Simple Summary
Oral cancer was considered a disease of old age. However, there has been a recent surge in the incidence of oral cancer in young individuals. Age dependence on survival outcomes such as overall survival, disease-free survival, recurrence, distant metastasis and second primary in surgically treated oral cancer has been investigated several times and the results differ. This systematic review and meta-analysis has been conducted to address this concern. The results of the present research may facilitate age-dependent prognosis stratification, which would assist in treatment planning in oral cancer patients.
Abstract
This systematic review and meta-analysis aims to address whether age can be a determinant of overall survival (OS), disease-free survival (DFS), recurrence, distant metastasis (DM) and second primary (SP) in surgically treated oral and oropharyngeal squamous cell carcinoma (OOPSCC). A total of 4981 cases and 44254 controls from 25 comparative observational studies were included in the analysis. A significantly better OS (matched subgroup analysis: OR 1.64; 95% CI 1.31–2.04, overall analysis: OR 1.48; 95% CI 1.09–2.01) was observed in young patients compared to older adults, with heterogeneity ranging from moderate to severe. Worse DFS (unmatched subgroup analysis OR 0.43; 95% CI 0.27–0.68) was observed in young patients compared to older adults with minimal to moderate heterogeneity. The frequency of recurrence (OR 1.49; 95% CI 1.10–2.02) and DM (OR 1.83; 95% CI 1.10–3.03) was significantly higher in the young patients, as found in unmatched and matched subgroup analysis, with the least heterogeneities. Young age can be considered as an independent prognostic factor for recurrence and distant metastases in OOP-SCC. Larger and methodologically robust observational studies with longer follow-up are needed to establish the definitive role of age as an independent prognostic factor on OS and DFS in OOPSCC.
1. Introduction
Oral and oropharyngeal cancers are the sixth-most common cancers worldwide and more than 90% of these cancers are histologically squamous cell carcinomas, termed as oropharyngeal squamous cell carcinoma (OPSCC) [1,2]. The “International Classification of Diseases and Related Health Problems (ICD-10)”, an international standard recommended by the World Health Organization (WHO currently in the 10th revision) [1,2], divides malignant neoplasms of the head and neck region into the codes C00 to C14. OSCC, according to their localizations, incorporate C01 to C6 (ICD-10), with C01: base of the tongue, C02: other and unspecified parts of the tongue, C03: alveolar mucosa and gingiva, C04: floor of the mouth, C05: hard palate, except C05.1: soft palate and C05.2: uvula, and C06: other and unspecified parts of the oral cavity including the buccal mucosa [1,2]. However, base of the tongue (C01), which belongs to oropharyngeal cancers embryologically as the posterior one-third, is developed from the third branchial arch [1,3]. OPSCC includes C01 and C10, which are driven by oncogenic variants of human papillomavirus (HPV) [1,2,4]. Smoking tobacco and alcohol consumption have been widely accepted as the major etiologic factors for OPSCC [1,2,3]. Other less common factors include betel quid chewing, a diet low in vegetables and fruits, poor nutrition, marijuana smoking, poor oral hygiene, and certain genetic mutations [1,2,3]. Clinicopathologic prognostic factors of OPSCC such as TNM staging, patient’s general health status, co-morbidities, primary tumor macrophage content, and lymph node metastasis are well studied [5,6,7,8,9]. The role of age as a prognostic factor has been proposed recently.
Literature on OOPSCC often does not follow the distinction between oral cavity and oropharynx. In fact, anatomic subsite definitions are at times vague, with some authors using the term “oral” for cancers of the oral cavity inclusive of the oropharynx [1]. Regardless, OOPSCCs are mostly referred to together [1,2,3,10], because of which we have used the same terminology. OOPSCCs are conventionally known to be a disease of the elderly population with an age predilection of more than 60 years [1,2,3,10]. Ablative surgery, with curative intent, has been the mainstay of treatment for these cancers for over a century. Surgical resection helps in accurate staging, with appropriate details on the status of margins and the spread of tumor that can help in deciding subsequent management based upon assessment of risk versus benefit. The recent shift in the demographic trend from old to young age has created controversies regarding the influence of age on the prognosis of OOPSCC after treatment [10,11,12]. Numerous early reports concluded that the disease is more aggressive, and the prognosis is poorer in young adults; hence, young adults are suggested as a distinct cohort with different risk factors and disease behavior [1,11,13]. On the contrary, certain other studies showed the absence of any difference in terms of survival in young patients [14]. Moreover, studies have also highlighted the importance of ageing in poorer prognosis and overall survival in older patients [1,2,3,10]. Therefore, we hypothesized that age can be a significant factor that can segregate the outcomes in surgically treated OOPSCC, and the objective of this systematic review and meta-analysis of observational studies is to provide a comprehensive analysis of current evidence on the impact of age on the survival outcomes commonly reported in oral oncology.
