Indirect Treatment Comparison of Larotrectinib versus Entrectinib in Treating Patients with TRK Gene Fusion Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Sample Selection
- Documented NTRK fusion as determined by an independent radiology committee;
- Patients were required to be 18 years or older;
- Patients were required to have Eastern Cooperative Oncology Group (ECOG) scores of 2 or less;
- Patients were required to be TRK inhibitor naïve.
2.3. Outcome Measures
2.3.1. Efficacy Outcomes
2.3.2. Safety Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Efficacy Outcomes
3.3. Safety Outcomes
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amatu, A.; Sartore-Bianchi, A.; Bencardino, K.; Pizzutilo, E.G.; Tosi, F.; Siena, S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann. Oncol. 2019, 30, viii5–viii15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoleru, B.; Popescu, A.M.; Tache, D.E.; Neamtu, O.M.; Emami, G.; Tataranu, L.G.; Buteica, A.S.; Dricu, A.; Purcaru, S.O. Tropomyosin-receptor-kinases signaling in the nervous system. Maedica 2013, 8, 43–48. [Google Scholar] [PubMed]
- Kheder, E.S.; Hong, D.S. Emerging Targeted Therapy for Tumors with NTRK Fusion Proteins. Clin. Cancer Res. 2018, 24, 5807–5814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, S.J.; Zehir, A.; Sireci, A.N.; Aisner, D.L. Detection of Tumor NTRK Gene Fusions to Identify Patients Who May Benefit from Tyrosine Kinase (TRK) Inhibitor Therapy. J. Mol. Diagn. 2019, 21, 553–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Pollack, M.; Keating, K.; Wissinger, E.; Jackson, L.; Sarnes, E.; Cuffel, B. Transforming approaches to treating TRK fusion cancer: Historical comparison of larotrectinib and histology-specific therapies. Curr. Med. Res. Opin. 2021, 37, 59–70. [Google Scholar] [CrossRef]
- Gatalica, Z.; Xiu, J.; Swensen, J.; Vranic, S. Molecular characterization of cancers with NTRK gene fusions. Mod. Pathol. 2019, 32, 147–153. [Google Scholar] [CrossRef]
- Rolfo, C.; De Braud, F.; Doebele, R.; Drilon, A.; Siena, S.; Patel, M.; Cho, B.; Liu, S.; Ahn, M.; Chiu, C.; et al. Efficacy and safety of entrectinib in patients (pts) with NTRK-fusion positive (NTRK-fp) solid tumors: An updated integrated analysis. J. Clin. Oncol. 2020, 38, 3605. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Food and Drug Administration. Highlights of Prescribing Information VITRAKVI® (Larotrectinib). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211710s000lbl.pdf (accessed on 20 December 2020).
- Hong, D.S.; Bauer, T.M.; Lee, J.J.; Dowlati, A.; Brose, M.S.; Farago, A.F.; Taylor, M.; Shaw, A.T.; Montez, S.; Meric-Bernstam, F.; et al. Larotrectinib in adult patients with solid tumours: A multi-centre, open-label, phase I dose-escalation study. Ann. Oncol. 2019, 30, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Offin, M.; Harnicar, S.; Li, B.T.; Drilon, A. Entrectinib: An orally available, selective tyrosine kinase inhibitor for the treatment of NTRK, ROS1, and ALK fusion-positive solid tumors. Ther. Clin. Risk Manag. 2018, 14, 1247–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Food and Drug Administration. Highlights of Prescribing Information: ROZLYTREK (Entrectinib). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212725s000lbl.pdf (accessed on 20 December 2020).
