A Multicenter, Prospective, Observational Study to Assess the Clinical Activity and Impact on Symptom Burden and Patients’ Quality of Life in Patients with Advanced Soft Tissue Sarcomas Treated with Trabectedin in a Real-World Setting in Greece
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Objectives
2.3. Endpoint Definitions and Assessments
2.4. Treatments
2.5. Sample Size Determination
2.6. Statistical Analysis Methods
3. Results
3.1. Patient Disposition and Characteristics
3.2. Treatment
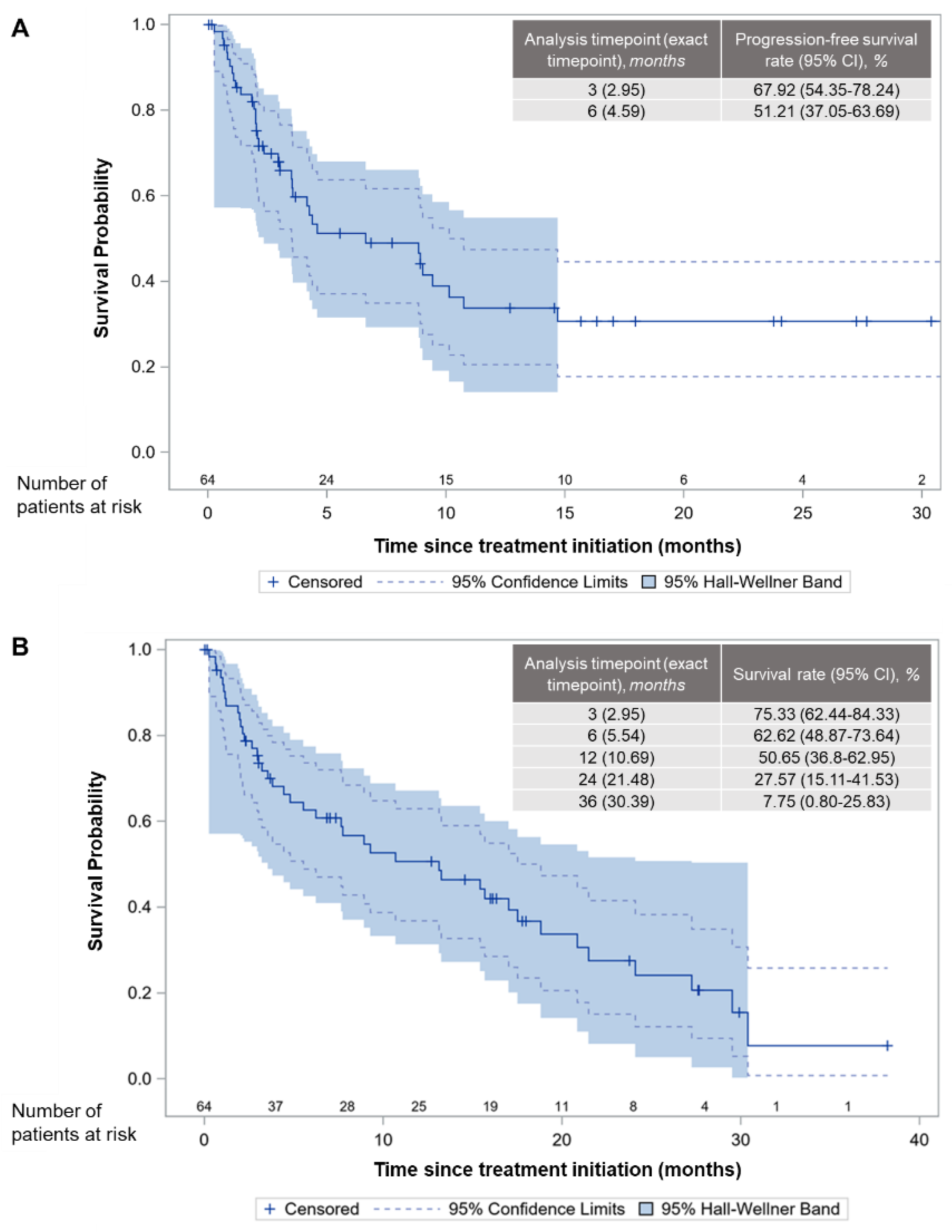
3.3. Effectiveness
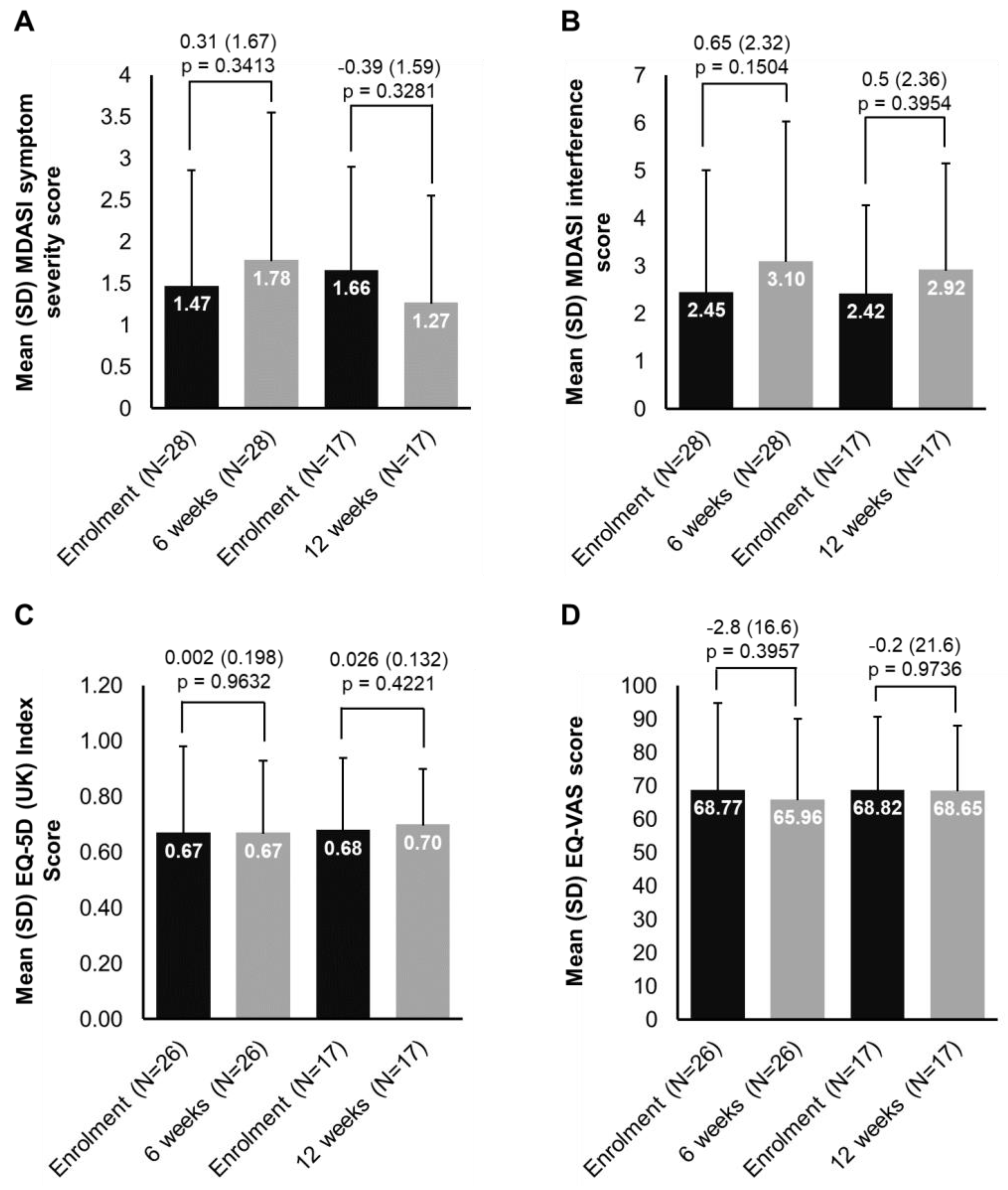
3.4. HRQoL
3.5. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, iv51–iv67. Available online: https://www.esmo.org/guidelines/sarcoma-and-gist/soft-tissue-and-visceral-sarcomas (accessed on 20 April 2021). [CrossRef] [PubMed]
- National Cancer Institute. SEER Cancer Stat Facts: Soft Tissue Cancer. Available online: https://seer.cancer.gov/statfacts/html/soft.html (accessed on 16 April 2021).