2. Materials and Methods
2.1. Data Sources and Search Strategy
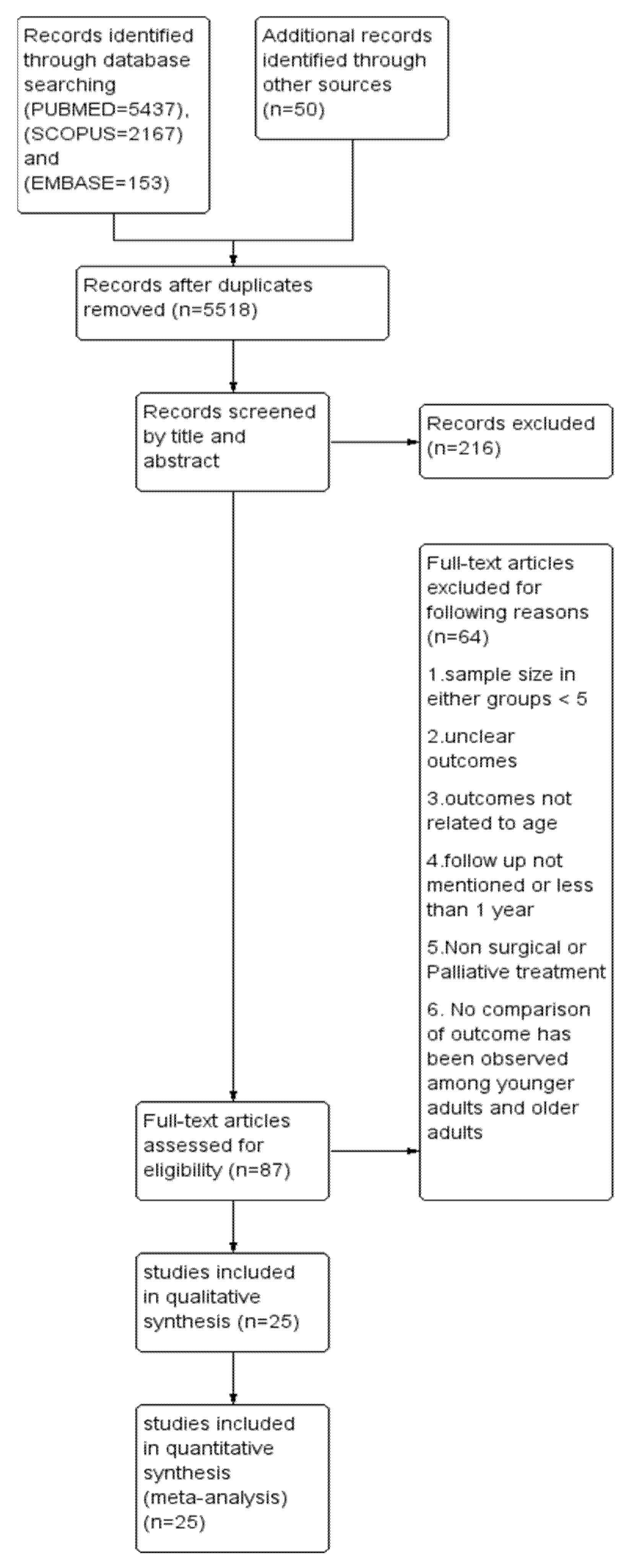
This systematic review and meta-analysis adhered to the Primary Reporting items for Systematic review and Meta-analysis (PRISMA) guidelines (Figure 1). A well-defined protocol was prepared and registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42020213023. A digitalized search was carried out in electronic databases, namely, PUBMED, SCOPUS and EMBASE using the following search string: ((((((oral cancer) OR (head and neck cancer)) OR cancer, oropharyngeal)) AND (((young adults) OR age) OR less than 40 years)) AND (((((((assessment, outcomes) OR disease-free survival) OR survival) OR recurrence) OR metastasis) OR second primary cancer) OR mean survival time)). The last search was conducted on 31 December 2021. In addition, a manual search was carried out in the recent issues of dental-related journals such as Oral Oncology, Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, European Journal of Cancer, Journal of Oral and Maxillofacial Surgery, British Journal of Cancer, and Cancer Research. The bibliography column of relevant clinical reports, potentially eligible studies, and reviews were also screened. Inclusiveness of studies followed the PICO (population, intervention, comparison, and outcome) format of observational studies.
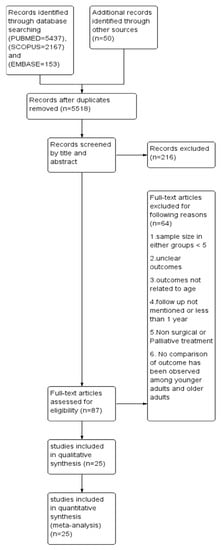
Figure 1.
Prisma flow chart demonstrating the selection of studies.
- Population: Young adult patients (≤40 years) with OSCC or OPSCC
- Intervention: Surgery with or without chemoradiotherapy
- Comparison: Older adult patients (>40 years) with OSCC or OPSCC
- Outcomes: Overall survival (OS), Disease-free survival (DFS), recurrence, distant metastasis (DM), and second primary (SP)
2.2. Study Selection
Criteria for selecting studies were based upon the age group of the target population, treatment strategies, and duration of follow-up. The target population consisted of oral cancer/oropharyngeal cancer patients in two age groups. Cases included patients aged 40 years old or younger (young patients) and controls included patients older than 40 years old (old patients). Reports on patients younger than 30 years or older than 70 years were excluded. Studies that reported the outcome of OOPSCC separately while addressing that of other sites in head and neck cancer were also included. Surgery alone, or in combination with radiotherapy and/or chemotherapy, was the treatment modality in the selected articles. Studies reporting outcome/outcomes such as three- to five-year overall survival (OS), disease-free survival (DFS), recurrence, distant metastasis (DM), and second primary (SP) were included. Studies that reported the events with a follow-up for less than one year were excluded. OS is defined as the time interval between primary treatment and death due to oral cancer or last follow-up. DFS is defined as the time interval between primary treatment and the first recurrence.
2.3. Data Extraction and Quality Assessment
Excel Spreadsheet (Microsoft, Redmond, WA, USA) was used to retrieve relevant information from the included studies for qualitative synthesis. Data extraction was carried out separately by three independent reviewers (S.P. (Swagatika Panda), S.P. (Saurav Panda), N.M.). The authors were reached over telephone or email to enquire about the details of missing or unclear information. Parameters like demographic characteristics, study design, sample size, clinical features such as age, gender, site, Tumor, Node, Metastasis (TNM) staging, grading, follow-up duration, treatment strategies and reported outcome/outcomes in the form of a number or percentage were recorded. Due to the non-uniform presentation of staging, we have categorized cT1, cT2, stage I and II as early and the rest as the advanced stage of presentation. Similarly, we have combined the well-differentiated tumors (Grade I) and moderately differentiated tumors (Grade II) as low grade and poorly differentiated and undifferentiated as high-grade tumors.
