MRI Response Assessment in Glioblastoma Patients Treated with Dendritic-Cell-Based Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
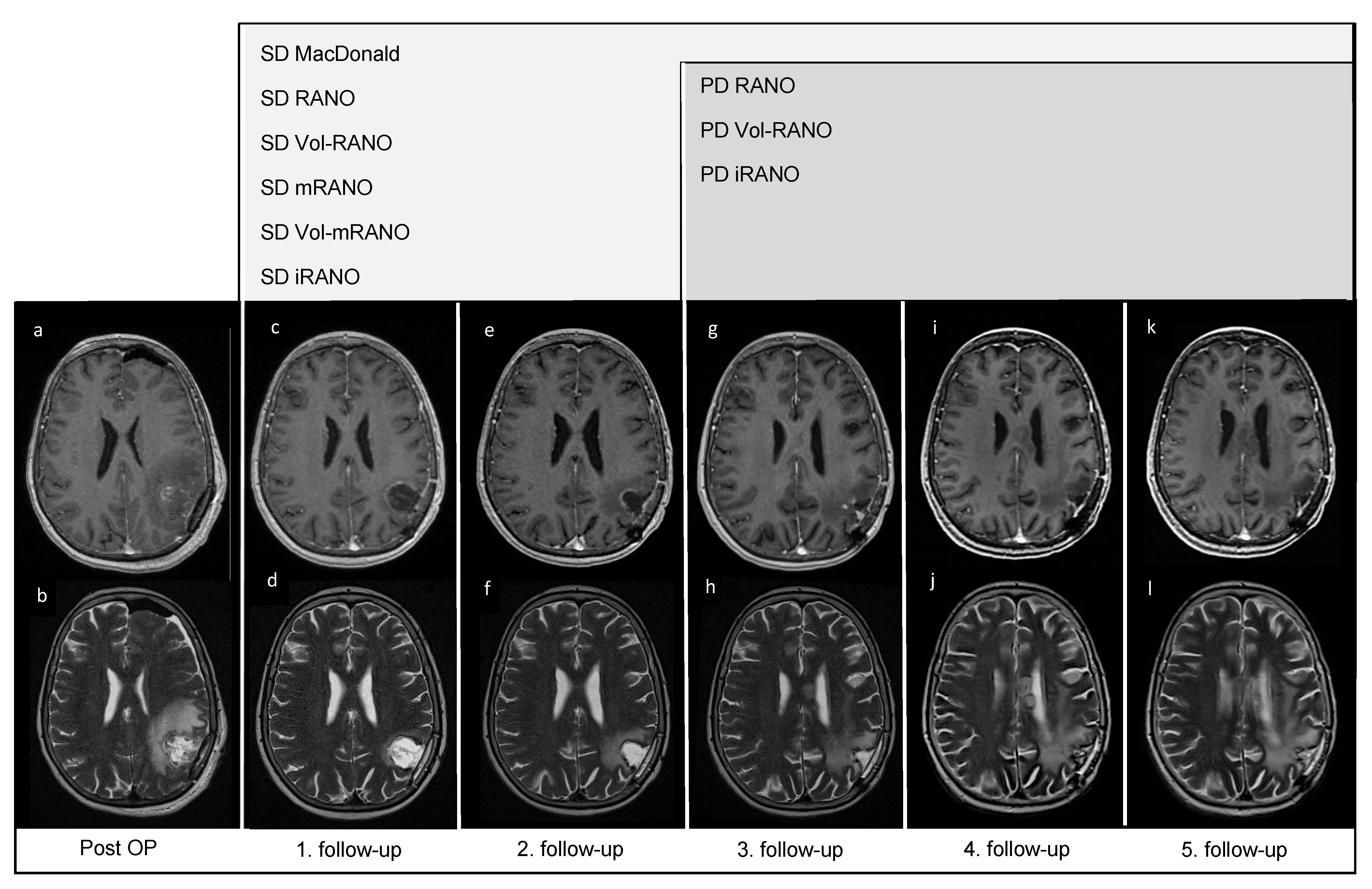
2.2. Magnetic Resonance Imaging
2.3. Radiologic Response Assessment
2.4. Volumetric Measurement
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Progression-Free Survival and Postprogression Survival
3.3. Progression-Free Survival and Correlation with Overall survival
3.4. Landmark Analysis
3.5. Non-Enhancing Abnormalities
3.6. Pseudoprogression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wöhrer, A.; Waldhör, T.; Heinzl, H.; Hackl, M.; Feichtinger, J.; Gruber-Mösenbacher, U.; Kiefer, A.; Maier, H.; Motz, R.; Reiner-Concin, A.; et al. The Austrian Brain Tumour Registry: A cooperative way to establish a population-based brain tumour registry. J. Neurooncol. 2009, 95, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22, iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.Y.; Reardon, D.A.; Armstrong, T.S.; Phuphanich, S.; Aiken, R.D.; Landolfi, J.C.; Curry, W.T.; Zhu, J.J.; Glantz, M.; Peereboom, D.M.; et al. A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients with Glioblastoma. Clin. Cancer Res. 2019, 25, 5799–5807. [Google Scholar] [CrossRef]
- Akasaki, Y.; Kikuchi, T.; Homma, S.; Koido, S.; Ohkusa, T.; Tasaki, T.; Hayashi, K.; Komita, H.; Watanabe, N.; Suzuki, Y.; et al. Phase I/II trial of combination of temozolomide chemotherapy and immunotherapy with fusions of dendritic and glioma cells in patients with glioblastoma. Cancer Immunol. Immunother. 2016, 65, 1499–1509. [Google Scholar] [CrossRef]
- Ardon, H.; Van Gool, S.W.; Verschuere, T.; Maes, W.; Fieuws, S.; Sciot, R.; Wilms, G.; Demaerel, P.; Goffin, J.; Van Calenbergh, F.; et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: Results of the HGG-2006 phase I/II trial. Cancer Immunol. Immunother. 2012, 61, 2033–2044. [Google Scholar] [CrossRef]
- Cao, J.X.; Zhang, X.Y.; Liu, J.L.; Li, D.; Li, J.L.; Liu, Y.S.; Wang, M.; Xu, B.L.; Wang, H.B.; Wang, Z.X. Clinical efficacy of tumor antigen-pulsed DC treatment for high-grade glioma patients: Evidence from a meta-analysis. PLoS ONE 2014, 9, e107173. [Google Scholar] [CrossRef] [PubMed]
- Inogés, S.; Tejada, S.; de Cerio, A.L.; Gállego Pérez-Larraya, J.; Espinós, J.; Idoate, M.A.; Domínguez, P.D.; de Eulate, R.G.; Aristu, J.; Bendandi, M.; et al. A phase II trial of autologous dendritic cell vaccination and radiochemotherapy following fluorescence-guided surgery in newly diagnosed glioblastoma patients. J. Transl. Med. 2017, 15, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapp, M.; Grauer, O.M.; Kamp, M.; Sevens, N.; Zotz, N.; Sabel, M.; Sorg, R.V. A randomized controlled phase II trial of vaccination with lysate-loaded, mature dendritic cells integrated into standard radiochemotherapy of newly diagnosed glioblastoma (GlioVax): Study protocol for a randomized controlled trial. Trials 2018, 19, 293. [Google Scholar] [CrossRef] [PubMed]