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossid, S.; Navarro, C.; Chirlaquee, M.D.; Casali, P.G.; The RARECARE Working Group. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Van Der Zwan, J.M.; Casali, P.G.; Siesling, S.; Dei Tos, A.P.; Kunkler, I.; Otter, R.; Licitra, L.; Mallone, S.; Tavilla, A.; et al. Rare cancers are not so rare: The rare cancer burden in Europe. Eur. J. Cancer 2011, 47, 2493–2511. [Google Scholar] [CrossRef] [PubMed]
- Rare Cancer Network in Europe. Available online: http://rarecarenet.istitutotumori.mi.it/ (accessed on 15 April 2021).
- Robinson, D.; Nersesyan, K.; Pomerantz, D. Epidemiology And Treatment Of Soft Tissue Sarcoma In The Eu5. Value Health 2015, 18, A439. [Google Scholar] [CrossRef] [Green Version]
- Cancer Research UK. Soft Tissue Sarcoma. Available online: https://www.cancerresearchuk.org/about-cancer/soft-tissue-sarcoma/survival (accessed on 15 April 2021).
- Stiller, C.A.; Botta, L.; Brewster, D.H.; Ho, V.K.Y.; Frezza, A.M.; Whelan, J.; Casali, P.G.; Trama, A.; Gatta, G.; EUROCARE-5 Working Group. Survival of adults with cancers of bone or soft tissue in Europe-Report from the EUROCARE-5 study. Cancer Epidemiol. 2018, 56, 146–153. [Google Scholar] [CrossRef]
- Nagar, S.P.; Mytelka, D.S.; Candrilli, S.D.; D’yachkova, Y.; Lorenzo, M.; Kasper, B.; Lopez-Martin, J.A.; Kaye, J.A. Treatment Patterns and Survival among Adult Patients with Advanced Soft Tissue Sarcoma: A Retrospective Medical Record Review in the United Kingdom, Spain, Germany, and France. Sarcoma 2018, 2018, 5467057. [Google Scholar] [CrossRef] [Green Version]
- Eichler, M.; Hentschel, L.; Richter, S.; Hohenberger, P.; Kasper, B.; Andreou, D.; Pink, D.; Jakob, J.; Singer, S.; Grützmann, R.; et al. The Health-Related Quality of Life of Sarcoma Patients and Survivors in Germany-Cross-Sectional Results of a Nationwide Observational Study (PROSa). Cancers 2020, 12, 3590. [Google Scholar] [CrossRef]
- Reichardt, P.; Leahy, M.; Garcia del Muro, X.; Ferrari, S.; Martin, J.; Gelderblom, H.; Wang, J.; Krishna, A.; Eriksson, J.; Staddon, A.; et al. Quality of Life and Utility in Patients with Metastatic Soft Tissue and Bone Sarcoma: The Sarcoma Treatment and Burden of Illness in North America and Europe (SABINE) Study. Sarcoma 2012, 2012, 740279. [Google Scholar] [CrossRef]
- NCCN. Clinical Practice Guidelines in Oncology, Soft Tissue Sarcoma, Version 1.2021, 30 October 2020. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1464 (accessed on 21 April 2021).
- Wesolowski, R.; Budd, G.T. Use of chemotherapy for patients with bone and soft-tissue sarcomas. Clevel. Clin. J. Med. 2010, 77 (Suppl. 1), S23–S26. [Google Scholar] [CrossRef]
- Cuevas, C.; Francesch, A. Development of Yondelis (trabectedin, ET-743). A semisynthetic process solves the supply problem. Nat. Prod. Rep. 2009, 26, 322–337. [Google Scholar] [CrossRef]
- European Medicines Agency. Yondelis® Summary of Product Characteristics. Last Updated on 9 October 2020. Available online: https://www.ema.europa.eu/en/documents/product-information/yondelis-epar-product-information_en.pdf (accessed on 13 April 2021).