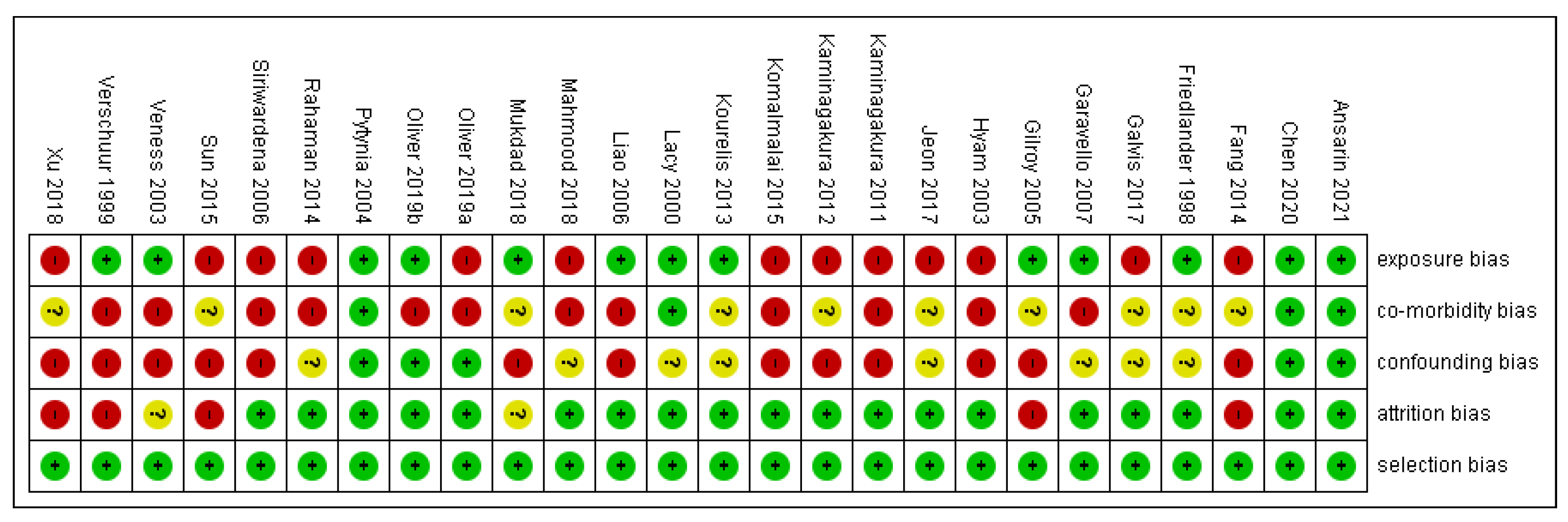
The risk of bias in methodological quality was assessed in Review Manager 5.3 Software (Copenhagen, Denmark). by two independent investigators (S.P. (Swagatika Panda) and S.P. (Saurav Panda)). Each study was evaluated for (1) selection bias, (2) exposure risk, (3) co-morbidity, (4) attrition bias, (5) confounding bias, and (6) immortal time bias. Two authors appraised nine points in every included study and colored ‘green’ for low risk, ‘yellow’ for unclear, and ‘red’ for high risk. The risk of bias was categorized as low when the study was showing more and equal to 60% of the ‘green’ score and high when there was 40% of either ‘yellow’ or ‘red’.
2.4. Data Synthesis and Analysis
A detailed qualitative analysis was carried out for all included studies. A Chi-square test was performed in SPSS (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY, USA: IBM Corp.) to compare the differences in staging and grading of OOPSCC between two age groups. A p-value of less than 0.05 was considered statistically significant. To conduct meta-analysis, Odd’s ratio (OR) was pooled from the number or percentages of events of OS, DFS, recurrence, DM, and SP in both the cohorts. Forest plots were constructed using Review manager 5.3 (Copenhagen, Denmark). Due to the diverse nature of interventions, the follow-up period and clinicopathologic factors heterogeneity was expected and therefore the random-effects model was chosen. The heterogeneity of the included studies was assessed using I2 statistics. Chi-square statistics were used to measure the variation in the effect size due to heterogeneity. Values of I2 greater than 50% represented significant heterogeneity. The risk of bias was mapped to express the heterogeneity among eligible studies [15]. To conduct subgroup analysis, studies were further segregated into matched and unmatched studies for one or many factors, including age, gender, site, TNM staging, and treatments provided. Publication bias was assessed using the Funnel plot in Revman.
3. Results
3.1. Characteristics of Included Studies
A total of 5247, 2167 and 153 articles were identified from three databases like PUBMED, SCOPUS and EMBASE, respectively, and duplicates were removed. After careful reading of abstracts and full texts, twenty-five articles [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] were selected for conducting the systematic review (SR), followed by a meta-analysis. A PRISMA flow chart depicting the selection of articles is shown in Figure 1. Out of 25 publications, ten [16,17,18,19,20,21,27,30,32,38] were studied in Asian populations and the rest in European, Australian and North American populations. Only two reports [18,26] were prospective in nature. A total of 4981 young patients and 44,254 old patients were studied. Male-to-female ratios in case and controls were found to be 1.4 and 1.7, respectively (p = 0.39). Tongue is the predominant site in eight studies [19,20,22,23,30,31,33,40], whereas ten studies [16,17,28,32,34,35,36,37,38,39] were conducted exclusively in tongue. Rahman et al. [21] did not report the specific site. The proportions of an early stage in the case and control group were found to be 43.6% and 44.4%, respectively (p = 0.35). Two studies [21,27] did not report the staging. Grading has been reported in all except five studies [25,26,27,33,39]. The proportion of low grades in case and control were found to be 70.5% and 71.2%, respectively (p = 0.62). The median follow-up period as mentioned in all studies, except one [18], was found to be 59.5 months and 57.7 months in case and control, respectively (p = 0.52). Clinicopathological and outcome details of included articles are listed in Table 1 and Table 2, respectively.

Table 1.
Clinicopathological features of eligible articles.

Table 2.
Details of follow up, treatment and outcomes of eligible studies.
3.2. Risk of Bias
Out of 25 studies, 13 studies showed a high risk of bias, as depicted in supplemental Figure 2. Comorbidity bias was the most common bias seen in 15 out of 25 articles. Selection and attrition bias were found to be the least common.
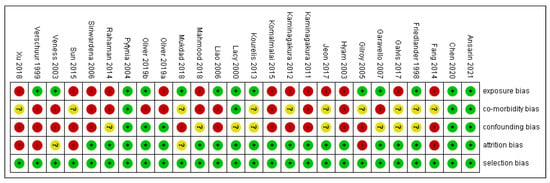
Figure 2.
Risk of bias.
