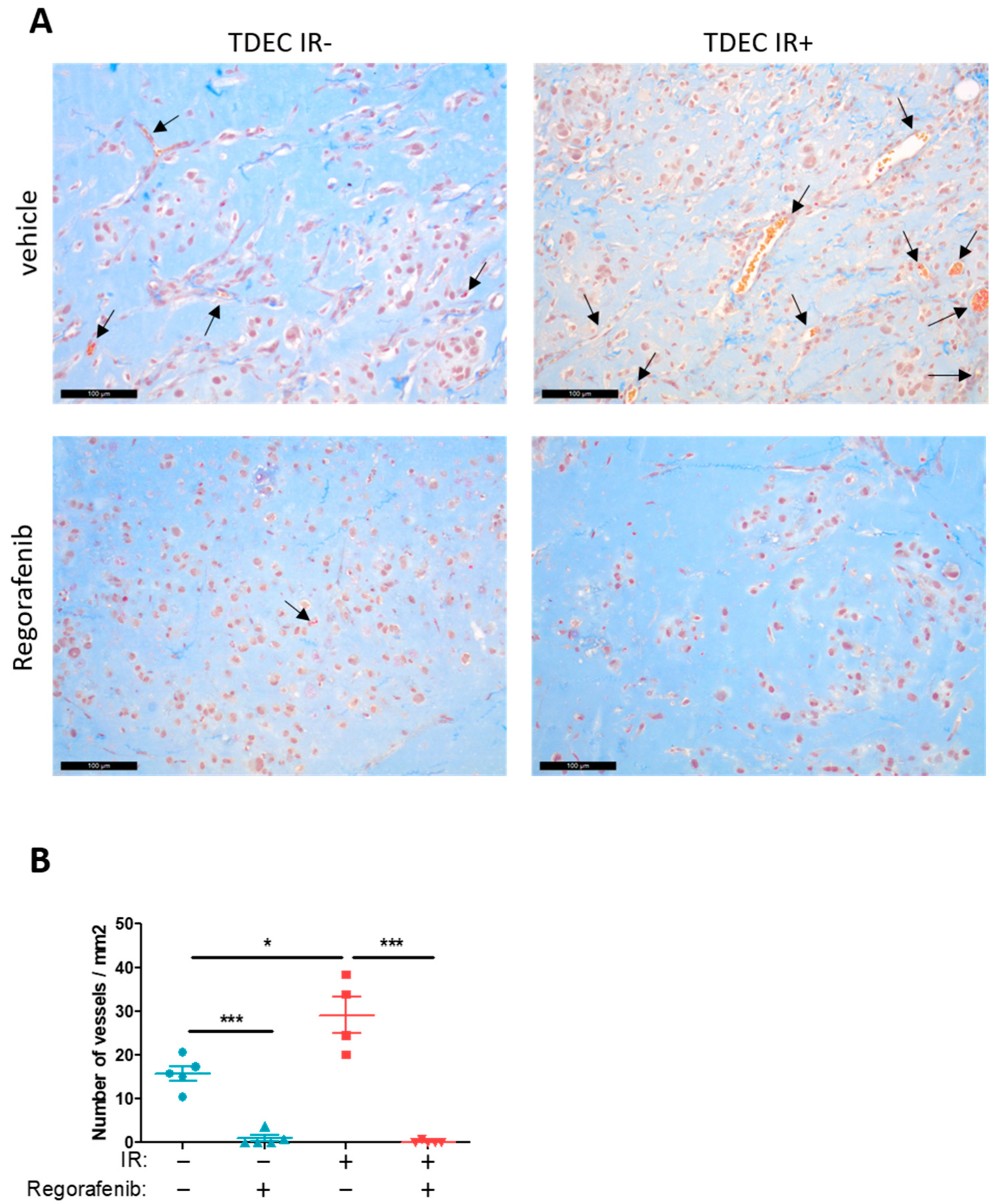
3.5. Regorafenib Inhibits Classical and IR-Induced Transdifferentiation In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K. World Health Organization Histological Classifications of Tumours of the Central Nervous System, 4th ed.; IARC Press: Lyon, France, 2016. [Google Scholar]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 8. [Google Scholar] [CrossRef]
- Tang, D.G. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012, 22, 457–472. [Google Scholar] [CrossRef]
- Nduom, E.K.-E.; Hadjipanayis, C.G.; Van Meir, E.G. Glioblastoma Cancer Stem-Like Cells: Implications for Pathogenesis and Treatment. Cancer J. 2012, 18, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Bao, S.; Rich, J.N. Potential therapeutic implications of cancer stem cells in glioblastoma. Biochem. Pharmacol. 2010, 80, 654–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchoghandjian, A.; Baeza, N.; Colin, C.; Cayre, M.; Metellus, P.; Beclin, C.; Ouafik, L.H.; Figarella-Branger, D. A2B5 Cells from Human Glioblastoma have Cancer Stem Cell Properties. Brain Pathol. 2010, 20, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Poppleton, H.; Kocak, M.; Hogg, T.L.; Fuller, C.; Hamner, B.; Oh, E.Y.; Gaber, M.W.; Finklestein, D.; Allen, M.; et al. A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell 2007, 11, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Hardee, M.E.; Zagzag, D. Mechanisms of Glioma-Associated Neovascularization. Am. J. Pathol. 2012, 181, 1126–1141. [Google Scholar] [CrossRef] [Green Version]
- Ricci-Vitiani, L.; Pallini, R.; Biffoni, M.; Todaro, M.; Invernici, G.; Cenci, T.; Maira, G.; Parati, E.A.; Stassi, G.; Larocca, L.M.; et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010, 468, 824–828. [Google Scholar] [CrossRef]
- Wang, R.; Chadalavada, K.; Wilshire, J.; Kowalik, U.; Hovinga, K.E.; Geber, A.; Fligelman, B.; Leversha, M.; Brennan, C.; Tabar, V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010, 468, 829–833. [Google Scholar] [CrossRef]
- Soda, Y.; Marumoto, T.; Friedmann-Morvinski, D.; Soda, M.; Liu, F.; Michiue, H.; Pastorino, S.; Yang, M.; Hoffman, R.M.; Kesari, S.; et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 4274–4280. [Google Scholar] [CrossRef] [Green Version]
