Prognostic Value of the Largest Lesion Size for Progression-Free Survival in Patients with NET Undergoing Salvage PRRT with [177Lu]Lu-DOTATOC
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- van Velthuysen, M.L.; Groen, E.J.; van der Noort, V.; van de Pol, A.; Tesselaar, M.E.; Korse, C.M. Grading of neuroendocrine neoplasms: Mitoses and Ki-67 are both essential. Neuroendocrinology 2014, 100, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Moss, S.F.; Gustafsson, B.I.; Lawrence, B.; Schimmack, S.; Kidd, M. The archaic distinction between functioning and nonfunctioning neuroendocrine neoplasms is no longer clinically relevant. Langenbecks Arch. Surg. 2011, 396, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Oberg, K.E.; Reubi, J.C.; Kwekkeboom, D.J.; Krenning, E.P. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology 2010, 139, 742–753.e1. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Kunz, P.L.; Hendifar, A.; Yao, J.; Bushnell, D.; Kulke, M.H.; Baum, R.P.; Caplin, M.; Ruszniewski, P.; Delpassand, E.; et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with (177)Lu-Dotatate: An analysis of the NETTER-1 study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2372–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruhwedel, T.; Rogasch, J.M.M.; Huang, K.; Jann, H.; Schatka, I.; Furth, C.; Amthauer, H.; Wetz, C. The Prognostic Value of the De Ritis Ratio for Progression-Free Survival in Patients with NET Undergoing [(177)Lu]Lu-DOTATOC-PRRT: A Retrospective Analysis. Cancers 2021, 13, 635. [Google Scholar] [CrossRef]
- Kim, Y.I. Salvage peptide receptor radionuclide therapy in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis. Nucl. Med. Commun. 2021, 42, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Rudisile, S.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Boning, G.; Gildehaus, F.J.; Fendler, W.P.; Auernhammer, C.J.; Spitzweg, C.; Bartenstein, P.; et al. Salvage PRRT with (177)Lu-DOTA-octreotate in extensively pretreated patients with metastatic neuroendocrine tumor (NET): Dosimetry, toxicity, efficacy, and survival. BMC Cancer 2019, 19, 788. [Google Scholar] [CrossRef] [Green Version]