3.3. Meta-Analysis
3.3.1. Overall Survival
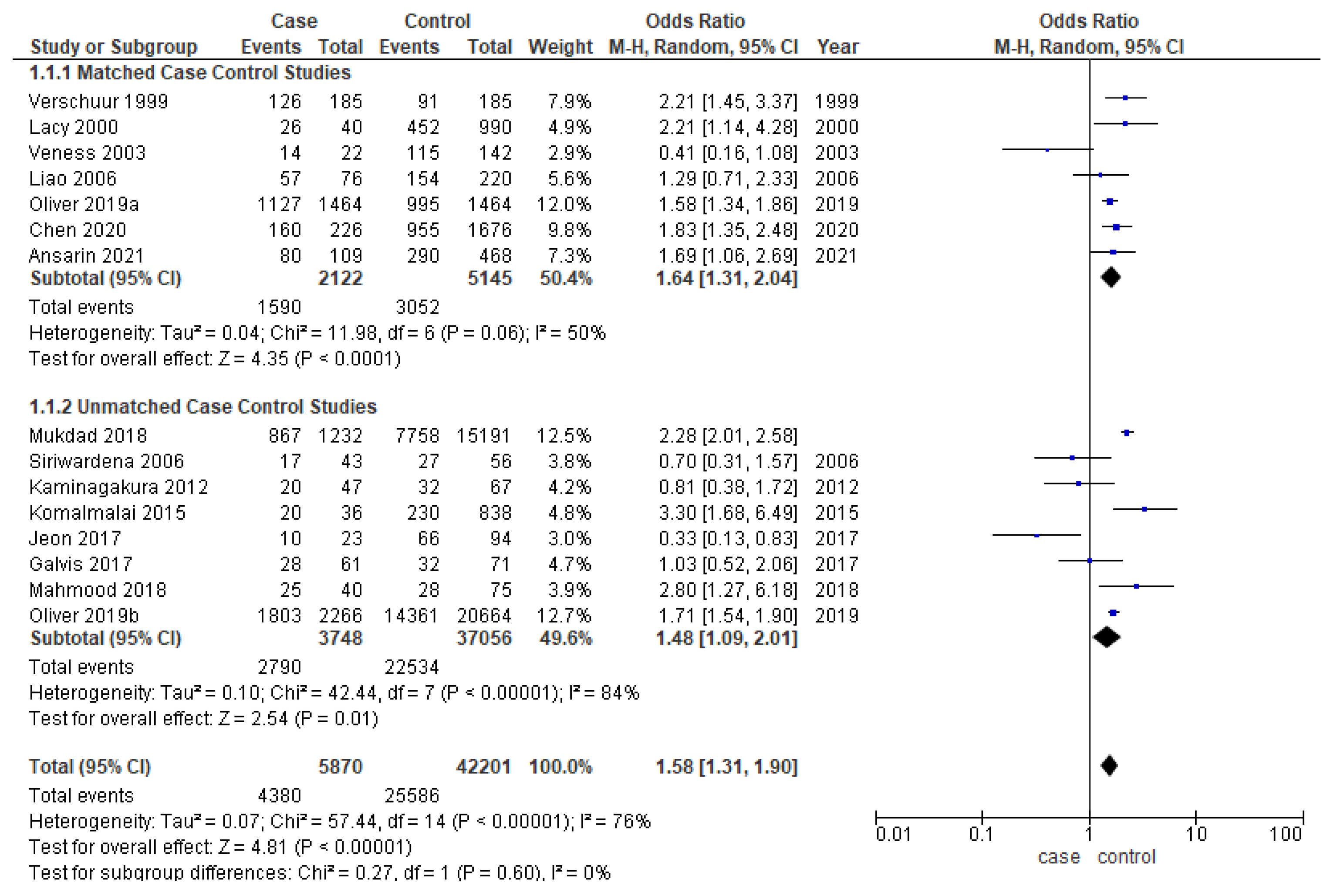
Younger patients had significantly better overall survival in the overall analysis (OR-1.58; 95% CI-1.31–1.90) (Figure 3). The cases and controls were matched for one or multiple factors such as age, gender, TNM staging, and grading in six out of thirteen studies [17,18,20,22,25,27,31,32,33,35,36,37,40]. The odds ratio for both matched (OR-1.64; 95% CI 1.31–2.04) and unmatched studies (OR-1.48; 95% CI 1.09–2.01) are similar to the overall OR. While meta-analysis of matched studies demonstrated moderate heterogeneity (50%), significant heterogeneity was identified in overall analysis (76%) and unmatched analysis (84%).
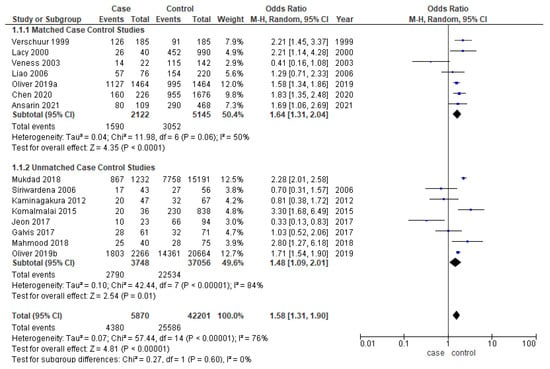
Figure 3.
Forest plot demonstrating the OR for overall survival in young patients.