- Roth, J.A.; Carlson, J.J.; Xia, F.; Williamson, T.; Sullivan, S.D. The Potential Long-Term Comparative Effectiveness of Larotrectinib and Entrectinib for Second-Line Treatment of TRK Fusion-Positive Metastatic Lung Cancer. J. Manag. Care Spec. Pharm. 2020, 26, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Signorovitch, J.E.; Wu, E.Q.; Yu, A.P.; Gerrits, C.M.; Kantor, E.; Bao, Y.; Gupta, S.R.; Mulani, P.M. Comparative effectiveness without head-to-head trials: A method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics 2010, 28, 935–945. [Google Scholar] [CrossRef]
- Signorovitch, J.E.; Sikirica, V.; Erder, M.H.; Xie, J.; Lu, M.; Hodgkins, P.S.; Betts, K.A.; Wu, E.Q. Matching-adjusted indirect comparisons: A new tool for timely comparative effectiveness research. Value Health 2012, 15, 940–947. [Google Scholar] [CrossRef] [Green Version]
- Phillippo, D.M.; Ades, A.; Dias, S.; Palmer, S.; Abrams, K.R.; Welton, N.J. NICE DSU Technical Support Document 18: Methods for Population-Adjusted Indirect Comparisons in Submissions to NICE; NICE Decision Support Unit: Sheffield, UK, 2016. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Wen, P.Y.; Chang, S.M.; Van den Bent, M.J.; Vogelbaum, M.A.; Macdonald, D.R.; Lee, E.Q. Response Assessment in Neuro-Oncology Clinical Trials. J. Clin. Oncol. 2017, 35, 2439–2449. [Google Scholar] [CrossRef]
- Guyot, P.; Ades, A.E.; Ouwens, M.J.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. Available online: https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5x7.pdf (accessed on 21 December 2020).
- Hall, A.R. Generalized Method of Moments; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Ishak, K.J.; Proskorovsky, I.; Benedict, A. Simulation and matching-based approaches for indirect comparison of treatments. Pharmacoeconomics 2015, 33, 537–549. [Google Scholar] [CrossRef]