- Yovine, A.; Riofrio, M.; Blay, J.Y.; Brain, E.; Alexandre, J.; Kahatt, C.; Taamma, A.; Jimeno, J.; Martin, C.; Salhi, Y.; et al. Phase II Study of Ecteinascidin-743 in Advanced Pretreated Soft Tissue Sarcoma Patients. J. Clin. Oncol. 2004, 22, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Le Cesne, A.; Blay, J.Y.; Judson, I.; Van Oosterom, A.; Verweij, J.; Radford, J.; Lorigan, P.; Rodenhuis, S.; Ray-Coquard, I.; Bonvalot, S.; et al. Phase II study of ET-743 in advanced soft tissue sarcomas: A European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 576–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Carbonero, R.; Supko, J.G.; Maki, R.G.; Manola, J.; Ryan, D.P.; Harmon, D.; Puchalski, T.A.; Goss, G.; Seiden, M.V.; Waxman, A.; et al. Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: Multicenter phase II and pharmacokinetic study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 5484–5492. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; Chawla, S.P.; von Mehren, M.; Ritch, P.; Baker, L.H.; Blay, J.Y.; Hande, K.R.; Keohan, M.L.; Samuels, B.L.; Schuetze, S.; et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: Results of a randomized phase II study of two different schedules. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 4188–4196. [Google Scholar] [CrossRef]
- Demetri, G.D.; Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef]
- Patel, S.; Mehren, M.; Reed, D.R.; Kaiser, P.; Charlson, J.; Ryan, C.W.; Rushing, D.; Livingston, M.; Singh, A.; Seth, R.; et al. Overall survival and histology-specific subgroup analyses from a phase 3, randomized controlled study of trabectedin or dacarbazine in patients with advanced liposarcoma or leiomyosarcoma. Cancer 2019, 125, 2610–2620. [Google Scholar] [CrossRef] [Green Version]
- Reichardt, P.; Grünwald, V.; Kasper, B.; Schuler, M.; Gelderblom, H. Efficacy of trabectedin in patients with some rare advanced soft tissue sarcoma subtypes other than liposarcoma and leiomyosarcoma. J. Med. Drug Rev. 2015, 5, 33–42. [Google Scholar]
- De Sanctis, R.; Marrari, A.; Marchetti, S.; Mussi, C.; Balzarini, L.; Lutman, F.R.; Daolio, P.; Bastoni, S.; Bertuzzi, A.F.; Quagliuolo, V.; et al. Efficacy of trabectedin in advanced soft tissue sarcoma: Beyond lipo- and leiomyosarcoma. Drug Des. Dev. Ther. 2015, 9, 5785–5791. [Google Scholar] [CrossRef] [Green Version]
- Fabbroni, C.; Fucà, G.; Ligorio, F.; Fumagalli, E.; Barisella, M.; Collini, P.; Morosi, C.; Gronchi, A.; Tos, A.P.; Casali, P.G.; et al. Impact of Pathological Stratification on the Clinical Outcomes of Advanced Well-Differentiated/Dedifferentiated Liposarcoma Treated with Trabectedin. Cancers 2021, 13, 1453. [Google Scholar] [CrossRef]
- Gounaris, I.; Hatcher, H.M.; Davidson, D.; Sherbourne, K.; Alam, S.; Zaki, K.A.; Horan, G.; Earl, H.M. Trabectedin for advanced soft tissue sarcomas: A single institution experience. Future Oncol. 2014, 10, 1843–1851. [Google Scholar] [CrossRef]