- Buchroithner, J.; Erhart, F.; Pichler, J.; Widhalm, G.; Preusser, M.; Stockhammer, G.; Nowosielski, M.; Iglseder, S.; Freyschlag, C.F.; Oberndorfer, S.; et al. Audencel Immunotherapy Based on Dendritic Cells Has No Effect on Overall and Progression-Free Survival in Newly Diagnosed Glioblastoma: A Phase II Randomized Trial. Cancers 2018, 10, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hygino da Cruz, L.C., Jr.; Rodriguez, I.; Domingues, R.C.; Gasparetto, E.L.; Sorensen, A.G. Pseudoprogression and pseudoresponse: Imaging challenges in the assessment of posttreatment glioma. AJNR Am. J. Neuroradiol. 2011, 32, 1978–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wick, W.; Chinot, O.L.; Bendszus, M.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Revil, C.; Kerloeguen, Y.; Cloughesy, T. Evaluation of pseudoprogression rates and tumor progression patterns in a phase III trial of bevacizumab plus radiotherapy/temozolomide for newly diagnosed glioblastoma. Neuro Oncol. 2016, 18, 1434–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanghera, P.; Perry, J.; Sahgal, A.; Symons, S.; Aviv, R.; Morrison, M.; Lam, K.; Davey, P.; Tsao, M.N. Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can. J. Neurol. Sci. 2010, 37, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Ellingson, B.M.; Chung, C.; Pope, W.B.; Boxerman, J.L.; Kaufmann, T.J. Pseudoprogression, radionecrosis, inflammation or true tumor progression? challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J. Neurooncol. 2017, 134, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Nowosielski, M.; Wen, P.Y. Imaging Criteria in Neuro-oncology. Semin. Neurol. 2018, 38, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, D.R.; Cascino, T.L.; Schold, S.C., Jr.; Cairncross, J.G. Response criteria for phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 1990, 8, 1277–1280. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.L.; Chang, S. Pseudoprogression and pseudoresponse: Challenges in brain tumor imaging. Curr. Neurol. Neurosci. Rep. 2009, 9, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Wen, P.Y.; Cloughesy, T.F. Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials. Neurotherapeutics 2017, 14, 307–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbé, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, H.; Weller, M.; Huang, R.; Finocchiaro, G.; Gilbert, M.R.; Wick, W.; Ellingson, B.M.; Hashimoto, N.; Pollack, I.F.; Brandes, A.A.; et al. Immunotherapy response assessment in neuro-oncology: A report of the RANO working group. Lancet Oncol. 2015, 16, e534–e542. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Somarouthu, B.; Alessandrino, F.; Kurra, V.; Shinagare, A.B. Atypical Response Patterns in Patients Treated With Nivolumab. AJR Am. J. Roentgenol. 2019, 212, 6. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Dong, Q.; Zhan, L.; Zhang, J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am. J. Cancer Res. 2019, 9, 1546–1553. [Google Scholar]