- Harada, G.; Gongora, A.B.L.; da Costa, C.M.; Santini, F.C. TRK Inhibitors in Non-Small Cell Lung Cancer. Curr. Treat. Options Oncol. 2020, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, A.; Giordano, S. From single- to multi-target drugs in cancer therapy: When aspecificity becomes an advantage. Curr. Med. Chem. 2008, 15, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Pettit, S.D.; Kirch, R. Do current approaches to assessing therapy related adverse events align with the needs of long-term cancer patients and survivors? Cardiooncology 2018, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.I.; Zerillo, J.A.; Stuver, S.O.; Siegel, J.H.; Jacobson, J.O.; McNiff, K.K. Role of Adverse Events in Unscheduled Hospitalizations Among Patients With Solid Tumors Who Receive Medical Oncology Treatment. J. Oncol. Pract. 2019, 15, e39–e45. [Google Scholar] [CrossRef] [PubMed]
| Variables | Entrectinib N = 74 | Larotrectinib N = 117 | ||
|---|---|---|---|---|
| Before Matching | After Matching a | |||
| N (%) | N (%) | p-Value b | % | |
| Male | 35 (47.3%) | 54 (46.2%) | >0.99 | 47.3% |
| Age above 57 years | 37 (50.0%) | 52 (44.4%) | 0.55 | 50.0% |
| Race | ||||
| White | 52 (70.3%) | 86 (73.5%) | 0.75 | 70.3% |
| Black | 2 (2.7%) | 5 (4.3%) | 0.71 | 2.7% |
| Asian | 13 (17.6%) | 14 (12.0%) | 0.38 | 17.6% |
| Other/Not reported | 7 (9.5%) | 12 (10.3%) | >0.99 | 9.4% |
| ECOG PS score | ||||
| 0 | 30 (40.5%) | 41 (35.0%) | 0.54 | 40.5% |
| 1 | 34 (45.9%) | 61 (52.1%) | 0.49 | 45.9% |
| 2 | 10 (13.5%) | 15 (12.8%) | >0.99 | 13.6% |
| Primary tumor type | ||||
| Thyroid | 7 (9.5) | 25 (21.4) | 0.05 | 9.5 |
| Salivary | 13 (17.6) | 21 (17.9) | >0.99 | 17.6 |
| Sarcoma | 16 (21.6) | 25 (21.4) | >0.99 | 21.6 |
| Lung | 13 (17.6) | 13 (11.1) | 0.29 | 17.6 |
| Other | 25 (33.8) | 33 (28.2) | 0.51 | 33.7 |
| Metastatic disease (vs. locally advanced, unresectable disease) | 52 (96.3) | 106 (90.6) | 0.23 | 96.3 |
| Central nervous system metastases (Yes) | 16 (21.6) | 14 (12.0) | 0.11 | 21.6 |
| NTRK gene fusion | ||||
| NTRK1 | 30 (40.5) | 52 (44.4) | 0.70 | 40.5 |
| NTRK2 | 2 (2.7) | 3 (2.6) | >0.99 | 2.7 |
| NTRK3 | 42 (56.8) | 62 (53.0) | 0.72 | 56.8 |
| Prior lines of systemic therapy for metastatic disease | ||||
| 0 | 20 (27.0) | 30 (25.6) | 0.97 | 27.0 |
| 1 | 21 (28.4) | 29 (24.8) | 0.70 | 28.4 |