- Hindi, N.; García, I.C.; Sánchez-Camacho, A.; Gutierrez, A.; Peinado, J.; Rincón, I.; Benedetti, J.; Sancho, P.; Santos, P.; Sánchez-Bustos, P.; et al. Trabectedin Plus Radiotherapy for Advanced Soft-Tissue Sarcoma: Experience in Forty Patients Treated at a Sarcoma Reference Center. Cancers 2020, 12, 3740. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.; Keller, E.; Dietrich, S.; Wuchter, P.; Ho, A.D.; Egerer, G. Trabectedin for metastatic soft tissue sarcoma: A retrospective single center analysis. Mar. Drugs 2010, 8, 2647–2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schur, S.; Lamm, W.; Köstler, W.J.; Hoetzenecker, K.; Nemecek, E.; Schwameis, K.; Klepetko, W.; Windhager, R.; Brodowicz, T. Trabectedin in patients with metastatic soft tissue sarcoma: A retrospective single center analysis. Anti-Cancer Drugs 2013, 24, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Sanfilippo, R.; Grignani, G.; Buonadonna, A.; Romanini, A.; Badalamenti, G.; Ferraresi, V.; Vincenzi, B.; Comandone, A.; Pizzolorusso, A.; et al. Trabectedin for Patients with Advanced Soft Tissue Sarcoma: A Non-Interventional, Retrospective, Multicenter Study of the Italian Sarcoma Group. Cancers 2021, 13, 1053. [Google Scholar] [CrossRef]
- Martínez-Trufero, J.; Sande-González, L.M.; Luna, P.; Martin-Broto, J.; Álvarez, R.; Marquina, G.; Beveridge, R.D.; Poveda, A.; Cano, J.M.; Cruz-Jurado, J.; et al. A Growth Modulation Index-Based GEISTRA Score as a New Prognostic Tool for Trabectedin Efficacy in Patients with Advanced Soft Tissue Sarcomas: A Spanish Group for Sarcoma Research (GEIS) Retrospective Study. Cancers 2021, 13, 792. [Google Scholar] [CrossRef]
- Schack, L.H.; Mouritsen, L.S.; Elowsson, C.; Krarup-Hansen, A.; Safwat, A. The Danish experience with trabectedin treatment for metastatic sarcoma: Importance of hyponatremia. Acta Oncol. (Stockh. Swed.) 2015, 54, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Ploner, F.; Lamm, W.; Schur, S.; Eisterer, W.; Kühr, T.; Lindorfer, A.; Tinchon, C.; Köstler, W.J.; Szkandera, J.; Brodowicz, T.; et al. The Austrian experience with trabectedin in non-selected patients with metastatic soft tissue sarcoma (STS). J. Cancer Res. Clin. Oncol. 2013, 139, 1337–1342. [Google Scholar] [CrossRef]
- Le Cesne, A.; Ray-Coquard, I.; Duffaud, F.; Chevreau, C.; Penel, N.; Nguyen, B.B.; Piperno-Neumann, S.; Delcambre, C.; Rios, M.; Chaigneau, L.; et al. Trabectedin in patients with advanced soft tissue sarcoma: A retrospective national analysis of the French Sarcoma Group. Eur. J. Cancer 2015, 51, 742–750. [Google Scholar] [CrossRef]
- Buonadonna, A.; Casanova, C.B.J.; Kasper, B.; Pousa, A.L.; Mazzeo, F.; Brodowicz, T.; Penel, N. A noninterventional, multicenter, prospective phase IV study of trabectedin in patients with advanced soft tissue sarcoma. Anti-Cancer Drugs 2017, 28, 1157–1165. [Google Scholar] [CrossRef]
- Hentschel, L.; Richter, S.; Kopp, H.G.; Kasper, B.; Kunitz, A.; Grünwald, V.; Kessler, T.; Chemnitz, J.M.; Pelzer, U.; Schuler, U.; et al. Quality of life and added value of a tailored palliative care intervention in patients with soft tissue sarcoma undergoing treatment with trabectedin: A multicentre, cluster-randomised trial within the German Interdisciplinary Sarcoma Group (GISG). BMJ Open 2020, 10, e035546. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Italiano, A.; Ray-Coquard, Ι.; Le Cesne, A.; Duffaud, F.; Rios, M.; Collard, O.; Bertucci, F.; Bompas, E.; Isambert, N.; et al. Long-term outcome and effect of maintenance therapy in patients with advanced sarcoma treated with trabectedin: An analysis of 181 patients of the French ATU compassionate use program. BMC Cancer 2013, 13, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Glabbeke, M.; Verweij, J.; Judson, I.; Nielsen, O.S. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur. J. Cancer 2002, 38, 543–549. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.; Elias, A.D.; Pierson, R.F.; Knoblauch, R.E.; Park, Y.C.; Wang, G.C.; et al. Patient-reported outcomes from randomized, phase-3 study of trabectedin (T) vs. dacarbazine (D) in advanced leiomyosarcoma (LMS) or liposarcoma (LPS). J. Clin. Oncol. 2016, 34, 11061. [Google Scholar] [CrossRef]
- Gough, N.; Koffman, J.; Ross, J.R.; Riley, J.; Judson, I. Symptom Burden in Advanced Soft-Tissue Sarcoma. J. Pain Symptom Manag. 2017, 53, 588–597. [Google Scholar] [CrossRef] [Green Version]
- Gough, N.J.; Smith, C.; Ross, J.R.; Riley, J.; Judson, I. Symptom burden, survival and palliative care in advanced soft tissue sarcoma. Sarcoma 2011, 2011, 325189. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Full Analysis Set (n = 64) |
|---|---|
| Age, median (IQR) | 58.3 (46.9–64.8) |
| Gender, n (%) | |
| Female | 40 (62.5) |
| Male | 24 (37.5) |
| Educational level, n (%) | |
| No education | 7 (10.9) |
| Primary education | 14 (21.9) |
| Secondary education | 26 (40.6) |
| Tertiary education | 17 (26.6) |
| Employment status, n (%) | |
| Unemployed | 10 (15.6) |
| Employed | 27 (42.2) |
| Retired | 13 (20.3) |
| Household duties | 11 (17.2) |
| Other | 3 (4.7) |
| BMI classification, n (%) | |
| <18.5 | 4 (6.3) |
| 18.5–24.9 | 24 (37.5) |
| 25.0–29.9 | 23 (35.9) |
| ≥30.0 | 13 (20.3) |
| PS (ECOG), n (%) | |
| 0 | 38 (59.4) |
| 1 | 22 (34.4) |
| 2 | 2 (3.1) |
| 3 | 2 (3.1) |
| Presence of at least one comorbidity, n (%) | 34 (53.1) |
| Histological Subtype | n (%) |
| Leiomyosarcoma | 21 (32.8) |
| UPS | 10 (15.6) |
| Liposarcoma | 7 (10.9) |
| Myxoid Liposarcoma | 4 (6.3) |
| Synovial Sarcoma | 3 (4.7) |
| Unclassified | 3 (4.7) |
| Fibrosarcoma | 2 (3.1) |
| Other 1 | 14 (21.9) |
| Metastasis sites, n (%) | 43 (67.2) |
| Lung | 32 (50.0) |
| Bone | 7 (10.9) |
| Liver | 7 (10.9) |
| Nodes | 5 (7.8) |
| Pelvis | 4 (6.3) |
| Brain | 1 (1.6) |
| Other | 7 (10.9) |
| Prior surgery, n (%) | 26 (40.6) 2 |
| Complete tumor excision, n/n (%) | 12 (18.9) |
| Prior radiotherapy, n (%) | 15 (23.4) 2 |
| Number of prior lines of chemotherapy, n (%) | |
| 0 | 11 (17.2) |
| 1 | 34 (53.1) |
| 2 | 16 (25.0) |
| 3 | 3 (4.7) |
| Number | Death or PD Events | Censored (30 Days Post-Treatment Discontinuation) |
|---|---|---|
| n | 35 | 29 |
| Variable | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| Age (Years) | 1.01 (0.98–1.03) | 0.5888 |
| Gender (Male vs. Female) | 0.81 (0.42–1.57) | 0.5357 |
| STS extent (Locally advanced vs. Metastatic) | 0.76 (0.36–1.560) | 0.4659 |
| ECOG PS (1–3 vs. 0) | 2.69 (1.43–5.05) | 0.0021 |
| Lung metastasis (Yes vs. No) | 0.97 (0.52–1.81) | 0.9317 |
| Prior surgery (Yes vs. No) | 1.47 (0.76–2.83) | 0.2508 |