- Huang, R.Y.; Rahman, R.; Ballman, K.V.; Felten, S.J.; Anderson, S.K.; Ellingson, B.M.; Nayak, L.; Lee, E.Q.; Abrey, L.E.; Galanis, E.; et al. The Impact of T2/FLAIR Evaluation per RANO Criteria on Response Assessment of Recurrent Glioblastoma Patients Treated with Bevacizumab. Clin. Cancer Res. 2016, 22, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Ellingson, B.M.; Sampson, J.; Achrol, A.S.; Aghi, M.K.; Bankiewicz, K.; Wang, C.; Bexon, M.; Brem, S.; Brenner, A.; Chowdhary, S.; et al. Modified RANO, Immunotherapy RANO, and Standard RANO Response to Convection-Enhanced Delivery of IL4R-Targeted Immunotoxin MDNA55 in Recurrent Glioblastoma. Clin. Cancer Res. 2021, 27, 3916–3925. [Google Scholar] [CrossRef]
- Kickingereder, P.; Isensee, F.; Tursunova, I.; Petersen, J.; Neuberger, U.; Bonekamp, D.; Brugnara, G.; Schell, M.; Kessler, T.; Foltyn, M.; et al. Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: A multicentre, retrospective study. Lancet Oncol. 2019, 20, 728–740. [Google Scholar] [CrossRef] [Green Version]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prados, M.; Cloughesy, T.; Samant, M.; Fang, L.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.K.; Paleologos, N.; et al. Response as a predictor of survival in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011, 13, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Lamborn, K.R.; Yung, W.K.; Chang, S.M.; Wen, P.Y.; Cloughesy, T.F.; DeAngelis, L.M.; Robins, H.I.; Lieberman, F.S.; Fine, H.A.; Fink, K.L.; et al. Progression-free survival: An important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008, 10, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Ren, M.; Wick, W.; Abrey, L.; Das, A.; Jin, J.; Reardon, D.A. Progression-free survival as a surrogate endpoint for overall survival in glioblastoma: A literature-based meta-analysis from 91 trials. Neuro Oncol. 2014, 16, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Gállego Pérez-Larraya, J.; Lahutte, M.; Petrirena, G.; Reyes-Botero, G.; González-Aguilar, A.; Houillier, C.; Guillevin, R.; Sanson, M.; Hoang-Xuan, K.; Delattre, J.Y. Response assessment in recurrent glioblastoma treated with irinotecan-bevacizumab: Comparative analysis of the Macdonald, RECIST, RANO, and RECIST + F criteria. Neuro Oncol. 2012, 14, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, D.; van den Bent, M.J. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr. Opin. Neurol. 2009, 22, 633–638. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Tensaouti, F.; Khalifa, J.; Lusque, A.; Plas, B.; Lotterie, J.A.; Berry, I.; Laprie, A.; Cohen-Jonathan Moyal, E.; Lubrano, V. Response Assessment in Neuro-Oncology criteria, contrast enhancement and perfusion MRI for assessing progression in glioblastoma. Neuroradiology 2017, 59, 1013–1020. [Google Scholar] [CrossRef]