- Sabet, A.; Haslerud, T.; Pape, U.F.; Sabet, A.; Ahmadzadehfar, H.; Grunwald, F.; Guhlke, S.; Biersack, H.J.; Ezziddin, S. Outcome and toxicity of salvage therapy with 177Lu-octreotate in patients with metastatic gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 205–210. [Google Scholar] [CrossRef] [PubMed]
- van der Zwan, W.A.; Brabander, T.; Kam, B.L.R.; Teunissen, J.J.M.; Feelders, R.A.; Hofland, J.; Krenning, E.P.; de Herder, W.W. Salvage peptide receptor radionuclide therapy with [(177)Lu-DOTA,Tyr(3)]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 704–717. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, E.; Machta, J.; Walker, M.; Toumpanakis, C.; Caplin, M.; Navalkissoor, S. Retreatment with peptide receptor radionuclide therapy in patients with progressing neuroendocrine tumours: Efficacy and prognostic factors for response. Br. J. Radiol. 2018, 91, 20180041. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kulkarni, H.R.; Singh, A.; Kaemmerer, D.; Mueller, D.; Prasad, V.; Hommann, M.; Robiller, F.C.; Niepsch, K.; Franz, H.; et al. Results and adverse events of personalized peptide receptor radionuclide therapy with (90)Yttrium and (177)Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget 2018, 9, 16932–16950. [Google Scholar] [CrossRef] [Green Version]
- Severi, S.; Sansovini, M.; Ianniello, A.; Bodei, L.; Nicolini, S.; Ibrahim, T.; Di Iorio, V.; D’Errico, V.; Caroli, P.; Monti, M.; et al. Feasibility and utility of re-treatment with (177)Lu-DOTATATE in GEP-NENs relapsed after treatment with (90)Y-DOTATOC. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1955–1963. [Google Scholar] [CrossRef]
- Yordanova, A.; Mayer, K.; Brossart, P.; Gonzalez-Carmona, M.A.; Strassburg, C.P.; Essler, M.; Ahmadzadehfar, H. Safety of multiple repeated cycles of (177)Lu-octreotate in patients with recurrent neuroendocrine tumour. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- van Essen, M.; Krenning, E.P.; Kam, B.L.; de Herder, W.W.; Feelders, R.A.; Kwekkeboom, D.J. Salvage therapy with (177)Lu-octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumors. J. Nucl. Med. 2010, 51, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Meira-Machado, L.; Cadarso-Suarez, C.; Gude, F.; Araujo, A. smoothHR: An R package for pointwise nonparametric estimation of hazard ratio curves of continuous predictors. Comput. Math. Methods Med. 2013, 2013, 745742. [Google Scholar] [CrossRef]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Gyorffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef] [Green Version]