| Prior radiotherapy (Yes vs. No) | 0.68 (0.34–1.38) | 0.2888 |
| Number | Death Events | Censored (at the Last Follow-Up Visit) |
|---|---|---|
| n | 41 | 23 |
| Trabectedin-Related AEs | Full Analysis Set (n = 64) | ||||
|---|---|---|---|---|---|
| Overall | Serious | Grade 1–2 | Grade 3–4 | ||
| nevents | npt (%) | npt (%) | npt (%) | npt (%) | |
| Trabectedin-related AEs in ≥2% of patients (>1 patient) | 87 | 30 (46.9) | 9 (14.1) | 20 (31.3) | 18 (28.1) |
| Fatigue | 17 | 14 (21.9) | 1 (1.6) | 4 (6.3) | 10 (15.6) |
| Anemia | 17 | 11 (17.2) | 5 (7.8) | 9 (14.1) | 4 (6.3) |
| Neutropenia | 8 | 6 (9.4) | - | 3 (4.7) | 4 (6.3) |
| Nausea | 6 | 6 (9.4) | - | 6 (9.4) | - |
| Thrombocytopenia | 5 | 5 (7.8) | 2 (3.1) | 3 (4.7) | 2 (3.1) |
| Vomiting | 5 | 5 (7.8) | - | 4 (6.3) | 1 (1.6) |
| Leukopenia | 3 | 3 (4.7) | - | 3 (4.7) | - |
| Febrile neutropenia | 2 1 | 2 (3.1) | 2 (3.1) | - | 1 (1.6) |
| Hepatitis | 2 1 | 2 (3.1) | 2 (3.1) | - | 1 (1.6) |
| Pancytopenia | 2 | 2 (3.1) | 2 (3.1) | - | 2 (3.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkali, S.; Boukovinas, I.; Samantas, E.; Papakotoulas, P.; Athanasiadis, I.; Andreadis, C.; Makrantonakis, P.; Samelis, G.; Timotheadou, E.; Vassilopoulos, G.; et al. A Multicenter, Prospective, Observational Study to Assess the Clinical Activity and Impact on Symptom Burden and Patients’ Quality of Life in Patients with Advanced Soft Tissue Sarcomas Treated with Trabectedin in a Real-World Setting in Greece. Cancers 2022, 14, 1879. https://doi.org/10.3390/cancers14081879
Kokkali S, Boukovinas I, Samantas E, Papakotoulas P, Athanasiadis I, Andreadis C, Makrantonakis P, Samelis G, Timotheadou E, Vassilopoulos G, et al. A Multicenter, Prospective, Observational Study to Assess the Clinical Activity and Impact on Symptom Burden and Patients’ Quality of Life in Patients with Advanced Soft Tissue Sarcomas Treated with Trabectedin in a Real-World Setting in Greece. Cancers. 2022; 14(8):1879. https://doi.org/10.3390/cancers14081879
Chicago/Turabian StyleKokkali, Stefania, Ioannis Boukovinas, Epaminondas Samantas, Pavlos Papakotoulas, Ilias Athanasiadis, Charalampos Andreadis, Parisis Makrantonakis, Georgios Samelis, Eleni Timotheadou, Georgios Vassilopoulos, and et al. 2022. "A Multicenter, Prospective, Observational Study to Assess the Clinical Activity and Impact on Symptom Burden and Patients’ Quality of Life in Patients with Advanced Soft Tissue Sarcomas Treated with Trabectedin in a Real-World Setting in Greece" Cancers 14, no. 8: 1879. https://doi.org/10.3390/cancers14081879
APA StyleKokkali, S., Boukovinas, I., Samantas, E., Papakotoulas, P., Athanasiadis, I., Andreadis, C., Makrantonakis, P., Samelis, G., Timotheadou, E., Vassilopoulos, G., Papadimitriou, C., Tzanninis, D., Ardavanis, A., Kotsantis, I., Karvounis-Marolachakis, K., Theodoropoulou, T., & Psyrri, A. (2022). A Multicenter, Prospective, Observational Study to Assess the Clinical Activity and Impact on Symptom Burden and Patients’ Quality of Life in Patients with Advanced Soft Tissue Sarcomas Treated with Trabectedin in a Real-World Setting in Greece. Cancers, 14(8), 1879. https://doi.org/10.3390/cancers14081879