- Dempsey, M.F.; Condon, B.R.; Hadley, D.M. Measurement of tumor “size” in recurrent malignant glioma: 1D, 2D, or 3D? AJNR Am. J. Neuroradiol. 2005, 26, 770–776. [Google Scholar] [PubMed]
- Gahrmann, R.; van den Bent, M.; van der Holt, B.; Vernhout, R.M.; Taal, W.; Vos, M.; de Groot, J.C.; Beerepoot, L.V.; Buter, J.; Flach, Z.H.; et al. Comparison of 2D (RANO) and volumetric methods for assessment of recurrent glioblastoma treated with bevacizumab-a report from the BELOB trial. Neuro Oncol. 2017, 19, 853–861. [Google Scholar] [CrossRef] [Green Version]
- Boxerman, J.L.; Zhang, Z.; Safriel, Y.; Larvie, M.; Snyder, B.S.; Jain, R.; Chi, T.L.; Sorensen, A.G.; Gilbert, M.R.; Barboriak, D.P. Early post-bevacizumab progression on contrast-enhanced MRI as a prognostic marker for overall survival in recurrent glioblastoma: Results from the ACRIN 6677/RTOG 0625 Central Reader Study. Neuro Oncol. 2013, 15, 945–954. [Google Scholar] [CrossRef]
- Galanis, E.; Buckner, J.C.; Maurer, M.J.; Sykora, R.; Castillo, R.; Ballman, K.V.; Erickson, B.J. Validation of neuroradiologic response assessment in gliomas: Measurement by RECIST, two-dimensional, computer-assisted tumor area, and computer-assisted tumor volume methods. Neuro Oncol. 2006, 8, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Shah, G.D.; Kesari, S.; Xu, R.; Batchelor, T.T.; O’Neill, A.M.; Hochberg, F.H.; Levy, B.; Bradshaw, J.; Wen, P.Y. Comparison of linear and volumetric criteria in assessing tumor response in adult high-grade gliomas. Neuro Oncol. 2006, 8, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Beers, A.L.; Bai, H.X.; Brown, J.M.; Ly, K.I.; Li, X.; Senders, J.T.; Kavouridis, V.K.; Boaro, A.; Su, C.; et al. Automatic assessment of glioma burden: A deep learning algorithm for fully automated volumetric and bidimensional measurement. Neuro Oncol. 2019, 21, 1412–1422. [Google Scholar] [CrossRef]
- Despotović, I.; Goossens, B.; Philips, W. MRI segmentation of the human brain: Challenges, methods, and applications. Comput. Math. Methods Med. 2015, 2015, 450341. [Google Scholar] [CrossRef] [Green Version]
- Li, X.T.; Huang, R.Y. Standardization of imaging methods for machine learning in neuro-oncology. Neurooncol. Adv. 2020, 2, iv49–iv55. [Google Scholar] [CrossRef]
- Klauschen, F.; Goldman, A.; Barra, V.; Meyer-Lindenberg, A.; Lundervold, A. Evaluation of automated brain MR image segmentation and volumetry methods. Hum. Brain Mapp. 2009, 30, 1310–1327. [Google Scholar] [CrossRef]
- Ung, T.H.; Ney, D.E.; Damek, D.; Rusthoven, C.G.; Youssef, A.S.; Lillehei, K.O.; Ormond, D.R. The Neurologic Assessment in Neuro-Oncology (NANO) Scale as an Assessment Tool for Survival in Patients With Primary Glioblastoma. Neurosurgery 2019, 84, 687–695. [Google Scholar] [CrossRef]