3.3.2. Disease-Free Survival
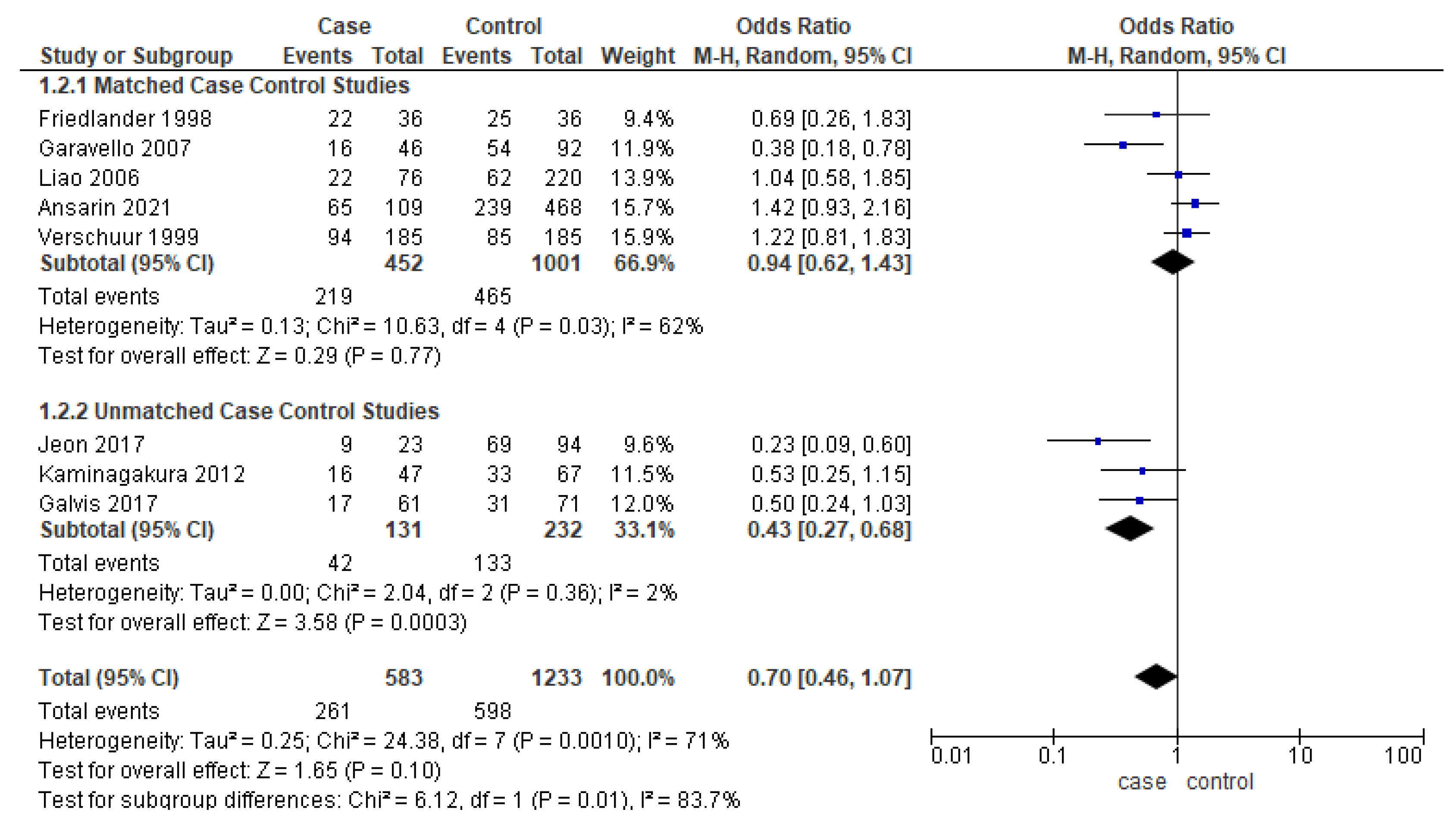
Eight studies [17,22,25,28,38,39,40] reported the DFS, out of which only five studies were matched for one or more clinicopathologic factors. Unmatched subgroup analysis demonstrated worse DFS in younger adults (Figure 4). The heterogeneity remained moderate (53%) and high (70%), respectively.
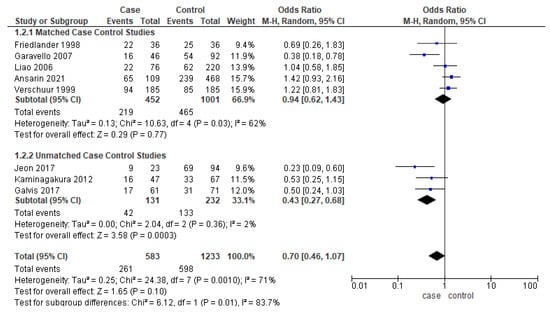
Figure 4.
Forest plot demonstrating the OR for DFS in young patients.
3.3.3. Events of Recurrence
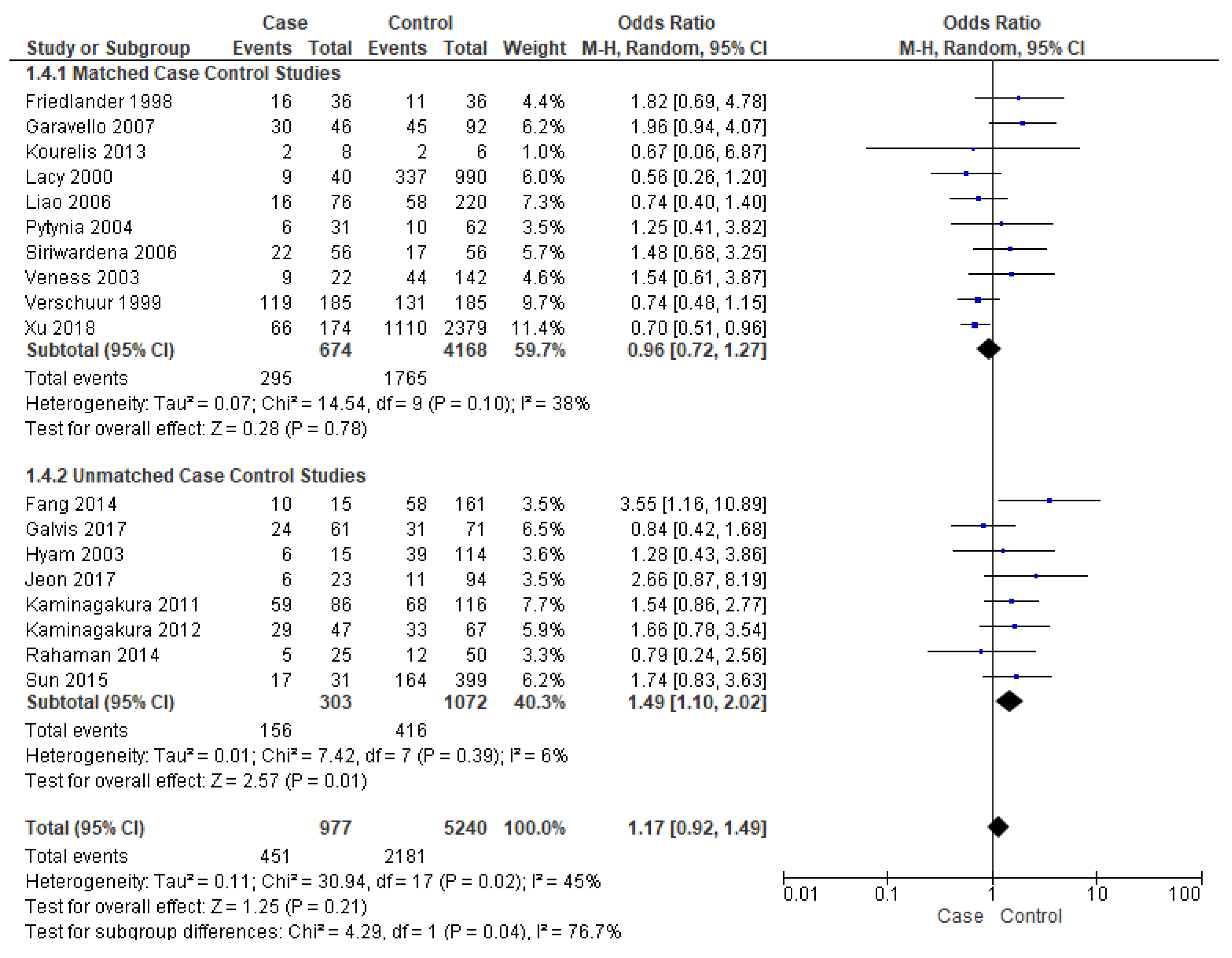
Events of recurrence were reported by eighteen studies [16,17,19,21,22,23,25,26,27,28,29,30,31,34,37,38,39,40]. Unmatched subgroup analysis comprised of eight studies [16,17,19,21,22,23,34,40] showed that young patients had a significantly high risk of recurrence, almost 49% (OR-1.49; 95% CI 1.10–2.02) (Figure 5), with a true population effect between 10% and 102%. The heterogeneity was very low (6%) for this comparison. However, the overall analysis and matched subgroup analysis did not find any difference.