| 2 | 20 (27.0) | 23 (19.7) | 0.31 | 27.0 |
| 3+ | 13 (17.6) | 35 (29.9) | 0.08 | 17.6 |
| Prior therapy (chemotherapy) c | 60 (81.1) | 68 (58.1%) | <0.01 * | 58.1 |
| p < 0.01 * | ||||
| Prior therapy (hormonal therapy) c | 9 (12.2) | 6 (5.1%) | 0.14 | 2.0 |
| p < 0.01 * | ||||
| Prior therapy (immunotherapy) c | 9 (12.2) | 14 (12.0%) | 1.00 | 15.9 |
| p = 0.53 | ||||
| Prior therapy (targeted therapy) c | 18 (24.3) | 31 (26.5%) | 0.87 | 18.5 |
| p = 0.36 | ||||
| Variables | Entrectinib N = 74 | Larotrectinib N = 147 | ||
|---|---|---|---|---|
| Before Matching | After Matching a | |||
| N (%) | N (%) | p-Value b | % | |
| Male | 35 (47.3) | 71 (48.3) | >0.99 | 47.3 |
| Age above 57 years | 37 (50.0) | 62 (42.2) | 0.34 | 50.0 |
| Race | ||||
| White | 52 (70.3) | 95 (64.6) | 0.49 | 70.3 |
| Black | 2 (2.7) | 5 (3.4) | >0.99 | 2.7 |
| Asian | 13 (17.6) | 33 (22.4) | 0.51 | 17.6 |
| Other/Not reported | 7 (9.5) | 14 (9.5) | >0.99 | 9.4 |
| ECOG PS score | ||||
| 0 | 30 (40.5) | 54 (36.7) | 0.69 | 40.5 |
| 1 | 34 (45.9) | 74 (50.3) | 0.63 | 45.9 |
| 2 | 10 (13.5) | 19 (12.9) | >0.99 | 13.6 |
| Primary tumor type | ||||
| Thyroid | 7 (9.5) | 29 (19.7) | 0.08 | 9.5 |
| Salivary | 13 (17.6) | 24 (16.3) | 0.96 | 17.6 |
| Sarcoma | 16 (21.6) | 29 (19.7) | 0.88 | 21.6 |
| Lung | 13 (17.6) | 19 (12.9) | 0.47 | 17.6 |
| Other | 25 (33.8) | 46 (31.3) | 0.82 | 33.7 |
| Metastatic disease (vs. locally advanced, unresectable disease) | 52 (96.3) | 128 (87.1) | 0.07 | 96.3 |
| Central nervous system metastases (Yes) | 16 (21.6) | 18 (12.2) | 0.10 | 21.6 |
| NTRK gene fusion | ||||
| NTRK1 | 30 (40.5) | 64 (43.5) | 0.78 | 40.5 |
| NTRK2 | 2 (2.7) | 9 (6.1) | 0.34 | 2.7 |
| NTRK3 | 42 (56.8) | 74 (50.3) | 0.45 | 56.8 |
| Prior lines of systemic therapy for metastatic disease | ||||
| 0 | 20 (27.0) | 39 (26.5) | >0.99 | 27.0 |
| 1 | 21 (28.4) | 37 (25.2) | 0.72 | 28.4 |
| 2 | 20 (27.0) | 30 (20.4) | 0.35 | 27.0 |
| 3+ | 13 (17.6) | 41 (27.9) | 0.13 | 17.6 |
| Prior therapy (chemotherapy) c | 60 (81.1) | 88 (59.9) | <0.01 * | 81.1 |
| p < 0.01 * | ||||
