The Prognostic Value of PI-RADS Score in CyberKnife Ultra-Hypofractionated Radiotherapy for Localized Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Pi-Rads Score
2.3. Treatment Protocol and Follow-Up
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salembier, C.; Villeirs, G.; De Bari, B.; Hoskin, P.; Pieters, B.R.; Van Vulpen, M.; Khoo, V.; Henry, A.; Bossi, A.; De Meerleer, G.; et al. ESTRO ACROP consensus guideline on CT- and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother. Oncol. 2018, 127, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Rasch, C.; Barillot, I.; Remeijer, P.; Touw, A.; Van Herk, M.; Lebesque, J.V. Definition of the prostate in CT and MRI: A multi-observer study. Int. J. Radiat. Oncol. 1999, 43, 57–66. [Google Scholar] [CrossRef]
- Hentschel, B.; Oehler, W.; Straufß, D.; Ulrich, A.; Malich, A. Definition of the CTV Prostate in CT and MRI by Using CT–MRI Image Fusion in IMRT Planning for Prostate Cancer. Strahlenther. Und Onkol. 2011, 187, 183–190. [Google Scholar] [CrossRef]
- Sander, L.; Langkilde, N.C.; Holmberg, M.; Carl, J. MRI target delineation may reduce long-term toxicity after prostate radiotherapy. Prostate Cancer 2014, 53, 809–814. [Google Scholar] [CrossRef] [Green Version]
- Padhani, A.R.; Weinreb, J.; Rosenkrantz, A.B.; Villeirs, G.; Turkbey, B.; Barentsz, J. Prostate Imaging-Reporting and Data System Steering Committee: PI-RADS v2 Status Update and Future Directions. Eur. Urol. 2019, 75, 385–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer, M.D.; Shih, J.H.; Lay, N.; Barrett, T.; Bittencourt, L.; Borofsky, S.; Kabakus, I.; Law, Y.M.; Marko, J.; Shebel, H.; et al. Interreader Variability of Prostate Imaging Reporting and Data System Version 2 in Detecting and Assessing Prostate Cancer Lesions at Prostate MRI. AJR. Am. J. Roentgenol. 2019, 212, 1197–1205. [Google Scholar] [CrossRef]
- Muller, B.G.; Shih, J.H.; Sankineni, S.; Marko, J.; Rais-Bahrami, S.; George, A.K.; De La Rosette, J.J.M.C.H.; Merino, M.J.; Wood, B.J.; Pinto, P.; et al. Prostate cancer: Interobserver agreement and accuracy with the revised prostate imaging reporting and data system at multiparametric mr imaging1. Radiology 2015, 277, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Girometti, R.; Giannarini, G.; Greco, F.; Isola, M.; Cereser, L.; Como, G.; Sioletic, S.; Pizzolitto, S.; Crestani, A.; Ficarra, V.; et al. Interreader agreement of PI-RADS v. 2 in assessing prostate cancer with multiparametric MRI: A study using whole-mount histology as the standard of reference. J. Magn. Reson. Imaging 2019, 49, 546–555. [Google Scholar] [CrossRef]