| Response Criteria | Complete Response | Partial Response | Stable Disease | Progressive Disease |
|---|---|---|---|---|
| MacDonald [20] | disappearance of all enhancing tumor | ≥50% decrease in cross-section area of measurable disease | not qualified for other | ≥25% increase in cross-section area; new lesion |
| Vol-RANO [30], RANO [21] | disappearance of measurable and nonmeasurable disease; no new lesion; stable/improved non-enhancing T2/FLAIR abnormalities | ≥50% decrease in cross-section area of measurable disease; no progress of nonmeasurable disease; stable/improved T2/FLAIR abnormalities | not qualified for other; stable T2/FLAIR abnormalities; best response for patients with nonmeasurable disease at baseline | ≥25% increase in cross-section area/≥40% increase in total volume; new lesion; significant increase or ≥100% increase in volume of T2/FLAIR abnormalities |
| Vol-mRANO, mRANO [23] | 1. MRI: Preliminary CR disappearance of all measurable and nonmeasurable disease 2. MRI (4–8 weeks later): if continuous disappearance: durable CR; if measurable disease: preliminary PD/ pseudoresponse | 1. MRI Preliminary PR. ≥50% decrease in cross-section area/≥65% decrease in total volume of measurable disease 2. MRI (4–8 weeks later): if SD, PR or CR: durable PR; if PD: preliminary PD/ pseudoresponse | not qualified for other; best response for patients with nonmeasurable disease at baseline | 1. MRI: Preliminary PD new measurable lesion; ≥25% increase in cross-section area/≥40% increase in total volume 2. MRI (4–8 weeks later): if subsequent ≥25% in cross-section area/≥40% increase in total volume: confirmed PD; if SD or PR/CR: pseudoprogression |
| iRANO [25] | disappearance of measurable and nonmeasurable disease; no new lesion; stable/improved non-enhancing T2/FLAIR abnormalities | ≥50% decrease in cross-section area of measurable disease; no progress of nonmeasurable disease; stable/improved T2/FLAIR abnormalities | not qualified for other; stable T2/FLAIR abnormalities; best response for patients with nonmeasurable disease at baseline | 1. MRI within 6 months of treatment start: ≥25% increase in cross-section area; new lesion; significant increase in non-enhancing T2/FLAIR abnormalities additional 2. MRI in ≥3 months: if RANO for PD met: PD; if RANO for SD, PD, CR met: pseudoprogression MRI after 6 months of treatment start: ≥25% increase in cross-section area; new lesion; significant increase in non-enhancing T2/FLAIR abnormalities |
| Characteristics | Audencel Group | Control Group | |
|---|---|---|---|
| Number of patients | 36 | 40 | |
| Sex, n (%) | male | 20 (55.6) | 29 (72.5) |
| female | 16 (44.4) | 11 (27.5) | |
| Median age at diagnosis, years (95% CI) | 59.4 (53.6–61.5) | 54.4 (50.5–57.0) | |