| Prior therapy (hormonal therapy) c | 9 (12.2) | 6 (4.1) | <0.05 * | 1.6 |
| p < 0.01 * | ||||
| Prior therapy (immunotherapy) c | 9 (12.2) | 16 (10.9) | 0.95 | 15.0 |
| p = 0.61 | ||||
| Prior therapy (targeted therapy) c | 18 (24.3) | 35 (23.8) | >0.99 | 19.6 |
| p = 0.46 | ||||
| Time-to-Event Outcomes | Entrectinib | Larotrectinib Before Matching | Larotrectinib After Matching a | ||||
|---|---|---|---|---|---|---|---|
| Median, Months (95% CI) | Median, Months (95% CI) | HR vs. Entrectinib (95% CI) | p-Value | Median, Months (95% CI) | HR vs. Entrectinib (95% CI) | p-Value | |
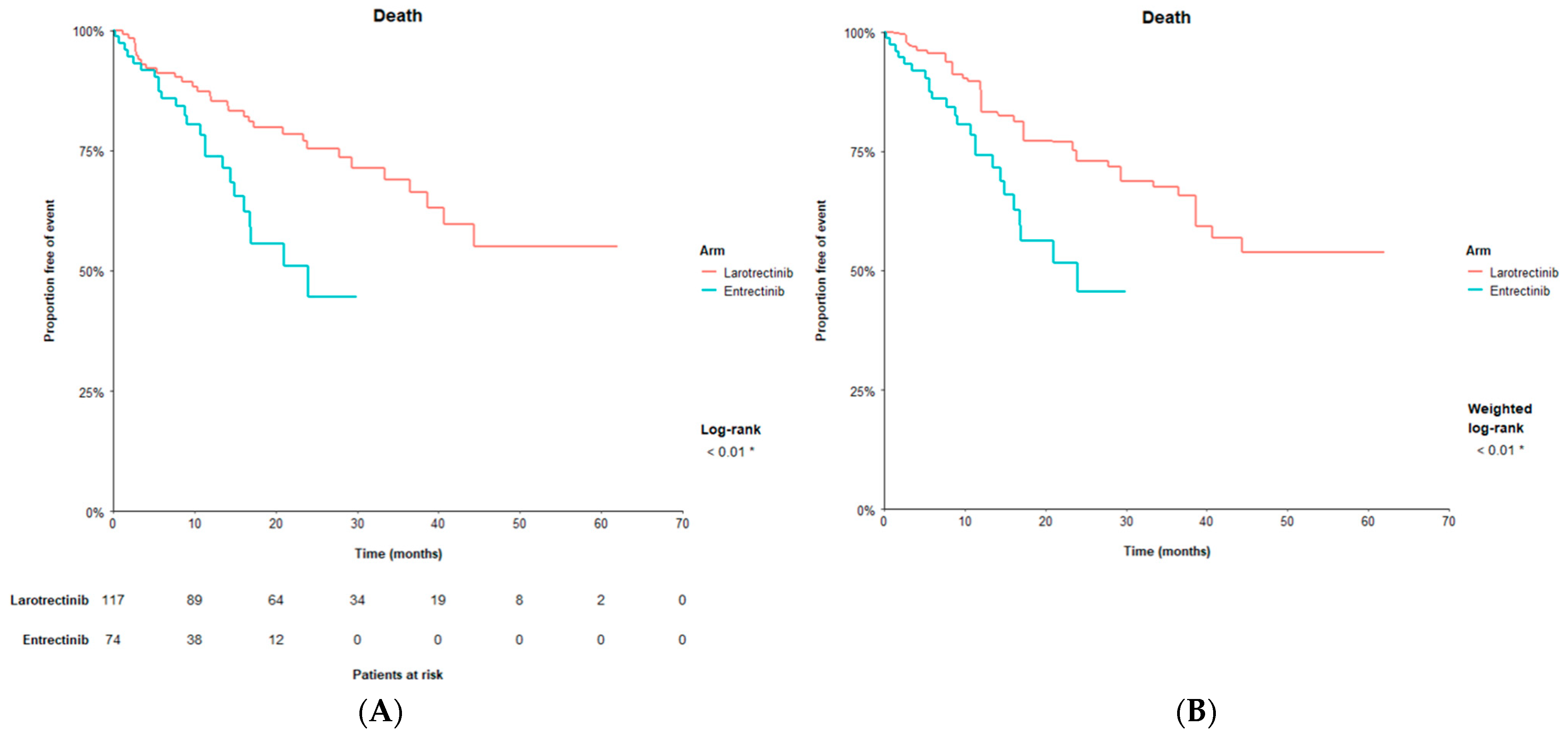
| OS | 23.9 | NR | 0.43 | <0.01 | NR | 0.43 | <0.05 * |
| (16.0, NE) | (40.7, NE) | (0.24, 0.76) | (38.7, NE) | (0.23, 0.83) | |||
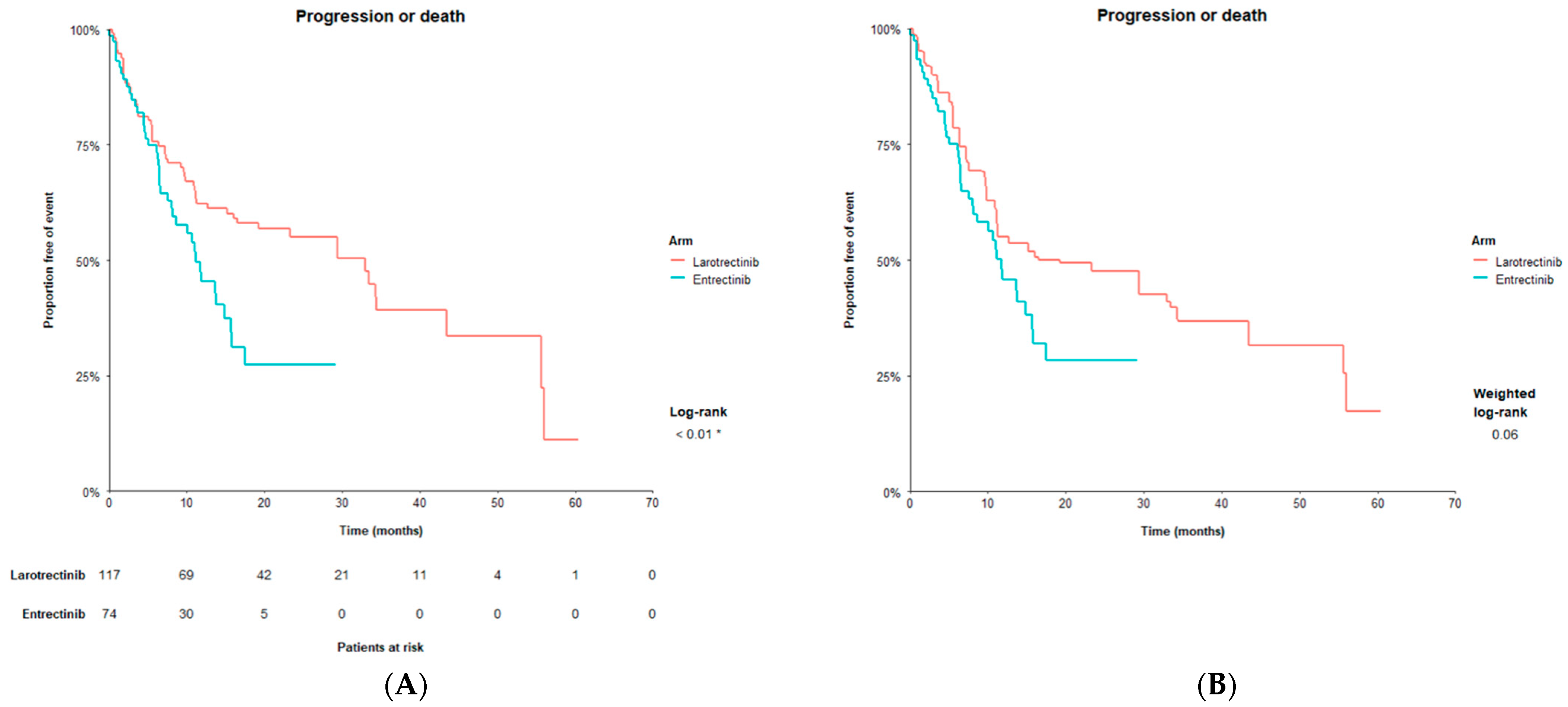
| PFS | 11.2 | 33.0 | 0.56 | <0.01 | 19.3 | 0.66 | 0.07 |
| (8.0, 15.7) | (16.6, NE) | (0.37, 0.86) | (11.5, 55.7) | (0.42, 1.03) | |||
| DoR b | 12.9 | 41.5 | 0.33 | <0.001 | 32.5 | 0.49 | <0.05 * |
| (9.3, NE) | (32.5, NE) | (0.17, 0.63) | (17.4, NE) | (0.25, 0.98) | |||
| Binary Outcomes | % | % | RD vs. entrectinib (95% CI) | p-value | % | RD vs. entrectinib (95% CI) | p-value |
| (95% CI) | (95% CI) | (95% CI) | |||||
| ORR | 63.5 | 65.0 | 1.5 | 0.84 | 67.3 | 3.8 | 0.63 |
| (51.5, 74.4) | (56.1, 73.2) | (−12.5, 15.4) | (55.6, 77.2) | (−11.7, 19.3) | |||
| CR | 6.8 | 19.7 | 12.9 | <0.01 | 20.3 | 13.5 | <0.05 * |
| (2.2, 15.1) | (13.2, 27.5) | (3.7, 22.1) | (12.8, 30.6) | (2.9, 24.1) | |||
| Variables | Entrectinib% (95% CI) | Larotrectinib Before Matching | Larotrectinib After Matching a | ||||
|---|---|---|---|---|---|---|---|
| % (95% CI) | RD vs. Entrectinib% (95% CI) | p-Value | % (95% CI) | RD vs. Entrectinib% (95% CI) | p-Value | ||
| Any serious TRAE | 10.0 | 5.4 | −4.6 | 0.27 | 6.3 | −3.7 | 0.40 |
| (4.2, 20.1) | (2.5, 9.9) | (−12.6, 3.5) | (3.0, 12.8) | (−12.1, 4.8) | |||
| TRAE leading to discontinuation | 4.0 | 0.7 | −3.3 | 0.18 | 0.7 | −3.3 | 0.18 |
| (0.9, 12.4) | (0.0, 3.0) | (−8.2, 1.5) | (0.1, 4.6) | (−8.2, 1.5) | |||
| Outcomes (Larotrectinib Relative to Entrectinib) | Primary Analysis | Sensitivity Analyses | ||