- Gandaglia, G.; Ploussard, G.; Valerio, M.; Marra, G.; Moschini, M.; Martini, A.; Roumiguié, M.; Fossati, N.; Stabile, A.; Beauval, J.B.; et al. Prognostic Implications of Multiparametric Magnetic Resonance Imaging and Concomitant Systematic Biopsy in Predicting Biochemical Recurrence After Radical Prostatectomy in Prostate Cancer Patients Diagnosed with Magnetic Resonance Imaging-targeted Biopsy. Eur. Urol. Oncol. 2020, 3, 739–747. [Google Scholar] [CrossRef]
- Martini, A.; Soeterik, T.F.W.; Haverdings, H.; Rahota, R.G.; Checcucci, E.; De Cillis, S.; Hermanns, T.; Fankhauser, C.D.; Afferi, L.; Moschini, M.; et al. An Algorithm to Personalize Nerve Sparing in Men with Unilateral High-Risk Prostate Cancer. J. Urol. 2022, 207, 350–357. [Google Scholar] [CrossRef]
- Turchan, W.T.; Kauffmann, G.; Patel, P.; Oto, A.; Liauw, S.L. PI-RADS score is associated with biochemical control and distant metastasis in men with intermediate-risk and high-risk prostate cancer treated with radiation therapy. Urol. Oncol. 2020, 38, 600.e1–600.e8. [Google Scholar] [CrossRef] [PubMed]
- Miszczyk, L.; Namysł-Kaletka, A.; Napieralska, A.; Kraszkiewicz, M.; Miszczyk, M.; Woźniak, G.; Stąpór-Fudzińska, M.; Głowacki, G.; Tukiendorf, A. Stereotactic Ablative Radiotherapy for Prostate Cancer-The Treatment Results of 500 Patients and Analysis of Failures. Technol. Cancer Res. Treat. 2019, 18, 1533033819870815. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.V.; Whittington, R.; Bruce Malkowicz, S.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical Outcome After Radical Prostatectomy, External Beam Radiation Therapy, or Interstitial Radiation Therapy for Clinically Localized Prostate Cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef]
- Austin, P.C.; Allignol, A.; Fine, J.P. The number of primary events per variable affects estimation of the subdistribution hazard competing risks model. J. Clin. Epidemiol. 2017, 83, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased recursive partitioning: A conditional inference framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]
- Ishaq, A.; Sadiq, S.; Umer, M.; Ullah, S.; Mirjalili, S.; Rupapara, V.; Nappi, M. Improving the Prediction of Heart Failure Patients’ Survival Using SMOTE and Effective Data Mining Techniques. IEEE Access 2021, 9, 39707–39716. [Google Scholar] [CrossRef]
- Strasser, H.; Weber, C. On the Asymptotic Theory of Permutation Statistics. 1999. Available online: https://epub.wu.ac.at/102/ (accessed on 16 March 2022).
- Uno, H.; Cai, T.; Pencina, M.J.; D’Agostino, R.B.; Wei, L.J. On the C-statistics for Evaluating Overall Adequacy of Risk Prediction Procedures with Censored Survival Data. Stat. Med. 2011, 30, 1105. [Google Scholar] [CrossRef] [Green Version]
- Supplementary Code to Paper Entatiled “The prognostic value of PI-RADS score in CyberKnife Ultra-Hypofractionated Radiotherapy for Localized Prostate Cancer”. by Miszyk et. al. Code by Konrad Stawiski MD, PhD ([email protected]. https://konsta.com.pl). Available online: https://gist.github.com/kstawiski/51eb34abc10d1d7d6a7afc7d4bbbedc6 (accessed on 16 March 2022).