| Median overall survival, months (95% CI) | 18.7 (17.7–27.0) | 19.3 (16.5–23.4) | |
| Survival at trial end, n (%) | death | 30 (83.3) | 31 (77.5) |
| alive | 4 (11.1) | 6 (15) | |
| unknown | 2 (5.6) | 3 (7.5) | |
| ECOG at baseline, n (%) | 0 | 11 (30.6) | 15 (37.5) |
| 1 | 25 (69.4) | 20 (50) | |
| 2 | 0 (0) | 5 (12.5) | |
| MGMT promoter, n (%) | samples measured | 20 | 17 |
| methylated | 7/20 (35) | 6/17 (35.3) | |
| unmethylated | 13/20 (65) | 11/17 (64.7) | |
| IDH 1 mutation, n (%) | yes | 0 (0) | 0 (0) |
| no | 36 (100) | 40 (100) | |
| Side of tumor bulk, n (%) | left | 16 (44.4) | 22 (55) |
| right | 18 (50) | 18 (45) | |
| central/bilateral | 2 (5.6) | 0 (0) | |
| Tumor location, n (%) | frontal | 10 (27.8) | 17 (42.5) |
| temporal | 4 (11.1) | 5 (12.5) | |
| parietal | 8 (22.2) | 8 (20) | |
| occipital | 14 (38.9) | 10 (25) | |
| Response Criteria | Median PFS, Months | 95% CI | Difference of PFS (p-Value) | |||||
|---|---|---|---|---|---|---|---|---|
| MacDonald | RANO | Vol-RANO | mRANO | Vol-mRANO | iRANO | |||
| SOC and SOC + Audencel Patients (n = 76) | ||||||||
| MacDonald | 4.0 | 5.2–8.8 | - | 1.000 | 1.000 | 0.001 | 0.000 | - |
| RANO | 4.2 | 5.3–8.6 | 1.000 | - | 1.000 | 0.003 | 0.001 | - |
| Vol-RANO | 5.4 | 5.4–8.2 | 1.000 | 1.000 | - | 0.022 | 0.008 | - |
| mRANO | 8.6 | 9.1–14.0 | 0.001 | 0.003 | 0.022 | - | 1.000 | - |
| Vol-mRANO | 8.6 | 9.7–14.9 | 0.000 | 1.000 | 0.008 | 1.000 | - | - |
| SOC + Audencel patients (n = 36) | ||||||||
| MacDonald | 4.2 | 4.2–10.3 | - | 1.000 | 1.000 | 0.034 | 0.020 | 1.000 |
| RANO | 4.7 | 4.6–10.6 | 1.000 | - | 1.000 | 0.105 | 0.066 | 1.000 |
| Vol-RANO | 5.4 | 4.5–9.0 | 1.000 | 1.000 | - | 0.154 | 0.095 | 1.000 |
| mRANO | 8.1 | 8.6–17.8 | 0.034 | 0.105 | 0.154 | - | 1.000 | 1.000 |
| Vol-mRANO | 8.6 | 9.4–19.1 | 0.020 | 0.066 | 0.154 | 1.000 | - | 1.000 |
| iRANO | 6.2 | 5.7–11.7 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | - |
| Response Criteria | Median PPS, Months | 95% CI | Difference of PPS (p-Value) | |||||
|---|---|---|---|---|---|---|---|---|
| MacDonald | RANO | Vol-RANO | mRANO | Vol-mRANO | iRANO | |||
| SOC and SOC + Audencel Patients (n = 76) | ||||||||
| MacDonald | 12.0 | 11.8–15.8 | - | 1.000 | 1.000 | 0.013 | 0.001 | - |
| RANO | 11.4 | 11.8–15.9 | 1.000 | - | 1.000 | 0.019 | 0.002 | - |
| Vol-RANO | 10.8 | 11.7–16.2 | 1.000 | 1.000 | - | 0.046 | 0.005 | - |
| mRANO | 8.8 | 7.8–11.2 | 0.013 | 0.019 | 0.046 | - | 1.000 | - |
| Vol-mRANO | 8.7 | 7.1–10.4 | 0.001 | 0.002 | 0.005 | 1.000 | - | - |
| SOC + Audencel patients (n = 36) | ||||||||
| MacDonald | 15.2 | 11.9–17.2 | - | 1.000 | 1.000 | 0.030 | 0.002 | 1.000 |
| RANO | 12.3 | 11.4–17.0 | 1.000 | - | 1.000 | 0.104 | 0.011 | 1.000 |
| Vol-RANO | 12.1 | 11.4–18.8 | 1.000 | 1.000 | - | 0.137 | 0.015 | 1.000 |
| mRANO | 7.3 | 6.6–11.6 | 0.030 | 0.104 | 0.137 | - | 1.000 | 0.351 |