|---|---|---|---|---|
| Replacing Number of Lines of Prior Therapy with Type of Prior Therapy | Adding GI Tumors to the Matching Factors | Simulated Treatment Comparison | ||
| Overall survival, HR (95% CI) | 0.43 (0.23, 0.83) | 0.44 (0.23, 0.83) | 0.44 (0.23, 0.84) | 0.48 (0.27, 0.77) |
| Progression-free survival, HR (95% CI) | 0.66 (0.42, 1.03) | 0.58 (0.36, 0.93) | 0.67 (0.42, 1.05) | 0.76 (0.56, 1.15) |
| Overall response rate, RD (95% CI) | 3.8 (−11.7, 19.3) | 1.5 (−12.5, 15.4) | 3.6 (−11.9, 19.1) | 9.5 (−7.4, 26.4) |
| Complete response rate, RD (95% CI) | 13.5 (2.9, 24.1) | 12.9 (3.7, 22.1) | 13.6 (3.0, 24.2) | 18.2 (5.4, 30.9) |
| Duration of response, HR (95% CI) | 0.49 (0.25, 0.98) | 0.41 (0.20, 0.82) | 0.50 (0.25, 0.98) | 0.47 (0.24, 0.96) |
| Any serious TRAE, RD(95% CI) | −3.7 (−12.1, 4.8) | −6.0 (−13.9, 1.9) | −3.4 (−12.0, 5.2) | 4.3 (−9.9, 18.5) |
| TRAE leading to discontinuation, RD (95% CI) | −3.3 (−8.2, 1.5) | −3.9 (−8.6, 0.7) | −3.3 (−8.2, 1.5) | −4.0 (−9.5, 1.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Foncillas, J.; Bokemeyer, C.; Italiano, A.; Keating, K.; Paracha, N.; Fellous, M.; Marian, M.; Fillbrunn, M.; Gao, W.; Ayyagari, R.; et al. Indirect Treatment Comparison of Larotrectinib versus Entrectinib in Treating Patients with TRK Gene Fusion Cancers. Cancers 2022, 14, 1793. https://doi.org/10.3390/cancers14071793
Garcia-Foncillas J, Bokemeyer C, Italiano A, Keating K, Paracha N, Fellous M, Marian M, Fillbrunn M, Gao W, Ayyagari R, et al. Indirect Treatment Comparison of Larotrectinib versus Entrectinib in Treating Patients with TRK Gene Fusion Cancers. Cancers. 2022; 14(7):1793. https://doi.org/10.3390/cancers14071793
Chicago/Turabian StyleGarcia-Foncillas, Jesus, Carsten Bokemeyer, Antoine Italiano, Karen Keating, Noman Paracha, Marc Fellous, Marisca Marian, Mirko Fillbrunn, Wei Gao, Rajeev Ayyagari, and et al. 2022. "Indirect Treatment Comparison of Larotrectinib versus Entrectinib in Treating Patients with TRK Gene Fusion Cancers" Cancers 14, no. 7: 1793. https://doi.org/10.3390/cancers14071793