- De Pascalis, I.; Morgante, L.; Pacioni, S.; D’Alessandris, Q.G.; Giannetti, S.; Martini, M.; Ricci-Vitiani, L.; Malinverno, M.; Dejana, E.; Larocca, L.M.; et al. Endothelial trans-differentiation in glioblastoma recurring after radiotherapy. Mod. Pathol. 2018, 31, 1361–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshors, P.; Toulas, C.; Arnauduc, F.; Malric, L.; Siegfried, A.; Nicaise, Y.; Lemarié, A.; Larrieu, D.; Tosolini, M.; Cohen-Jonathan Moyal, E.; et al. Ionizing radiation induces endothelial transdifferentiation of glioblastoma stem-like cells through the Tie2 signaling pathway. Cell Death Dis. 2019, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Schütz, G.; Thierauch, K.H.; Zopf, D. Regorafenib (BAY 734506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 2011, 129, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Zopf, D.; Fichtner, I.; Bhargava, A.; Steinke, W.; Thierauch, K.H.; Diefenbach, K.; Wilhelm, S.; Hafner, F.T.; Gerisch, M. Pharmacologic activity and pharmacokinetics of metabolites of regorafenib in preclinical models. Cancer Med. 2016, 5, 3176–3185. [Google Scholar] [CrossRef]
- Grothey, A.; Blay, J.-Y.; Pavlakis, N.; Yoshino, T.; Bruix, J. Evolving role of regorafenib for the treatment of advanced cancers. Cancer Treat. Rev. 2020, 86, 101993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daudigeos-Dubus, E.; Le Dret, L.; Lanvers-Kaminsky, C.; Bawa, O.; Opolon, P.; Vievard, A.; Villa, I.; Pagès, M.; Bosq, J.; Vassal, G.; et al. Regorafenib: Antitumor Activity upon Mono and Combination Therapy in Preclinical Pediatric Malignancy Models. PLoS ONE 2015, 10, e0142612. [Google Scholar] [CrossRef]
- Lombardi, G.; De Salvo, G.L.; Brandes, A.A.; Eoli, M.; Rudà, R.; Faedi, M.; Lolli, I.; Pace, A.; Daniele, B.; Pasqualetti, F.; et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019, 20, 110–119. [Google Scholar] [CrossRef]
- Malric, L.; Monferran, S.; Delmas, C.; Arnauduc, F.; Dahan, P.; Boyrie, S.; Deshors, P.; Lubrano, V.; Da Mota, D.F.; Gilhodes, J.; et al. Inhibiting Integrin β8 to Differentiate and Radiosensitize Glioblastoma-Initiating Cells. Mol. Cancer Res. 2019, 17, 384–397. [Google Scholar] [CrossRef] [Green Version]
- Dahan, P.; Martinez Gala, J.; Delmas, C.; Monferran, S.; Malric, L.; Zentkowski, D.; Lubrano, V.; Toulas, C.; Cohen-Jonathan Moyal, E.; Lemarie, A. Ionizing radiations sustain glioblastoma cell dedifferentiation to a stem-like phenotype through survivin: Possible involvement in radioresistance. Cell Death Dis. 2014, 5, e1543. [Google Scholar] [CrossRef] [Green Version]