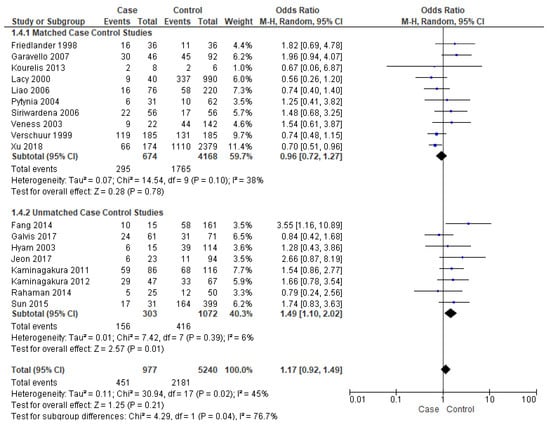
Figure 5.
Forest plot demonstrating the OR for events of recurrence in young patients.
3.3.4. Distant Metastasis
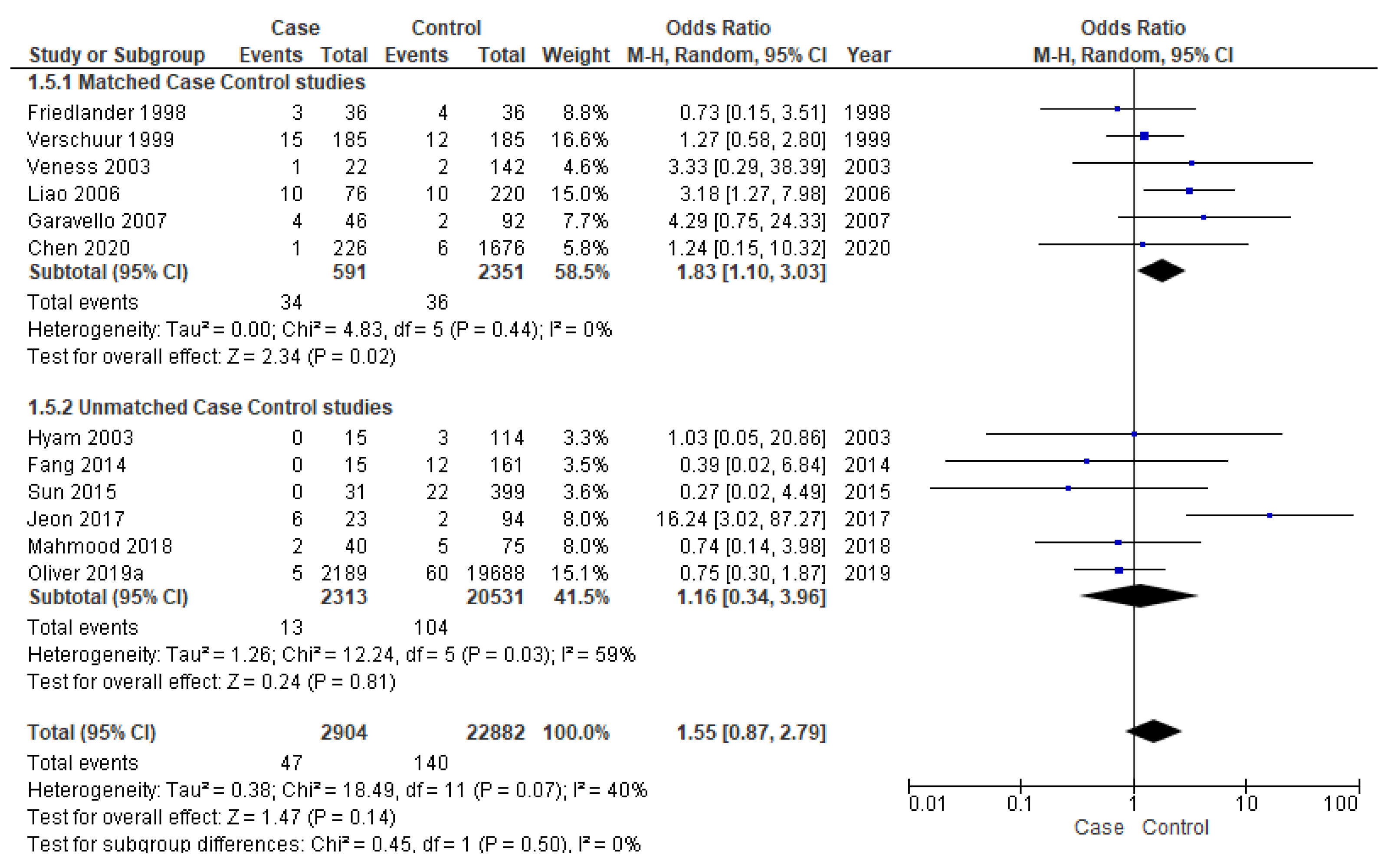
Twelve studies [16,17,18,19,25,28,33,34,36,37,38,39] were included for meta-analysis of DM, out of which six [25,28,33,37,38,39] were matched studies. The matched subgroup analysis illustrated a significantly higher risk (90%) of developing DM in young patients (OR-1.83; 95% CI 1.10–3.03) compared to the old ones. The heterogeneity remained nil (I2 = 0%) (Figure 6).