- Strosberg, J.; Leeuwenkamp, O.; Siddiqui, M.K. Peptide receptor radiotherapy re-treatment in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis (vol 93, 102141, 2021). Cancer Treat Rev. 2021, 97. [Google Scholar] [CrossRef]
- O’Donoghue, J.A.; Bardies, M.; Wheldon, T.E. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J. Nucl. Med. 1995, 36, 1902–1909. [Google Scholar]
- de Jong, M.; Breeman, W.A.; Bernard, B.F.; Bakker, W.H.; Visser, T.J.; Kooij, P.P.; van Gameren, A.; Krenning, E.P. Tumor response after [(90)Y-DOTA(0),Tyr(3)]octreotide radionuclide therapy in a transplantable rat tumor model is dependent on tumor size. J. Nucl. Med. 2001, 42, 1841–1846. [Google Scholar] [PubMed]
- de Jong, M.; Breeman, W.A.; Valkema, R.; Bernard, B.F.; Krenning, E.P. Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. J. Nucl. Med. 2005, 46 (Suppl. 1), 13S–17S. [Google Scholar] [PubMed]
- Proskuryakov, S.Y.; Konoplyannikov, A.G.; Gabai, V.L. Necrosis: A specific form of programmed cell death? Exp. Cell Res. 2003, 283, 1–16. [Google Scholar] [CrossRef]
- Takumi, K.; Fukukura, Y.; Higashi, M.; Ideue, J.; Umanodan, T.; Hakamada, H.; Kanetsuki, I.; Yoshiura, T. Pancreatic neuroendocrine tumors: Correlation between the contrast-enhanced computed tomography features and the pathological tumor grade. Eur. J. Radiol. 2015, 84, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Li, Y.W.; Shi, Y.; Zhang, Z.; Zheng, M.Y.; Zhang, S.W. Clinical and pathological characteristics and prognosis of 132 cases of rectal neuroendocrine tumors. World J. Gastrointest. Oncol. 2020, 12, 893–902. [Google Scholar] [CrossRef]
- Neperud, J.; Mahvash, A.; Garg, N.; Murthy, R.; Szklaruk, J. Can imaging patterns of neuroendocrine hepatic metastases predict response yttruim-90 radioembolotherapy? World J. Radiol. 2013, 5, 241–247. [Google Scholar] [CrossRef]
- Botros, M.; Sikaris, K.A. The de ritis ratio: The test of time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar] [PubMed]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar] [CrossRef]
