Targeting GLI1 Transcription Factor for Restoring Iodine Avidity with Redifferentiation in Radioactive-Iodine Refractory Thyroid Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. siRNA Transfection
2.3. Chemical
2.4. Cell Viability Assay
2.5. Protein Extraction
2.6. Western Blot Analysis
2.7. Immunofluorescence
2.8. I-125 Uptake Assay
2.9. I-131 Clonogenic Assay
2.10. Statistical Analysis
3. Results
3.1. Comparison of Factors of Hedgehog Signaling Pathway including GLI1 in Thyroid Cancer-Derived Cell Lines
3.2. Robust Effect of GLI1 Knockdown on Upregulation of Endogenous NIS Expression and Its Localization in Thyroid Cancer Cells
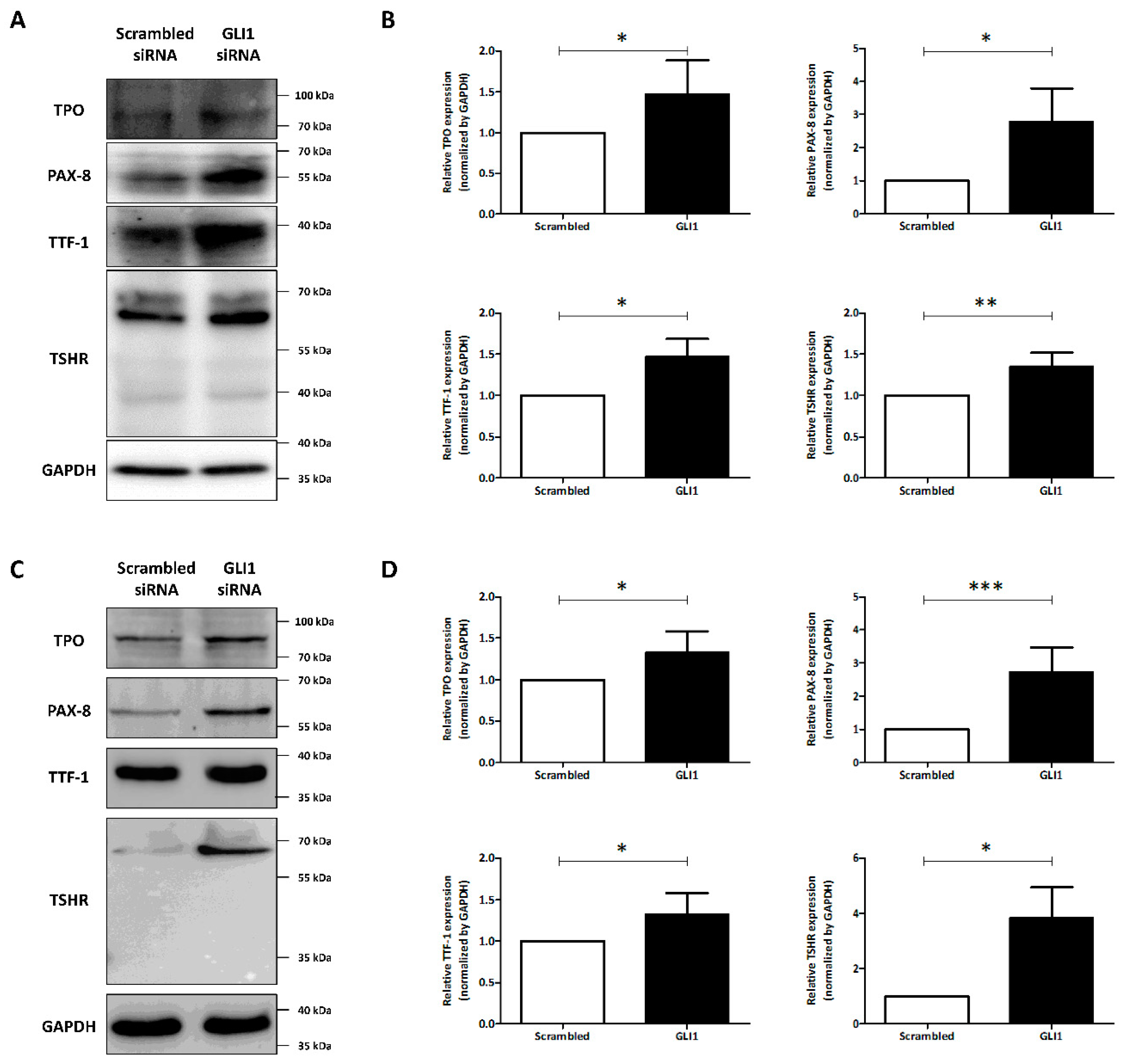
3.3. The Change of Thyroid-Specific Proteins and Transcription Factors via GLI1 Knockdown for Redifferentiation
3.4. Restoration of RAI Avidity and I-131-Mediated Cytotoxicity Effects Caused by GLI1 Knock Down
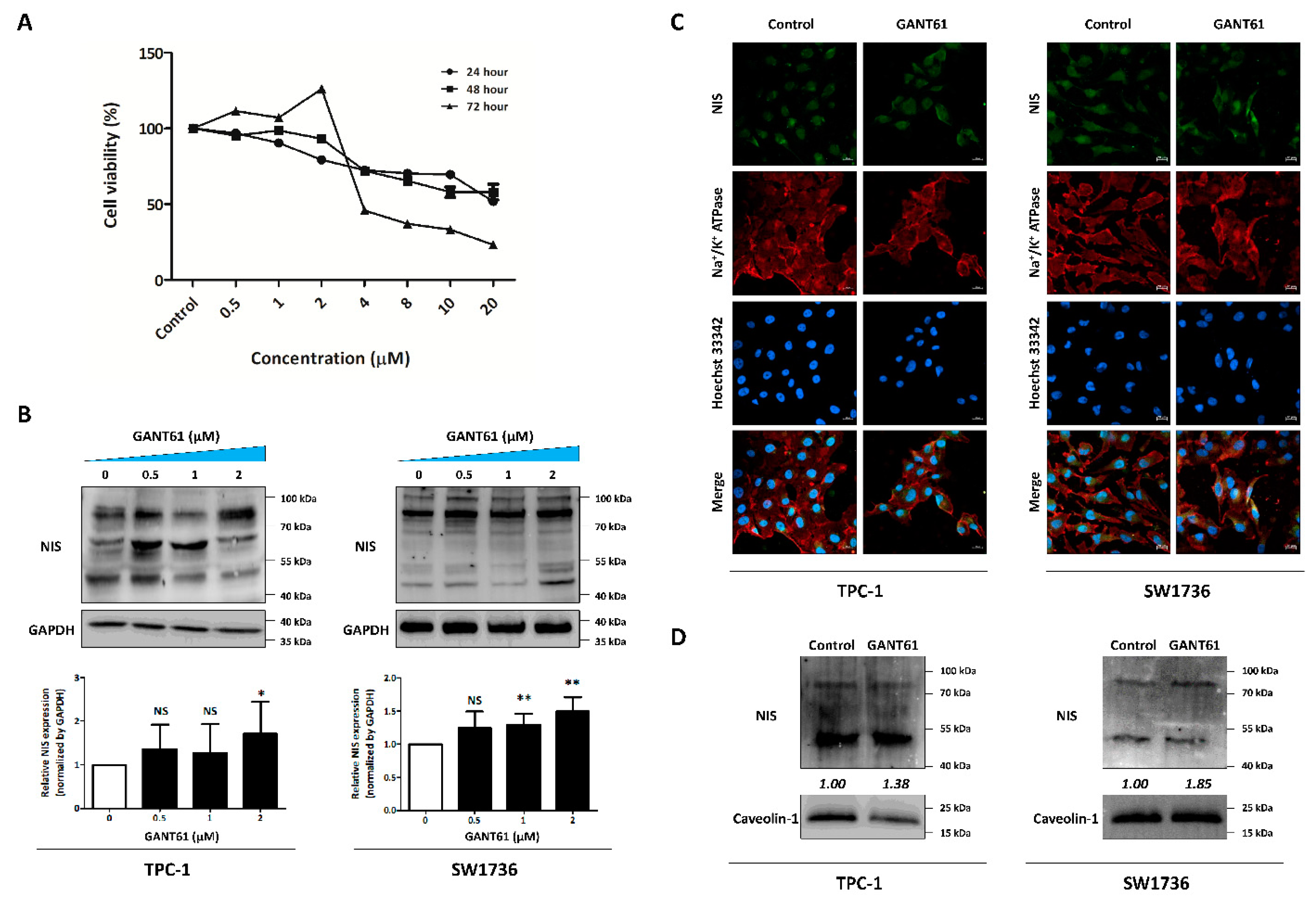
3.5. Identification of GLI1 Inhibitor Efficacy for NIS Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Oh, J.M.; Ahn, B.-C. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics 2021, 11, 6251–6277. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Lin, Y.; Liang, J. Radioactive Iodine-Refractory Differentiated Thyroid Cancer and Redifferentiation Therapy. Endocrinol. Metab. 2019, 34, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.M.; Ahn, B.-C. Redifferentiation of Radioiodine Refractory Differentiated Thyroid Cancer for Reapplication of I-131 Therapy. Front. Endocrinol. 2017, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Riobo-Del Galdo, N.A.; Lara Montero, Á.; Wertheimer, E.V. Role of Hedgehog Signaling in Breast Cancer: Pathogenesis and Therapeutics. Cells 2019, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Varjosalo, M.; Taipale, J. Hedgehog: Functions and mechanisms. Genes Dev. 2008, 22, 2454–2472. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Johnson, R.L.; Zhang, X.; Bare, J.W.; Waldman, F.M.; Cogen, P.H.; Menon, A.G.; Warren, R.S.; Chen, L.C.; Scott, M.P.; et al. Mutations of the PATCHED gene in several types of sporadic extracutaneous tumors. Cancer Res. 1997, 57, 2369–2372. [Google Scholar] [PubMed]