- Kowalski-Chauvel, A.; Modesto, A.; Gouaze-Andersson, V.; Baricault, L.; Gilhodes, J.; Delmas, C.; Lemarie, A.; Toulas, C.; Cohen-Jonathan-Moyal, E.; Seva, C. Alpha-6 Integrin Promotes Radioresistance of Glioblastoma by Modulating DNA Damage Response and the Transcription Factor Zeb1. Available online: http://www.nature.com/articles/s41419-018-0853-x (accessed on 14 February 2019).
- Gouazé-Andersson, V.; Ghérardi, M.J.; Lemarié, A.; Gilhodes, J.; Lubrano, V.; Arnauduc, F.; Moyal, E.C.; Toulas, C. FGFR1/FOXM1 Pathway: A Key Regulator of Glioblastoma Stem Cells Radioresistance and a Prognosis Biomarker. Available online: http://www.oncotarget.com/fulltext/25827 (accessed on 14 February 2019).
- Evrard, S.M.; D’Audigier, C.; Mauge, L.; Israël-Biet, D.; Guerin, C.L.; Bieche, I.; Kovacic, J.C.; Fischer, A.M.; Gaussem, P.; Smadja, D.M. The profibrotic cytokine transforming growth factor-β1 increases endothelial progenitor cell angiogenic properties. J. Thromb. Haemost. 2012, 10, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Dirks, P.B. Brain tumour stem cells: The undercurrents of human brain cancer and their relationship to neural stem cells. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 139–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Pastrana, E.; Silva-Vargas, V.; Doetsch, F. Eyes Wide Open: A Critical Review of Sphere-Formation as an Assay for Stem Cells. Cell Stem Cell 2011, 8, 486–498. [Google Scholar] [CrossRef] [Green Version]
- Oprita, A.; Baloi, S.C.; Staicu, G.A.; Alexandru, O.; Tache, D.E.; Danoiu, S.; Micu, E.S.; Sevastre, A.S. Updated Insights on EGFR Signaling Pathways in Glioma. Int. J. Mol. Sci. 2021, 22, 587. [Google Scholar] [CrossRef]
- Fischer, I.; Gagner, J.-P.; Law, M.; Newcomb, E.W.; Zagzag, D. Angiogenesis in Gliomas: Biology and Molecular Pathophysiology. Brain Pathol. 2006, 15, 297–310. [Google Scholar] [CrossRef]
- Folkins, C.; Shaked, Y.; Man, S.; Tang, T.; Lee, C.R.; Zhu, Z.; Hoffman, R.M.; Kerbel, R.S. Glioma Tumor Stem-Like Cells Promote Tumor Angiogenesis and Vasculogenesis via Vascular Endothelial Growth Factor and Stromal-Derived Factor 1. Cancer Res. 2009, 69, 7243–7251. [Google Scholar] [CrossRef] [Green Version]