- Rajwa, P.; Mori, K.; Huebner, N.A.; Martin, D.T.; Sprenkle, P.C.; Weinreb, J.C.; Ploussard, G.; Pradere, B.; Shariat, S.F.; Leapman, M.S. The Prognostic Association of Prostate MRI PI-RADSTM v2 Assessment Category and Risk of Biochemical Recurrence after Definitive Local Therapy for Prostate Cancer: A Systematic Review and Meta-Analysis. J. Urol. 2021, 206, 507–516. [Google Scholar] [CrossRef]
- Gharzai, L.A.; Jiang, R.; Wallington, D.; Jones, G.; Birer, S.; Jairath, N.; Jaworski, E.M.; McFarlane, M.R.; Mahal, B.A.; Nguyen, P.L.; et al. Intermediate clinical endpoints for surrogacy in localised prostate cancer: An aggregate meta-analysis. Lancet Oncol. 2021, 22, 402–410. [Google Scholar] [CrossRef]
- Martorana, E.; Pirola, G.M.; Aisa, M.C.; Scialpi, P.; Di Blasi, A.; Saredi, G.; D’Aandrea, A.; Signore, S.; Grisanti, R.; Scialpi, M. Prostate MRI and transperineal TRUS/MRI fusion biopsy for prostate cancer detection: Clinical practice updates. Turkish J. Urol. 2019, 45, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Simpson, B.S.; Freeman, A.; Kirkham, A.; Whitaker, H.C.; Emberton, M. Conspicuity of prostate cancer on multiparametric magnetic resonance imaging: A cross-disciplinary translational hypothesis. FASEB J. 2020, 34, 14150–14159. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Simpson, B.S.; Parry, M.A.; Allen, C.; Ball, R.; Freeman, A.; Kelly, D.; Kim, H.L.; Kirkham, A.; You, S.; et al. Genetic Landscape of Prostate Cancer Conspicuity on Multiparametric Magnetic Resonance Imaging: A Systematic Review and Bioinformatic Analysis. Eur. Urol. Open Sci. 2020, 20, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Purysko, A.S.; Magi-Galluzzi, C.; Mian, O.Y.; Sittenfeld, S.; Davicioni, E.; du Plessis, M.; Buerki, C.; Bullen, J.; Li, L.; Madabhushi, A.; et al. Correlation between MRI phenotypes and a genomic classifier of prostate cancer: Preliminary findings. Eur. Radiol. 2019, 29, 4861–4870. [Google Scholar] [CrossRef]
- Salmasi, A.; Said, J.; Shindel, A.W.; Khoshnoodi, P.; Felker, E.R.; Sisk, A.E.; Grogan, T.; McCullough, D.; Bennett, J.; Bailey, H.; et al. A 17-Gene Genomic Prostate Score Assay Provides Independent Information on Adverse Pathology in the Setting of Combined Multiparametric Magnetic Resonance Imaging Fusion Targeted and Systematic Prostate Biopsy. J. Urol. 2018, 200, 564–572. [Google Scholar] [CrossRef]
- Houlahan, K.E.; Salmasi, A.; Sadun, T.Y.; Pooli, A.; Felker, E.R.; Livingstone, J.; Huang, V.; Raman, S.S.; Ahuja, P.; Sisk, A.E.; et al. Molecular Hallmarks of Multiparametric Magnetic Resonance Imaging Visibility in Prostate Cancer. Eur. Urol. 2019, 76, 18–23. [Google Scholar] [CrossRef]