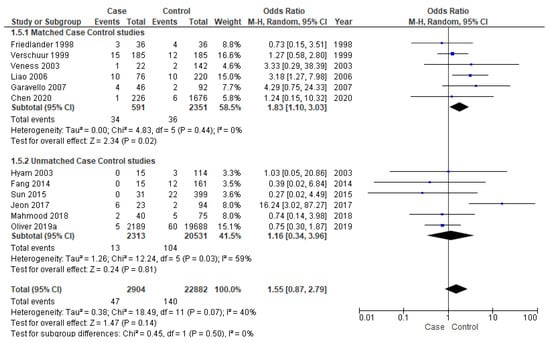
Figure 6.
Forest plot demonstrating the OR for distant metastasis in young patients.
3.3.5. Second Primary
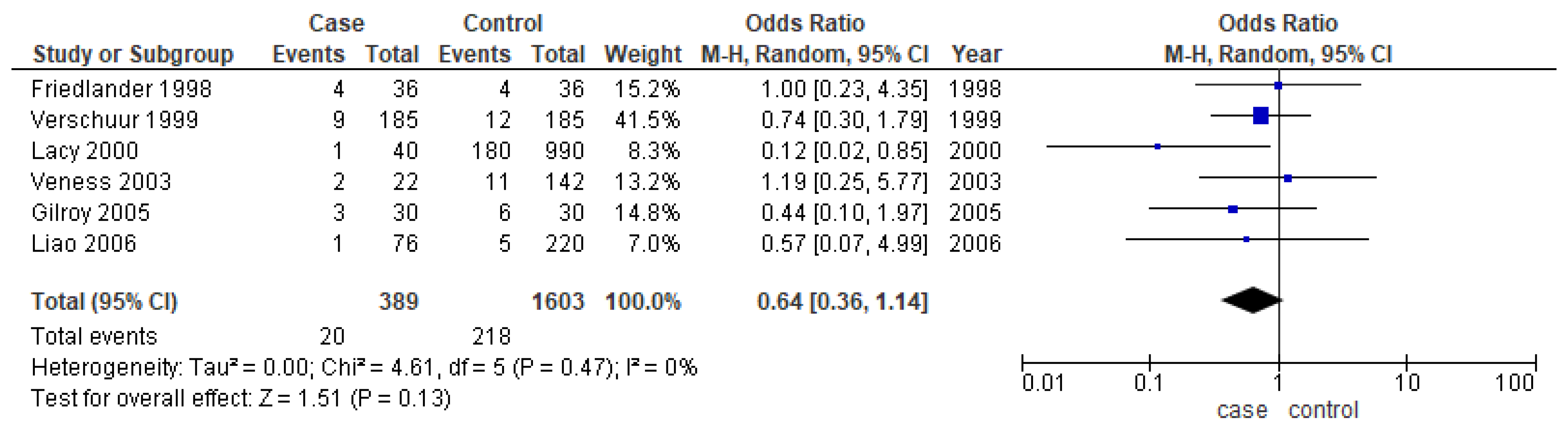
Only six studies [24,25,31,37,38,39] reported the second primary as one of their outcomes. The meta-analysis did not show any conclusive difference between the two age groups (OR-0.64; 95% CI: 0.36–1.14) (Figure 7).
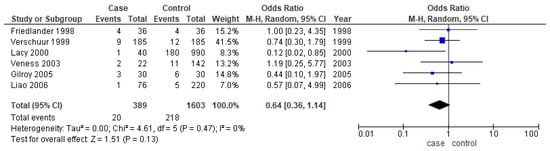
Figure 7.
Forest plot demonstrating the OR for the second primary in young patients.
3.3.6. Publication Bias
The symmetrical funnel plot of five negative outcomes (Figure A1, Figure A2, Figure A3, Figure A4 and Figure A5) indicates minimal publication bias in the matched subgroup analysis of recurrence and distant metastasis. The overall analysis of recurrence and second primary also demonstrated minimal publication bias.
4. Discussion
This systematic review and meta-analysis provides the cumulate evidence on age as a risk factor in stratifying negative outcomes in OOPSCC based on the observations from 25 comparative observational studies. One similar meta-analysis that compared the outcomes in older and younger adults [41] included the cutoff age for young patients as less than 30, 40, and 45 years. The present systematic review and meta-analysis has reasonably chosen less and equal to 40 years as the cutoff age of younger adults, which had been objectively identified by Marchiano et al. [42] as a transition point defining the young cohort as individuals with less and equal to 40 years. Another systematic review without meta-analysis has been reported by Sarode et al. [43], who has not determined the cutoff value of young age. The most recent meta-analysis on oral cavity squamous cell carcinoma, as reported by Lee et al. [44], suggested similar 5-year OS and DFS in both younger and older cohorts. To the best of our knowledge, this meta-analysis is the first to report a comprehensive comparison of five outcomes of OOPSCC between young and old patients. Demographic and clinicopathological features of young and old patients were also analyzed.
The present meta-analysis suggested that OS and DFS were significantly dependent upon age. Younger patients were found to present with better OS and worse DFS as compared to older adults. However, the heterogeneity incurred in the above analysis was high except for unmatched subgroup analysis for DFS (I2 = 2%) and matched subgroup analysis for OS (50%). The potential source of heterogeneity may be the absence of site specificity. In spite of the site-specific differences in molecular signature, most of the studies have pooled the tumors in different subsites of the oral cavity [45]. Other possible reasons for heterogeneity could be methodological issues such as patient selection (comorbidities and range of age group), type and extent of neck dissection, and large variation in the follow- up period. The significant heterogeneity could also be attributed to the differences in sample sizes, population features, and study setting. The present result does not coincide with Lee’s meta-analysis, which suggested that OS and DFS are similar in both the cohorts [44]. The difference in opinion may be attributed to Lee’s describing only oral cavity squamous cell carcinoma, in contrast to the present meta-analysis, which compared both oral cavity and oropharyngeal squamous cell carcinoma. In fact, better OS in younger adults was also observed in colorectal cancers, too. Considering the limited matched studies and heterogeneity, further research is warranted. Essentially, OS is comprised of DFS plus post-progression survival. Therefore, DFS has been suggested as a potential surrogate for OS in several cancers [46,47,48]. The correlation approach has been widely adopted to theoretically validate the efficiency of this surrogate endpoint [49]. However, better OS and worse DFS in young patients as revealed by the present result of meta-analysis may suggest that this may not prevail in OOPSCC.