- Thayer, S.P.; di Magliano, M.P.; Heiser, P.W.; Nielsen, C.M.; Roberts, D.J.; Lauwers, G.Y.; Qi, Y.P.; Gysin, S.; Fernández-del Castillo, C.; Yajnik, V.; et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 2003, 425, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.N.; Berman, D.M.; Burkholder, S.G.; Wang, B.; Beachy, P.A.; Baylin, S.B. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 2003, 422, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Qualtrough, D.; Buda, A.; Gaffield, W.; Williams, A.C.; Paraskeva, C. Hedgehog signalling in colorectal tumour cells: Induction of apoptosis with cyclopamine treatment. Int. J. Cancer 2004, 110, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, P.; Hernández, A.M.; Stecca, B.; Kahler, A.J.; DeGueme, A.M.; Barrett, A.; Beyna, M.; Datta, M.W.; Datta, S.; Ruiz i Altaba, A. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc. Natl. Acad. Sci. USA 2004, 101, 12561–12566. [Google Scholar] [CrossRef]
- Didiasova, M.; Schaefer, L.; Wygrecka, M. Targeting GLI Transcription Factors in Cancer. Molecules 2018, 23, 1003. [Google Scholar] [CrossRef]
- Rimkus, T.K.; Carpenter, R.L.; Qasem, S.; Chan, M.; Lo, H.-W. Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers 2016, 8, 22. [Google Scholar] [CrossRef]
- Parascandolo, A.; Laukkanen, M.O.; De Rosa, N.; Ugolini, C.; Cantisani, M.C.; Cirafici, A.M.; Basolo, F.; Santoro, M.; Castellone, M.D. A dual mechanism of activation of the Sonic Hedgehog pathway in anaplastic thyroid cancer: Crosstalk with RAS-BRAF-MEK pathway and ligand secretion by tumor stroma. Oncotarget 2018, 9, 4496–4510. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, S.; Lee, C.R.; Ku, C.R.; Kang, S.-W.; Jeong, J.J.; Nam, K.-H.; Shin, D.Y.; Chung, W.Y.; Lee, E.J.; et al. GLI1 Transcription Factor Affects Tumor Aggressiveness in Patients With Papillary Thyroid Cancers. Medicine 2015, 94, e998. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Sui, Y.; Wang, Y. LARP7 in papillary thyroid carcinoma induces NIS expression through suppression of the SHH signaling pathway. Mol. Med. Rep. 2018, 17, 7521–7528. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, H.; Wang, M.; Li, N.; Tian, T.; Wu, Y.; Xu, P.; Yang, S.; Zhai, Z.; Zhou, L.; et al. Global Burden of Thyroid Cancer From 1990 to 2017. JAMA Netw. Open 2020, 3, e208759. [Google Scholar] [CrossRef]
- Eustatia-Rutten, C.F.A.; Corssmit, E.P.M.; Biermasz, N.R.; Pereira, A.M.; Romijn, J.A.; Smit, J.W. Survival and death causes in differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 313–319. [Google Scholar] [CrossRef]