- Kamiike, W.; Fujikawa, M.; Koseki, M.; Sumimura, J.; Miyata, M.; Kawashima, Y.; Wada, H.; Tagawa, K. Different patterns of leakage of cytosolic and mitochondrial enzymes. Clin. Chim. Acta 1989, 185, 265–270. [Google Scholar] [CrossRef]
- Zhang, J.; Pavlova, N.N.; Thompson, C.B. Cancer cell metabolism: The essential role of the nonessential amino acid, glutamine. EMBO J. 2017, 36, 1302–1315. [Google Scholar] [CrossRef] [Green Version]
- Thornburg, J.M.; Nelson, K.K.; Clem, B.F.; Lane, A.N.; Arumugam, S.; Simmons, A.; Eaton, J.W.; Telang, S.; Chesney, J. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 2008, 10, R84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.S.; Stampouloglou, E.; Kingston, N.M.; Zhang, L.; Monti, S.; Varelas, X. Glutamine-utilizing transaminases are a metabolic vulnerability of TAZ/YAP-activated cancer cells. EMBO Rep. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Lyssiotis, C.A.; Son, J.; Cantley, L.C.; Kimmelman, A.C. Pancreatic cancers rely on a novel glutamine metabolism pathway to maintain redox balance. Cell Cycle 2013, 12, 1987–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Choi, Y.H.; Sung, H.H.; Han, D.H.; Jeon, H.G.; Chang Jeong, B.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Choi, H.Y. De Ritis Ratio (AST/ALT) as a Significant Prognostic Factor in Patients With Upper Tract Urothelial Cancer Treated With Surgery. Clin. Genitourin Cancer 2017, 15, e379–e385. [Google Scholar] [CrossRef] [PubMed]
- Riedl, J.M.; Posch, F.; Prager, G.; Eisterer, W.; Oehler, L.; Sliwa, T.; Wilthoner, K.; Petzer, A.; Pichler, P.; Hubmann, E.; et al. The AST/ALT (De Ritis) ratio predicts clinical outcome in patients with pancreatic cancer treated with first-line nab-paclitaxel and gemcitabine: Post hoc analysis of an Austrian multicenter, noninterventional study. Ther. Adv. Med. Oncol. 2020, 12, 1758835919900872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Fang, K.; Zhang, J.; Jiang, Y.; Wang, G.; Zhang, H.; Chen, T.; Shi, X.; Li, Y.; Duan, F.; et al. The significance of De Ritis (aspartate transaminase/alanine transaminase) ratio in predicting pathological outcomes and prognosis in localized prostate cancer patients. Int. Urol. Nephrol. 2017, 49, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Goessling, W.; Massaro, J.M.; Vasan, R.S.; D’Agostino, R.B., Sr.; Ellison, R.C.; Fox, C.S. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology 2008, 135, 1935–1944. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Chen, C.; Lu, J.; Zheng, J.; Ma, X.C.; Yuan, X.Y.; Huo, K.; Han, J.F. De Ritis ratio (AST/ALT) as an independent predictor of poor outcome in patients with acute ischemic stroke. Neuropsychiatr Dis. Treat 2017, 13, 1551–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rief, P.; Pichler, M.; Raggam, R.; Hafner, F.; Gerger, A.; Eller, P.; Brodmann, M.; Gary, T. The AST/ALT (De-Ritis) ratio: A novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. Medicine 2016, 95, e3843. [Google Scholar] [CrossRef] [PubMed]
- Ezziddin, S.; Khalaf, F.; Vanezi, M.; Haslerud, T.; Mayer, K.; Al Zreiqat, A.; Willinek, W.; Biersack, H.J.; Sabet, A. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 925–933. [Google Scholar] [CrossRef] [PubMed]
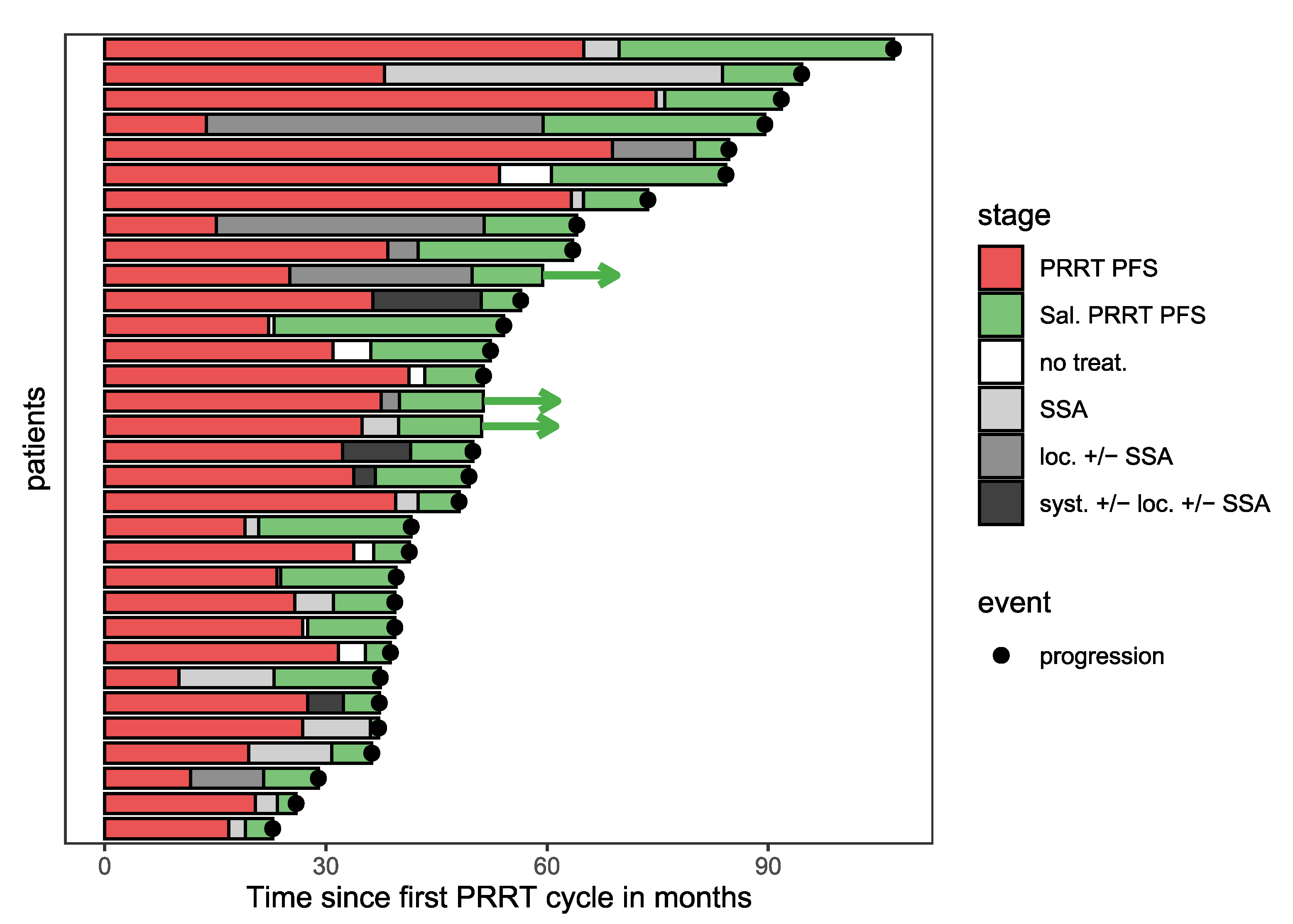
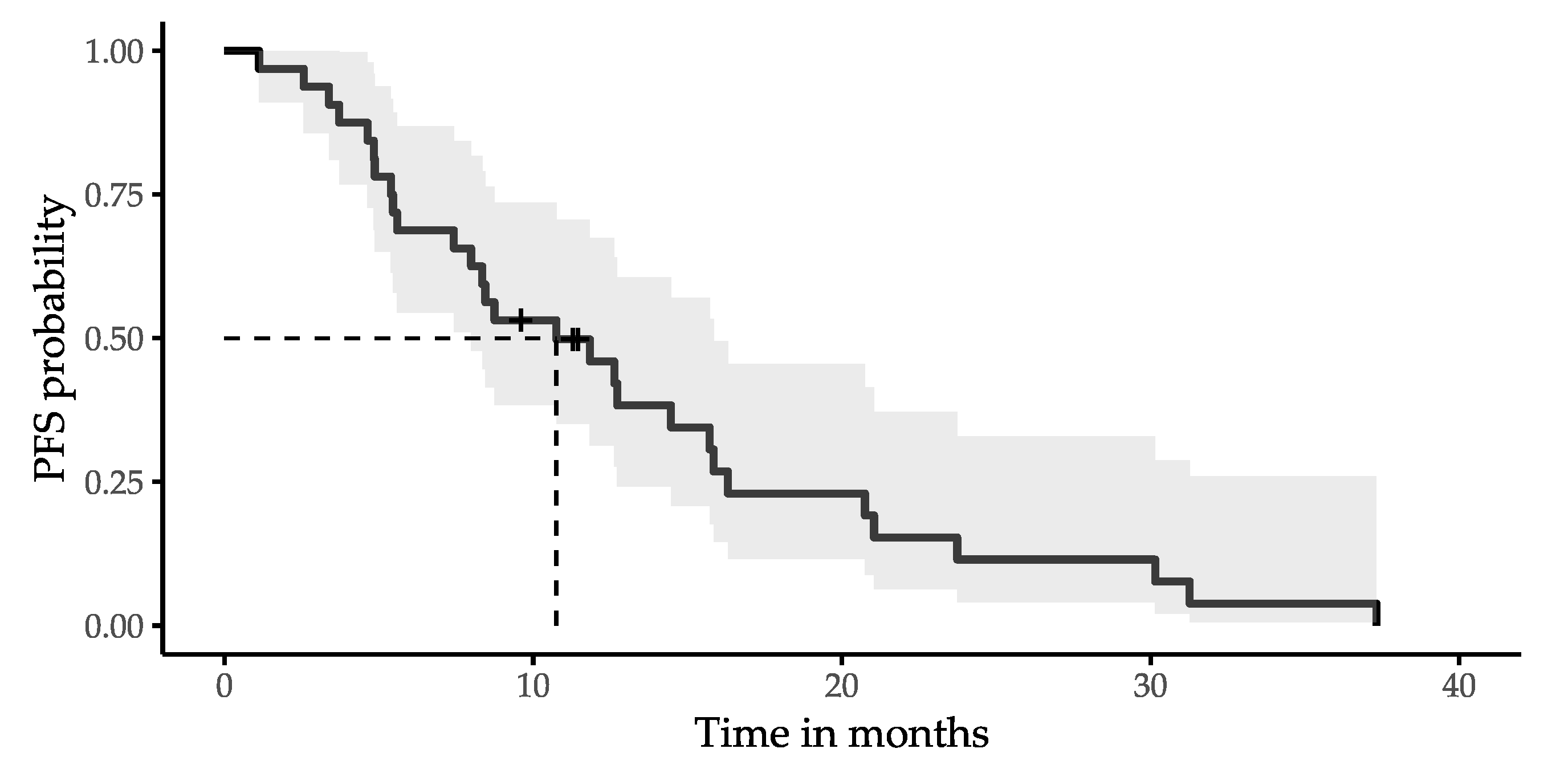
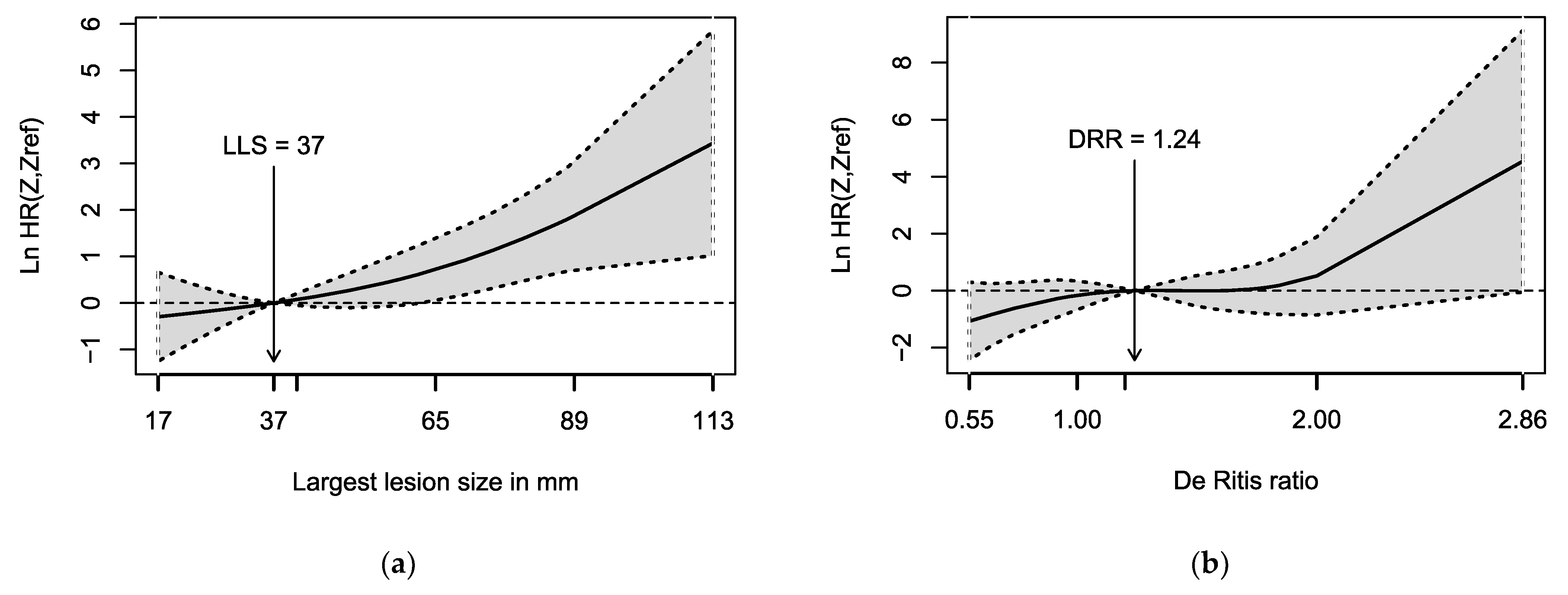
| Variable | n (%) or Median (Range) |
|---|---|
| Patient count | 32 |
| Sex | |
| Men | 19 (59%) |
| Women | 13 (41%) |
| Age in years | 67 (32–81) |
| Charlson comorbidity index | 3 (0–10) |
| Type 2 diabetes | 7 (22%) |
| Occlusive peripheral arterial disease | 0 (0%) |
| Ischemic stroke <1 month before salvage PRRT | 0 (0%) |
| Primary location | |
| Gastrointestinal | 18 (57%) |
| Pancreas | 5 (15%) |
| Lungs | 1 (3%) |
| Kidney | 1 (3%) |
| Unknown (CUP) | 7 (22%) |
| Grade | |
| G1 | 6 (19%) |
| G2 | 25 (78%) |
| G3 | 1 (3%) |
| Functional NET | 12 (38%) |
| Add. treatment before/after initial PRRT | |
| Operative treatment | 20 (63%)/2 (6%) |
| Somatostatin analogues | 19 (59%)/21 (66%) |
| Chemotherapy | 8 (25%)/1 (3%) |
| mTOR inhibitor | 5 (16%)/3 (9%) |
| Tyrosine kinase inhibitor | 1 (3%)/0 (0%) |
| Radiation therapy | 3 (9%)/4 (13%) |
| Local ablative therapy | 4 (13%)/3 (9%) |
| Number of initial PRRT cycles | 3 (3–5) |
| Initial PRRT performed with 177Lu | 30 (94%) |
| Cumulative activity during initial PRRT (GBq) | 21.1 (12.0–30.3) |
| PFS after initial PRRT in months | 31 (10–75) |
| Time between end of initial PRRT and salvage PRRT in months | 30 (11–70) |
| Metastatic sites before retreatment | |