- Kauffmann, G.; Arif, F.; Patel, P.; Oto, A.; Liauw, S.L. Pretreatment multiparametric MRI is independently associated with biochemical outcome in men treated with radiation therapy for prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 471.e11–471.e18. [Google Scholar] [CrossRef]
- Kim, R.; Kim, C.K.; Park, J.J.; Kim, J.H.; Seo, S.I.; Jeon, S.S.; Lee, H.M. Prognostic Significance for Long-Term Outcomes Following Radical Prostatectomy in Men with Prostate Cancer: Evaluation with Prostate Imaging Reporting and Data System Version 2. Korean J. Radiol. 2019, 20, 256. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Wu, C.J.; Bao, M.L.; Li, H.; Wang, X.N.; Liu, X.S.; Shi, H. Bin MR-based prognostic nomogram for prostate cancer after radical prostatectomy. J. Magn. Reson. Imaging 2017, 45, 586–596. [Google Scholar] [CrossRef]
- Takeuchi, N.; Sakamoto, S.; Nishiyama, A.; Horikoshi, T.; Yamada, Y.; Iizuka, J.; Maimaiti, M.; Imamura, Y.; Kawamura, K.; Imamoto, T.; et al. Biparametric Prostate Imaging Reporting and Data System version2 and International Society of Urological Pathology Grade Predict Biochemical Recurrence after Radical Prostatectomy. Clin. Genitourin. Cancer 2018, 16, e817–e829. [Google Scholar] [CrossRef]
- Park, S.Y.; Oh, Y.T.; Jung, D.C.; Cho, N.H.; Choi, Y.D.; Rha, K.H.; Hong, S.J. Prediction of biochemical recurrence after radical prostatectomy with PI-RADS version 2 in prostate cancers: Initial results. Eur. Radiol. 2016, 26, 2502–2509. [Google Scholar] [CrossRef]
- Tan, N.; Shen, L.; Khoshnoodi, P.; Alcalá, H.E.; Yu, W.; Hsu, W.; Reiter, R.E.; Lu, D.Y.; Raman, S.S. Pathological and 3 Tesla Volumetric Magnetic Resonance Imaging Predictors of Biochemical Recurrence after Robotic Assisted Radical Prostatectomy: Correlation with Whole Mount Histopathology. J. Urol. 2018, 199, 1218–1223. [Google Scholar] [CrossRef]
- Shin, T.J.; Jung, W.; Ha, J.Y.; Kim, B.H.; Kim, Y.H. The significance of the visible tumor on preoperative magnetic resonance imaging in localized prostate cancer. Prostate Int. 2021, 9, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Janoray, G.; Reynaud-Bougnoux, A.; Ruffier-Loubière, A.; Bernadou, G.; Pointreau, Y.; Calais, G. Stereotactic body re-irradiation therapy for locally recurrent prostate cancer after external-beam radiation therapy: Initial report. Cancer/Radiothérapie 2016, 20, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Billis, A.; Meirelles, L.R.; Freitas, L.L.L.; Polidoro, A.S.; Fernandes, H.A.; Padilha, M.M.; Magna, L.A.; Ferreira, U. Prostate Total Tumor Extent Versus Index Tumor Extent—Which is Predictive of Biochemical Recurrence Following Radical Prostatectomy? J. Urol. 2013, 189, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Spohn, S.K.B.; Bettermann, A.S.; Bamberg, F.; Benndorf, M.; Mix, M.; Nicolay, N.H.; Fechter, T.; Hölscher, T.; Grosu, R.; Chiti, A.; et al. Radiomics in prostate cancer imaging for a personalized treatment approach—Current aspects of methodology and a systematic review on validated studies. Theranostics 2021, 11, 8027. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, N.; Wang, Y.; Xie, H.; Duan, S.; Zhang, X.; Wang, B. A Radiomics nomogram for predicting bone metastasis in newly diagnosed prostate cancer patients. Eur. J. Radiol. 2020, 128, 109020. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, B.; Zhong, F.; Guo, Q.; Li, K.; Hou, Y.; Lin, N. MRI-based texture analysis of the primary tumor for pre-treatment prediction of bone metastases in prostate cancer. Magn. Reson. Imaging 2019, 60, 76–84. [Google Scholar] [CrossRef]

| Clinical Characteristics | PI-RADS ≤ 3 | PI-RADS = 4 | PI-RADS = 5 | Total | |
|---|---|---|---|---|---|
| Number of cases | 22 | 57 | 73 | 152 | |
| Age | (years) * | 69 (64–73) | 67 (62–71) | 68 (61–74) | 67 (62–73) |
| Max PSA | (ng/mL) * | 7.5 (5.2–9.3) | 7 (5.4–8.8) | 7.7 (6.4–9.7) | 7.5 (6–9.5) |
| Gleason Grade Group | I (3 + 3) | 21 (95.5%) | 52 (91.2%) | 62 (84.9%) | 135 (88.8%) |
| II (3 + 4) | 1 (4.5%) | 5 (8.8%) | 11 (15.1%) | 17 (11.2%) | |
| TNM T stage & | T1c | 14 (63.6%) | 26 (45.6%) | 42 (57.5%) | 82 (53.9%) |
| T2a | 3 (13.6%) | 12 (21.1%) | 17 (23.3%) | 32 (21.1%) | |
| T2b | 3 (13.6%) | 12 (21.1%) | 10 (13.7%) | 25 (16.4%) | |
| T2c | 2 (8.6%) | 7 (12.3%) | 4 (5.5%) | 13 (8.6%) | |
| Risk group # | Low | 13 (59.1%) | 28 (49.1%) | 40 (54.8%) | 81 (53.3%) |
| Intermediate | 9 (40.9%) | 29 (50.9%) | 33 (45.2%) | 71 (46.7%) | |
| Prostate volume | (cc) * | 31.8 (26.4–35.7) | 35.5 (25.2–44.4) | 30.5 (25.6–40) | 32.5 (25.4–40.9) |
| PSA density | (ng/mL/cc) * | 0.23 (0.16–0.27) | 0.21 (0.15–0.29) | 0.25 (0.19–0.34) | 0.23 (0.16–0.32) |
| Short-term ADT ## | % receiving | 4 (18.2%) | 5 (8.8%) | 0 (0%) | 9 (5.9%) |
| PI-RADS v2.1 components ^ | |||||
| Number of lesions | 1 | 10 (45.5%) | 55 (96.5%) | 66 (90.4%) | 131 (86.2%) |
| 2 | 2 (3.5%) | 7 (9.6%) | 9 (5.9%) | ||
| Axial dimensions of the index lesion * | (1) greatest [mm] * | 9.5 (7–13) | 12 (10–14) | 20 (17–24) | 16 (12–21) |
| (2) perpendicular [mm] * | 5.5 (5–10) | 8 (6–9) | 11 (10–13) | 9.5 (7–12) | |
| 1 × 2 * [mm2] | 51 (35–130) | 90 (63–112) | 230 (180–300) | 160 (84–234.5) | |
| Localization of the index lesion within the prostate (zone) | Peripheral | 7 (31.8%) | 51 (89.5%) | 52 (71.2%) | 110 (72.4%) |
| Transitional | 2 (9.1%) | 5 (8.8%) | 16 (21.9%) | 23 (15.1%) | |
| Peripheral + transitional | 0 (0%) | 1 (1.8%) | 3 (4.1%) | 4 (2.6%) | |
| Transitional + central | 1 (4.5%) | 0 (0%) | 2 (2.7%) | 3 (2%) | |
| Localization of the index lesion within the prostate (lobe) | Right | 5 (22.7%) | 22 (38.6%) | 32 (43.8%) | 59 (38.8%) |
| Left | 4 (18.2%) | 28 (49.1%) | 27 (37%) | 59 (38.8%) | |