| Vol-mRANO | 6.2 | 5.6–10.5 | 0.002 | 0.011 | 0.015 | 1.000 | - | 0.048 |
| iRANO | 13.0 | 10.6–16.2 | 1.000 | 1.000 | 1.000 | 0.351 | 0.048 | - |
| Response Criteria | 4-Month Landmark | 8-Month Landmark | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| MacDonald | 1.30 | 0.79–2.13 | 0.310 | 2.29 | 1.34–3.91 | 0.002 |
| RANO | 1.41 | 0.86–2.33 | 0.175 | 2.04 | 1.18–3.55 | 0.011 |
| Vol-RANO | 1.30 | 0.78–2.15 | 0.312 | 1.81 | 1.06–3.10 | 0.031 |
| mRANO | 1.69 | 0.96–2.96 | 0.068 | 2.57 | 1.48–4.46 | 0.001 |
| Vol-mRANO | 1.82 | 1.01–3.27 | 0.045 | 2.79 | 1.59–4.89 | 0.001 |
| iRANO | 2.07 | 0.98–4.37 | 0.057 | 1.20 | 0.88–4.53 | 0.098 |
| Response Criteria | Median OS, Months (95% CI) | |||
|---|---|---|---|---|
| 4-Month Landmark | 8-Month Landmark | |||
| SD | PD | SD | PD | |
| MacDonald | 20.5 (18.5–26.9) | 18.6 (15.8–22.8) | 23.7 (21.4–30.7) | 18.0 (15.5–20.9) |
| RANO | 21.5 (19.6–27.7) | 15.0 (14.8–21.8) | 24.1 (22.5–33.7) | 18.1 (15.9–21.0) |
| Vol-RANO | 20.7 (19.3–27.1) | 15.0 (14.6–21.8) | 23.5 (21.8–31.4) | 17.9 (16.1–22.4) |
| mRANO | 20.4 (19.0–25.4) | 13.6 (12.5–22.0) | 22.8 (21.4–28.6) | 13.7 (13.1–19.0) |
| Vol-mRANO | 20.6 (19.1–25.4) | 12.8 (11.2–21.5) | 23.1 (22.1–29.3) | 12.0 (12.5–17.9) |
| iRANO | 21.7 (19.1–31.0) | 12.7 (11.0–20.9) | 23.4 (19.2–40.5) | 17.3 (15.0–22.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heugenhauser, J.; Galijasevic, M.; Mangesius, S.; Goebel, G.; Buchroithner, J.; Erhart, F.; Pichler, J.; Widhalm, G.; Stockhammer, G.; Iglseder, S.; et al. MRI Response Assessment in Glioblastoma Patients Treated with Dendritic-Cell-Based Immunotherapy. Cancers 2022, 14, 1579. https://doi.org/10.3390/cancers14061579
Heugenhauser J, Galijasevic M, Mangesius S, Goebel G, Buchroithner J, Erhart F, Pichler J, Widhalm G, Stockhammer G, Iglseder S, et al. MRI Response Assessment in Glioblastoma Patients Treated with Dendritic-Cell-Based Immunotherapy. Cancers. 2022; 14(6):1579. https://doi.org/10.3390/cancers14061579
Chicago/Turabian StyleHeugenhauser, Johanna, Malik Galijasevic, Stephanie Mangesius, Georg Goebel, Johanna Buchroithner, Friedrich Erhart, Josef Pichler, Georg Widhalm, Günther Stockhammer, Sarah Iglseder, and et al. 2022. "MRI Response Assessment in Glioblastoma Patients Treated with Dendritic-Cell-Based Immunotherapy" Cancers 14, no. 6: 1579. https://doi.org/10.3390/cancers14061579
APA StyleHeugenhauser, J., Galijasevic, M., Mangesius, S., Goebel, G., Buchroithner, J., Erhart, F., Pichler, J., Widhalm, G., Stockhammer, G., Iglseder, S., Freyschlag, C. F., Oberndorfer, S., Bordihn, K., von Campe, G., Czech, T., Surböck, B., Urbanic Purkart, T., Marosi, C., Felzmann, T., & Nowosielski, M. (2022). MRI Response Assessment in Glioblastoma Patients Treated with Dendritic-Cell-Based Immunotherapy. Cancers, 14(6), 1579. https://doi.org/10.3390/cancers14061579