- Francescone, R.; Scully, S.; Bentley, B.; Yan, W.; Taylor, S.L.; Oh, D.; Moral, L.; Shao, R. Glioblastoma-derived Tumor Cells Induce Vasculogenic Mimicry through Flk-1 Protein Activation. J. Biol. Chem. 2012, 287, 24821–24831. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.B.; Yang, S.; Weng, H.Y.; Chen, Q.; Zhao, X.L.; Fu, W.J.; Niu, Q.; Ping, Y.F.; Wang, J.M.; Zhang, X.; et al. Autophagy-induced KDR/VEGFR-2 activation promotes the formation of vasculogenic mimicry by glioma stem cells. Autophagy 2017, 13, 1528–1542. [Google Scholar] [CrossRef] [Green Version]
- Soda, Y.; Myskiw, C.; Rommel, A.; Verma, I.M. Mechanisms of neovascularization and resistance to anti-angiogenic therapies in glioblastoma multiforme. J. Mol. Med. 2013, 91, 439–448. [Google Scholar] [CrossRef] [Green Version]
- Chinot, O.L.; Henriksson, R.; Carpentier, A.F.; Kavan, P.; Hilton, M.; Abrey, L. Bevacizumab plus Radiotherapy–Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Romero-López, M.; Benitez, L.I.; Di, K.; Frieboes, H.B.; Hughes, C.C.; Bota, D.A.; Lowengrub, J.S. 3D Mathematical Modeling of Glioblastoma Suggests that Transdifferentiated Vascular Endothelial Cells Mediate Resistance to Current Standard-of-Care Therapy. Cancer Res. 2017, 77, 4171–4184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. A Trial to Evaluate Multiple Regimens in Newly Diagnosed and Recurrent Glioblastoma (GBM AGILE). Available online: https://clinicaltrials.gov/ct2/show/NCT03970447 (accessed on 28 December 2021).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshors, P.; Arnauduc, F.; Boëlle, B.; Cohen-Jonathan Moyal, E.; Courtade-Saïdi, M.; Evrard, S.M. Impact of Regorafenib on Endothelial Transdifferentiation of Glioblastoma Stem-like Cells. Cancers 2022, 14, 1551. https://doi.org/10.3390/cancers14061551
Deshors P, Arnauduc F, Boëlle B, Cohen-Jonathan Moyal E, Courtade-Saïdi M, Evrard SM. Impact of Regorafenib on Endothelial Transdifferentiation of Glioblastoma Stem-like Cells. Cancers. 2022; 14(6):1551. https://doi.org/10.3390/cancers14061551
Chicago/Turabian StyleDeshors, Pauline, Florent Arnauduc, Betty Boëlle, Elizabeth Cohen-Jonathan Moyal, Monique Courtade-Saïdi, and Solène M. Evrard. 2022. "Impact of Regorafenib on Endothelial Transdifferentiation of Glioblastoma Stem-like Cells" Cancers 14, no. 6: 1551. https://doi.org/10.3390/cancers14061551
APA StyleDeshors, P., Arnauduc, F., Boëlle, B., Cohen-Jonathan Moyal, E., Courtade-Saïdi, M., & Evrard, S. M. (2022). Impact of Regorafenib on Endothelial Transdifferentiation of Glioblastoma Stem-like Cells. Cancers, 14(6), 1551. https://doi.org/10.3390/cancers14061551