- Jin, Y.; Van Nostrand, D.; Cheng, L.; Liu, M.; Chen, L. Radioiodine refractory differentiated thyroid cancer. Crit. Rev. Oncol. Hematol. 2018, 125, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liu, R.; Zhu, G.; Wang, H.; Xing, M. Robust Thyroid Gene Expression and Radioiodine Uptake Induced by Simultaneous Suppression of BRAF V600E and Histone Deacetylase in Thyroid Cancer Cells. J. Clin. Endocrinol. Metab. 2016, 101, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Baek, S.H.; Gangadaran, P.; Hong, C.M.; Rajendran, R.L.; Lee, H.W.; Zhu, L.; Gopal, A.; Kalimuthu, S.; Jeong, S.Y.; et al. A Novel Tyrosine Kinase Inhibitor Can Augment Radioactive Iodine Uptake Through Endogenous Sodium/Iodide Symporter Expression in Anaplastic Thyroid Cancer. Thyroid 2020, 30, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ding, H.; Rao, G.; Arora, S.; Saclarides, C.P.; Esparaz, J.; Gattuso, P.; Solorzano, C.C.; Prinz, R.A. Activation of the Sonic Hedgehog pathway in thyroid neoplasms and its potential role in tumor cell proliferation. Endocr. Relat. Cancer 2012, 19, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Riobó, N.A.; Lu, K.; Ai, X.; Haines, G.M.; Emerson, C.P. Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 4505–4510. [Google Scholar] [CrossRef] [PubMed]
- Riobo, N.A.; Haines, G.M.; Emerson, C.P. Protein kinase C-delta and mitogen-activated protein/extracellular signal-regulated kinase-1 control GLI activation in hedgehog signaling. Cancer Res. 2006, 66, 839–845. [Google Scholar] [CrossRef]
- Ji, Z.; Mei, F.C.; Xie, J.; Cheng, X. Oncogenic KRAS activates hedgehog signaling pathway in pancreatic cancer cells. J. Biol. Chem. 2007, 282, 14048–14055. [Google Scholar] [CrossRef]
- Song, L.; Li, Z.-Y.; Liu, W.-P.; Zhao, M.-R. Crosstalk between Wnt/β-catenin and Hedgehog/Gli signaling pathways in colon cancer and implications for therapy. Cancer Biol. Ther. 2015, 16, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Q.; Yen, C.-J.; Xia, W.; Izzo, J.G.; Lang, J.-Y.; Li, C.-W.; Hsu, J.L.; Miller, S.A.; Wang, X.; et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell 2012, 21, 374–387. [Google Scholar] [CrossRef]
- Xu, X.; Lu, Y.; Li, Y.; Prinz, R.A. Sonic Hedgehog Signaling in Thyroid Cancer. Front. Endocrinol. 2017, 8, 284. [Google Scholar] [CrossRef]
- Heiden, K.B.; Williamson, A.J.; Doscas, M.E.; Ye, J.; Wang, Y.; Liu, D.; Xing, M.; Prinz, R.A.; Xu, X. The sonic hedgehog signaling pathway maintains the cancer stem cell self-renewal of anaplastic thyroid cancer by inducing snail expression. J. Clin. Endocrinol. Metab. 2014, 99, E2178–E2187. [Google Scholar] [CrossRef]
- Bohinc, B.; Michelotti, G.; Diehl, A.M. Hedgehog signaling in human medullary thyroid carcinoma: A novel signaling pathway. Thyroid 2013, 23, 1119–1126. [Google Scholar] [CrossRef]