| Liver | 31 (97%) |
| Lymph node | 22 (69%) |
| Bone | 16 (50%) |
| Peritoneum | 7 (22%) |
| Lungs | 3 (9%) |
| Others (muscle, ovary, spleen) | 3 (9%) |
| Largest lesion size in mm | 37.5 (17–113) |
| Largest lesion position | |
| Liver | 24 (75%) |
| Lymph nodes | 5 (16%) |
| Bone | 1 (3%) |
| Others | 2 (6%) |
| AST in U/L | 26.5 (15–136) |
| ALT in U/L | 21 (10–91) |
| De Ritis ratio | 1.24 (0.55–2.86) |
| Number of retreatment cycles | 2 (1–3) |
| Univariable Cox Regression | |||
|---|---|---|---|
| Variable | Hazard Ratio | 95% CI | p-Value |
| Largest lesion size (LLS) in mm | 1.03 | 1.01–1.05 | 0.002 |
| De Ritis ratio | 2.64 | 1.01–6.87 | 0.047 |
| PFS after initial PRRT in months | 0.99 | 0.97–1.01 | 0.400 |
| Multivariable Cox Regression | |||
| Variable | Hazard Ratio | 95% CI | p-Value |
| Largest lesion size (LLS) in mm | 1.03 | 1.01–1.05 | 0.004 |
| De Ritis ratio | 2.46 | 0.94–6.43 | 0.066 |
| PFS after initial PRRT in months | 0.99 | 0.97–1.02 | 0.578 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galler, M.; Rogasch, J.M.M.; Huang, K.; Jann, H.; Plehm, K.; Wetz, C.; Amthauer, H. Prognostic Value of the Largest Lesion Size for Progression-Free Survival in Patients with NET Undergoing Salvage PRRT with [177Lu]Lu-DOTATOC. Cancers 2022, 14, 1768. https://doi.org/10.3390/cancers14071768
Galler M, Rogasch JMM, Huang K, Jann H, Plehm K, Wetz C, Amthauer H. Prognostic Value of the Largest Lesion Size for Progression-Free Survival in Patients with NET Undergoing Salvage PRRT with [177Lu]Lu-DOTATOC. Cancers. 2022; 14(7):1768. https://doi.org/10.3390/cancers14071768
Chicago/Turabian StyleGaller, Markus, Julian M. M. Rogasch, Kai Huang, Henning Jann, Kristina Plehm, Christoph Wetz, and Holger Amthauer. 2022. "Prognostic Value of the Largest Lesion Size for Progression-Free Survival in Patients with NET Undergoing Salvage PRRT with [177Lu]Lu-DOTATOC" Cancers 14, no. 7: 1768. https://doi.org/10.3390/cancers14071768
APA StyleGaller, M., Rogasch, J. M. M., Huang, K., Jann, H., Plehm, K., Wetz, C., & Amthauer, H. (2022). Prognostic Value of the Largest Lesion Size for Progression-Free Survival in Patients with NET Undergoing Salvage PRRT with [177Lu]Lu-DOTATOC. Cancers, 14(7), 1768. https://doi.org/10.3390/cancers14071768