| Both | 1 (4.5%) | 7 (12.3%) | 14 (19.2%) | 22 (14.5%) |
| Variables Included | Training C-Index | Internal Validation C-Index | 95% CI of Validation C-Index |
|---|---|---|---|
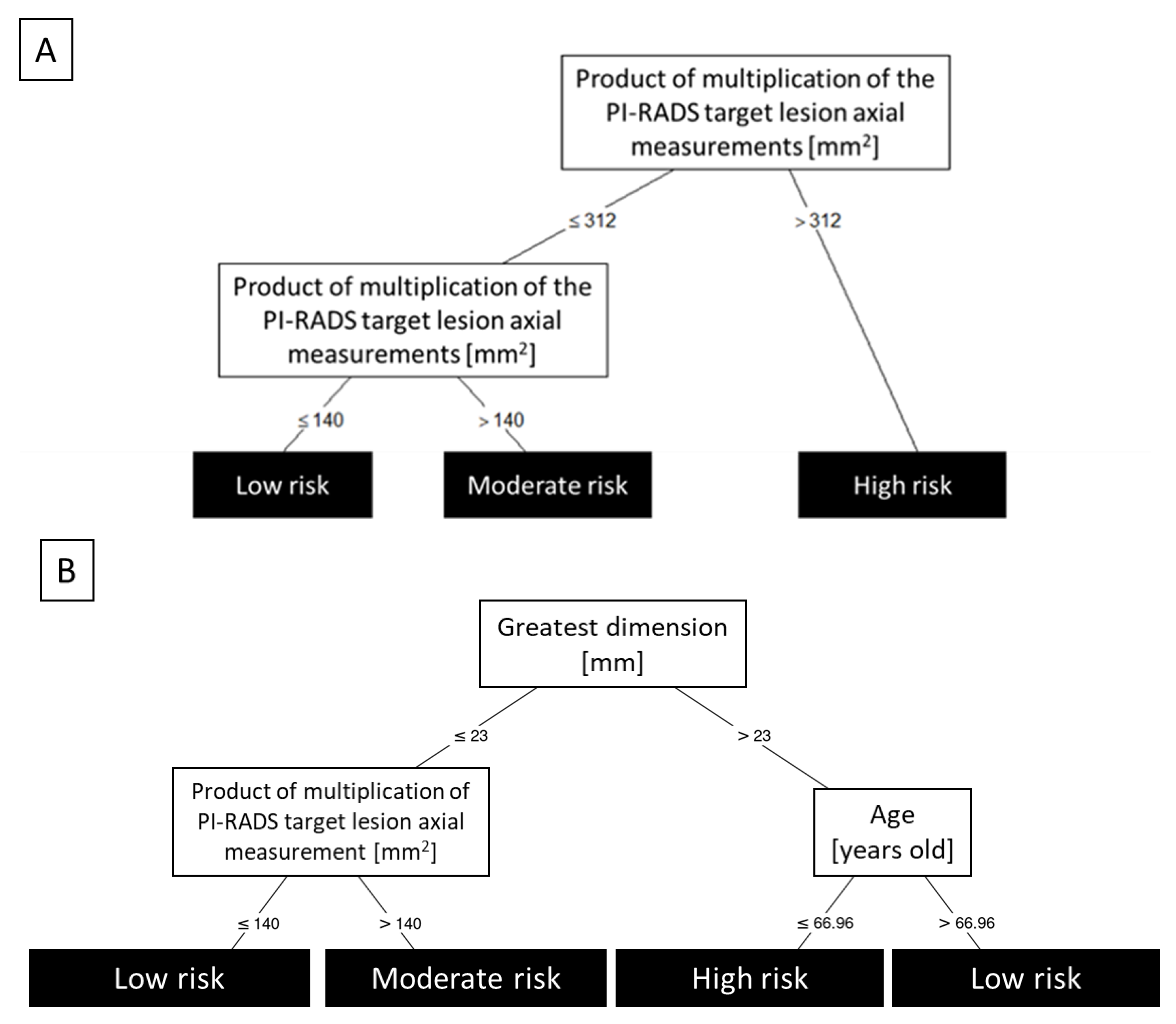
| Greatest dimension + Product of multiplication of PI-RADS target lesion axial measurements + age | 0.903 | 0.896 | 0.848–0.901 |
| Greatest dimension + Product of multiplication of PI-RADS target lesion axial measurements | 0.896 | 0.885 | 0.845–0.892 |
| Greatest dimension + T2 weighted imaging (T2W) score | 0.897 | 0.874 | 0.837–0.895 |
| Greatest dimension + age + PIRADS | 0.906 | 0.873 | 0.838–0.897 |
| Greatest dimension + PIRADS | 0.900 | 0.870 | 0.845–0.900 |
| Sum of PI-RADS target lesion axial measurements + age + PIRADS | 0.913 | 0.863 | 0.797–0.877 |
| Greatest dimension + T2 weighted imaging (T2W) score + age | 0.904 | 0.852 | 0.834–0.900 |
| Sum of PI-RADS target lesion axial measurements + age | 0.902 | 0.847 | 0.804–0.878 |
| Greatest dimension + Sum of PI-RADS target lesion axial measurements | 0.893 | 0.843 | 0.842–0.891 |
| Sum of PI-RADS target lesion axial measurements + T2 weighted imaging (T2W) score | 0.874 | 0.839 | 0.820–0.865 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miszczyk, M.; Rembak-Szynkiewicz, J.; Magrowski, Ł.; Stawiski, K.; Namysł-Kaletka, A.; Napieralska, A.; Kraszkiewicz, M.; Woźniak, G.; Stąpór-Fudzińska, M.; Głowacki, G.; et al. The Prognostic Value of PI-RADS Score in CyberKnife Ultra-Hypofractionated Radiotherapy for Localized Prostate Cancer. Cancers 2022, 14, 1613. https://doi.org/10.3390/cancers14071613
Miszczyk M, Rembak-Szynkiewicz J, Magrowski Ł, Stawiski K, Namysł-Kaletka A, Napieralska A, Kraszkiewicz M, Woźniak G, Stąpór-Fudzińska M, Głowacki G, et al. The Prognostic Value of PI-RADS Score in CyberKnife Ultra-Hypofractionated Radiotherapy for Localized Prostate Cancer. Cancers. 2022; 14(7):1613. https://doi.org/10.3390/cancers14071613
Chicago/Turabian StyleMiszczyk, Marcin, Justyna Rembak-Szynkiewicz, Łukasz Magrowski, Konrad Stawiski, Agnieszka Namysł-Kaletka, Aleksandra Napieralska, Małgorzata Kraszkiewicz, Grzegorz Woźniak, Małgorzata Stąpór-Fudzińska, Grzegorz Głowacki, and et al. 2022. "The Prognostic Value of PI-RADS Score in CyberKnife Ultra-Hypofractionated Radiotherapy for Localized Prostate Cancer" Cancers 14, no. 7: 1613. https://doi.org/10.3390/cancers14071613
APA StyleMiszczyk, M., Rembak-Szynkiewicz, J., Magrowski, Ł., Stawiski, K., Namysł-Kaletka, A., Napieralska, A., Kraszkiewicz, M., Woźniak, G., Stąpór-Fudzińska, M., Głowacki, G., Pradere, B., Laukhtina, E., Rajwa, P., & Majewski, W. (2022). The Prognostic Value of PI-RADS Score in CyberKnife Ultra-Hypofractionated Radiotherapy for Localized Prostate Cancer. Cancers, 14(7), 1613. https://doi.org/10.3390/cancers14071613