- Dohán, O.; Baloch, Z.; Bánrévi, Z.; Livolsi, V.; Carrasco, N. Rapid communication: Predominant intracellular overexpression of the Na+/I− symporter (NIS) in a large sampling of thyroid cancer cases. J. Clin. Endocrinol. Metab. 2001, 86, 2697–2700. [Google Scholar] [CrossRef] [PubMed]
- Wapnir, I.L.; van de Rijn, M.; Nowels, K.; Amenta, P.S.; Walton, K.; Montgomery, K.; Greco, R.S.; Dohán, O.; Carrasco, N. Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J. Clin. Endocrinol. Metab. 2003, 88, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Riesco-Eizaguirre, G.; Gutiérrez-Martínez, P.; García-Cabezas, M.A.; Nistal, M.; Santisteban, P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I− targeting to the membrane. Endocr. Relat. Cancer 2006, 13, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.E.; Read, M.L.; Turnell, A.S.; Watkins, R.J.; Watkinson, J.C.; Lewy, G.D.; Fong, J.C.W.; James, S.R.; Eggo, M.C.; Boelaert, K.; et al. A novel mechanism of sodium iodide symporter repression in differentiated thyroid cancer. J. Cell Sci. 2009, 122, 3393–3402. [Google Scholar] [CrossRef]
- Smith, V.E.; Sharma, N.; Watkins, R.J.; Read, M.L.; Ryan, G.A.; Kwan, P.P.; Martin, A.; Watkinson, J.C.; Boelaert, K.; Franklyn, J.A.; et al. Manipulation of PBF/PTTG1IP phosphorylation status; a potential new therapeutic strategy for improving radioiodine uptake in thyroid and other tumors. J. Clin. Endocrinol. Metab. 2013, 98, 2876–2886. [Google Scholar] [CrossRef]
- Amit, M.; Na’ara, S.; Francis, D.; Matanis, W.; Zolotov, S.; Eisenhaber, B.; Eisenhaber, F.; Weiler Sagie, M.; Malkin, L.; Billan, S.; et al. Post-translational Regulation of Radioactive Iodine Therapy Response in Papillary Thyroid Carcinoma. J. Natl. Cancer Inst. 2017, 109, djx092. [Google Scholar] [CrossRef]
- Lacoste, C.; Hervé, J.; Bou Nader, M.; Dos Santos, A.; Moniaux, N.; Valogne, Y.; Montjean, R.; Dorseuil, O.; Samuel, D.; Cassio, D.; et al. Iodide transporter NIS regulates cancer cell motility and invasiveness by interacting with the Rho guanine nucleotide exchange factor LARG. Cancer Res. 2012, 72, 5505–5515. [Google Scholar] [CrossRef]
- Feng, F.; Yehia, L.; Ni, Y.; Chang, Y.S.; Jhiang, S.M.; Eng, C. A Nonpump Function of Sodium Iodide Symporter in Thyroid Cancer via Cross-talk with PTEN Signaling. Cancer Res. 2018, 78, 6121–6133. [Google Scholar] [CrossRef]
- Feng, F.; Yehia, L.; Eng, C. Pro-tumorigenic non-pump function of sodium iodide symporter: A reimagined Trojan horse? Oncotarget 2019, 10, 688–689. [Google Scholar] [CrossRef]
- Fletcher, A.; Read, M.L.; Thornton, C.E.M.; Larner, D.P.; Poole, V.L.; Brookes, K.; Nieto, H.R.; Alshahrani, M.; Thompson, R.J.; Lavery, G.G.; et al. Targeting Novel Sodium Iodide Symporter Interactors ADP-Ribosylation Factor 4 and Valosin-Containing Protein Enhances Radioiodine Uptake. Cancer Res. 2020, 80, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Cheng, X.; Wu, J.; Wang, Y.; Wang, X.; Wu, L.; Yu, H.; Bao, J.; Zhang, L. Capsaicin restores sodium iodine symporter-mediated radioiodine uptake through bypassing canonical TSH–TSHR pathway in anaplastic thyroid carcinoma cells. J. Mol. Cell Biol. 2022, 13, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Domingues, R.; Bugalho, M.J.; Silva, A.L.; Matos, P. Analysis of NIS Plasma Membrane Interactors Discloses Key Regulation by a SRC/RAC1/PAK1/PIP5K/EZRIN Pathway with Potential Implications for Radioiodine Re-Sensitization Therapy in Thyroid Cancer. Cancers 2021, 13, 5460. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Domingues, R.; Bugalho, M.J.; Matos, P.; Silva, A.L. MAPK Inhibition Requires Active RAC1 Signaling to Effectively Improve Iodide Uptake by Thyroid Follicular Cells. Cancers 2021, 13, 5861. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, S.; Cheng, X.; Wu, J.; Wang, X.; Wu, L.; Yu, H.; Bao, J. Curcumin enhances the membrane trafficking of the sodium iodide symporter and augments radioiodine uptake in dedifferentiated thyroid cancer cells via suppression of the PI3K-AKT signaling pathway. Food Funct. 2021, 12, 8260–8273. [Google Scholar] [CrossRef]
- Martín, M.; Salleron, L.; Peyret, V.; Geysels, R.C.; Darrouzet, E.; Lindenthal, S.; Bernal Barquero, C.E.; Masini-Repiso, A.M.; Pourcher, T.; Nicola, J.P. The PDZ protein SCRIB regulates sodium/iodide symporter (NIS) expression at the basolateral plasma membrane. FASEB J. 2021, 35, e21681. [Google Scholar] [CrossRef]
- Lauth, M.; Bergström, A.; Shimokawa, T.; Toftgård, R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc. Natl. Acad. Sci. USA 2007, 104, 8455–8460. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, R.; Zeng, C.; Lu, Q.; Huang, D.; Shi, C.; Zhang, W.; Deng, L.; Yan, R.; Rao, H.; et al. Down-regulation of Gli transcription factor leads to the inhibition of migration and invasion of ovarian cancer cells via integrin β4-mediated FAK signaling. PLoS ONE 2014, 9, e88386. [Google Scholar] [CrossRef]
- Wickström, M.; Dyberg, C.; Shimokawa, T.; Milosevic, J.; Baryawno, N.; Fuskevåg, O.M.; Larsson, R.; Kogner, P.; Zaphiropoulos, P.G.; Johnsen, J.I. Targeting the hedgehog signal transduction pathway at the level of GLI inhibits neuroblastoma cell growth in vitro and in vivo. Int. J. Cancer 2013, 132, 1516–1524. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, Y.; Deng, S.; Chen, Y.; Li, W.; Sun, J.; Xu, X. Targeting the Sonic Hedgehog Pathway to Suppress the Expression of the Cancer Stem Cell (CSC)-Related Transcription Factors and CSC-Driven Thyroid Tumor Growth. Cancers 2021, 13, 418. [Google Scholar] [CrossRef]
- Williamson, A.J.; Doscas, M.E.; Ye, J.; Heiden, K.B.; Xing, M.; Li, Y.; Prinz, R.A.; Xu, X. The sonic hedgehog signaling pathway stimulates anaplastic thyroid cancer cell motility and invasiveness by activating Akt and c-Met. Oncotarget 2016, 7, 10472–10485. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J.; Lu, Y.; Zhao, Y.; Prinz, R.A.; Xu, X. Inhibition of the sonic hedgehog pathway activates TGF-β-activated kinase (TAK1) to induce autophagy and suppress apoptosis in thyroid tumor cells. Cell Death Dis. 2021, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Alimoghaddam, K. A review of arsenic trioxide and acute promyelocytic leukemia. Int. J. Hematol. Oncol. Stem Cell Res. 2014, 8, 44–54. [Google Scholar]
- Mastrangelo, E.; Milani, M. Role and inhibition of GLI1 protein in cancer. Lung Cancer 2018, 9, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-W.; Lin, J.-H.; Chen, Y.-A.; Ho, S.-Y.; Wang, Y.-J. Combination treatment with arsenic trioxide and irradiation enhances cell-killing effects in human fibrosarcoma cells in vitro and in vivo through induction of both autophagy and apoptosis. Autophagy 2010, 6, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Knox, S.J. Increased cure rate of glioblastoma using concurrent therapy with radiotherapy and arsenic trioxide. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Knox, S.J. Optimization of combination therapy of arsenic trioxide and fractionated radiotherapy for malignant glioma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 493–498. [Google Scholar] [CrossRef]
- Liu, H.; Tao, X.; Ma, F.; Qiu, J.; Wu, C.; Wang, M. Radiosensitizing effects of arsenic trioxide on MCF-7 human breast cancer cells exposed to 89 strontium chloride. Oncol. Rep. 2012, 28, 1894–1902. [Google Scholar] [CrossRef][Green Version]
- Modak, S.; Zanzonico, P.; Carrasquillo, J.A.; Kushner, B.H.; Kramer, K.; Cheung, N.-K.V.; Larson, S.M.; Pandit-Taskar, N. Arsenic Trioxide as a Radiation Sensitizer for 131I-Metaiodobenzylguanidine Therapy: Results of a Phase II Study. J. Nucl. Med. 2016, 57, 231–237. [Google Scholar] [CrossRef]
- Fröhlich, E.; Czarnocka, B.; Brossart, P.; Wahl, R. Antitumor effects of arsenic trioxide in transformed human thyroid cells. Thyroid 2008, 18, 1183–1193. [Google Scholar] [CrossRef]
- Fröhlich, E.; Brossart, P.; Wahl, R. Induction of iodide uptake in transformed thyrocytes: A compound screening in cell lines. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Montero-Conde, C.; Ruiz-Llorente, S.; Dominguez, J.M.; Knauf, J.A.; Viale, A.; Sherman, E.J.; Ryder, M.; Ghossein, R.A.; Rosen, N.; Fagin, J.A. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013, 3, 520–533. [Google Scholar] [CrossRef]
- Cheng, L.; Jin, Y.; Liu, M.; Ruan, M.; Chen, L. HER inhibitor promotes BRAF/MEK inhibitor-induced redifferentiation in papillary thyroid cancer harboring BRAFV600E. Oncotarget 2017, 8, 19843–19854. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, D. Synergistic inhibition of MEK/ERK and BRAF V600E with PD98059 and PLX4032 induces sodium/iodide symporter (NIS) expression and radioiodine uptake in BRAF mutated papillary thyroid cancer cells. Thyroid. Res. 2018, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.; Gaweł, D.; Godlewska, M. Novel Inhibitor-Based Therapies for Thyroid Cancer—An Update. Int. J. Mol. Sci. 2021, 22, 11829. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.M.; Rajendran, R.L.; Gangadaran, P.; Hong, C.M.; Jeong, J.H.; Lee, J.; Ahn, B.-C. Targeting GLI1 Transcription Factor for Restoring Iodine Avidity with Redifferentiation in Radioactive-Iodine Refractory Thyroid Cancers. Cancers 2022, 14, 1782. https://doi.org/10.3390/cancers14071782
Oh JM, Rajendran RL, Gangadaran P, Hong CM, Jeong JH, Lee J, Ahn B-C. Targeting GLI1 Transcription Factor for Restoring Iodine Avidity with Redifferentiation in Radioactive-Iodine Refractory Thyroid Cancers. Cancers. 2022; 14(7):1782. https://doi.org/10.3390/cancers14071782
Chicago/Turabian StyleOh, Ji Min, Ramya Lakshmi Rajendran, Prakash Gangadaran, Chae Moon Hong, Ju Hye Jeong, Jaetae Lee, and Byeong-Cheol Ahn. 2022. "Targeting GLI1 Transcription Factor for Restoring Iodine Avidity with Redifferentiation in Radioactive-Iodine Refractory Thyroid Cancers" Cancers 14, no. 7: 1782. https://doi.org/10.3390/cancers14071782
APA StyleOh, J. M., Rajendran, R. L., Gangadaran, P., Hong, C. M., Jeong, J. H., Lee, J., & Ahn, B.-C. (2022). Targeting GLI1 Transcription Factor for Restoring Iodine Avidity with Redifferentiation in Radioactive-Iodine Refractory Thyroid Cancers. Cancers, 14(7), 1782. https://doi.org/10.3390/cancers14071782