This study also revealed 49% greater odds of recurrence (95% CI-1.10–2.02) in unmatched subgroup analysis and 90% greater odds of metastasis in matched subgroup analysis in young subjects. Frequent events of recurrence and DM in younger adults as evident from the results may be the reason for worse DFS compared to older patients. This inverse relationship of age with recurrence and DM can be hypothetically explained with two reasons. First, the metastatic process can be protected by the deterioration of the immune system, which happens in old age [50]. Second, the age-dependent reduction in matrix-modifying protease activity of the extracellular matrix may also prevent metastasis in old patients [51]. Age dependency of survival outcomes may be attributed to the differences at the molecular level. Few studies illustrated distinctive molecular events in the young [52,53], whereas others reported similar molecular profiles in both age groups [54,55]. Since young patients are exposed to etiological factors such as tobacco and alcohol for a shorter period, it has been speculated that risk factors other than these two could play a role in that age group [56]. A systematic review and meta-analysis recently demonstrated the role of another prognostic factor, HPV, in shortening OS and lowering DM, with it having no effect on recurrence and DFS [4]. The results indicated that young age may be considered as an independent determining factor for recurrence and DM, though more matched studies are required to reinforce the association with recurrence.
Empirical studies on follow-up on OOPSCC are scarce and most studies reported the combined data for all head and neck cancers, which encompass a group of malignancies with distinct etiology, prognosis and frequency and timing of second primaries [56,57]. The available studies have shown that SPs can adversely influence OS in older patients with OOPSCC [58,59]. However, our analysis could not generate any conclusive evidence regarding differences in the frequency of SP in the two age groups. While old age (>60 years) was found to be independently associated with frequent events of SP in head and neck carcinoma [56], another study reported that young patients (<65 years) were prone to develop SP in esophageal cancers [58]. Establishing a standardized age group classification for risk stratification is essential for conclusive evidence.
Evaluation of the demographic characteristics of the selected studies revealed that there was a paucity of studies on the Asian population, even though these countries report the highest incidence of OOPSCC in the world, which is attributed to the chewing of areca nut and smokeless tobacco, both “homemade” and commercial, and which vary considerably in composition, mode of use, and toxicity [59]. Further, a female preponderance, though non-significant, was found in our review, which is supported by a few others as well [60,61,62], whereas it is contradicted by others [11,63,64]. The staging and grading of the tumors did not show significant differences between the two groups, making both of them comparable. However, Troeltzsch et al. [65] reported that age, in general, was shown to influence staging, supported by Sasaki et al. [66] and a similar review by Pitman et al. [67]. Nevertheless, similar to our results, grading was not influenced by age in the study by Troeltzsch et al. [65], whereas our results were contradicted by Sasaki et al. [66]. So it is safer to infer that there may be the least influence of age on staging and grading in these tumors. Furthermore, the tongue was the most common subsite in the included studies and was exclusively studied in nine articles, which may have compromised the precision of this, though Bell et al. [68] reported a minimal influence of sites upon the outcome.
We present the most extensive analysis of the pooled data from twenty-three studies with subgroup analysis on matched and unmatched case-control studies along with a comprehensive comparison of both clinicopathologic and treatment-related outcomes in younger and older patients. The publication bias was minimal in the study, as illustrated by the funnel plots for all the outcomes. Nevertheless, this study has certain limitations. First, there were only a handful of case-control studies that were matched for any of the demographic parameters. Case-control studies matched based on demographic characters as well as sites, staging, grading and treatment modalities would have improved the precision of this study. Second, the information regarding the differential application of elective and therapeutic neck dissection was lacking in the included studies. Immortal time bias is not reported in any of the included studies, and this has become an unavoidable bias. Co-morbidity in either age group was not reported in ten articles, which is possibly the source of a major bias, especially while reporting OS. Inconsistency in the follow-up period in the included studies may also act as a confounding factor. While recurrence can be more frequently observed during 1 to 60 months [69], time to DM was reported to range from less than 12 months to more than 2 years [70]. Similarly, the risk of developing SP continued even for over 5 years after the diagnosis of primary oral cancer [71]. Therefore, the results of the present meta-analysis may be interpreted keeping these limitations under consideration.
5. Conclusions
Our results provided a comprehensive review of the differences in clinicopathologic features and survival outcomes in younger and older patients with OOPSCC. Although not sufficiently robust to recommend age-specific therapeutic and follow-up strategies, the preliminary evidence of better OS and worse DFS in young subjects mandates larger and methodologically stronger observational studies with longer follow-up periods and matched comparisons. Young age can be considered as an independent prognostic factor for assessing recurrence and DM.
Author Contributions
Conceptualization, S.P. (Swagatika Panda) and N.M.; methodology, S.P. (Swagatika Panda), S.P. (Saurav Panda), D.G., A.S. and S.K.N.; software, S.P. (Swagatika Panda) and S.P. (Saurav Panda); validation, N.M., S.K.N. and L.M.; formal analysis, S.P. (Swagatika Panda), D.G., L.M. and B.L.; investigation, S.P. (Saurav Panda) and D.G.; resources, S.P. (Swagatika Panda), A.S. and B.L.; data curation, L.M.; writing—original draft preparation, S.P. (Swagatika Panda); writing—review and editing, S.P. (Saurav Panda) and B.L.; visualization, N.M.; supervision, N.M.; project administration, S.P. (Swagatika Panda). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are very thankful to Siksha ‘O’ Anusandhan deemed to be University and Medical University of Lodz for the support in conducting the study.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
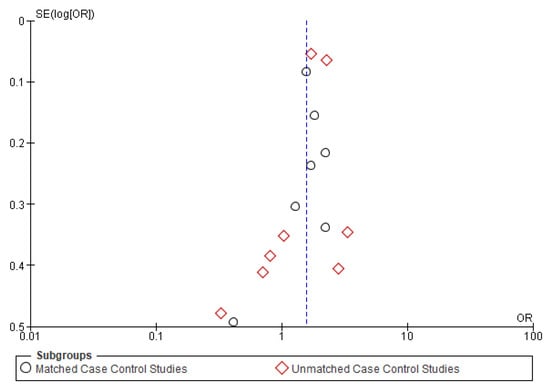
Figure A1.
Funnel plot of overall survival.
Figure A1.
Funnel plot of overall survival.
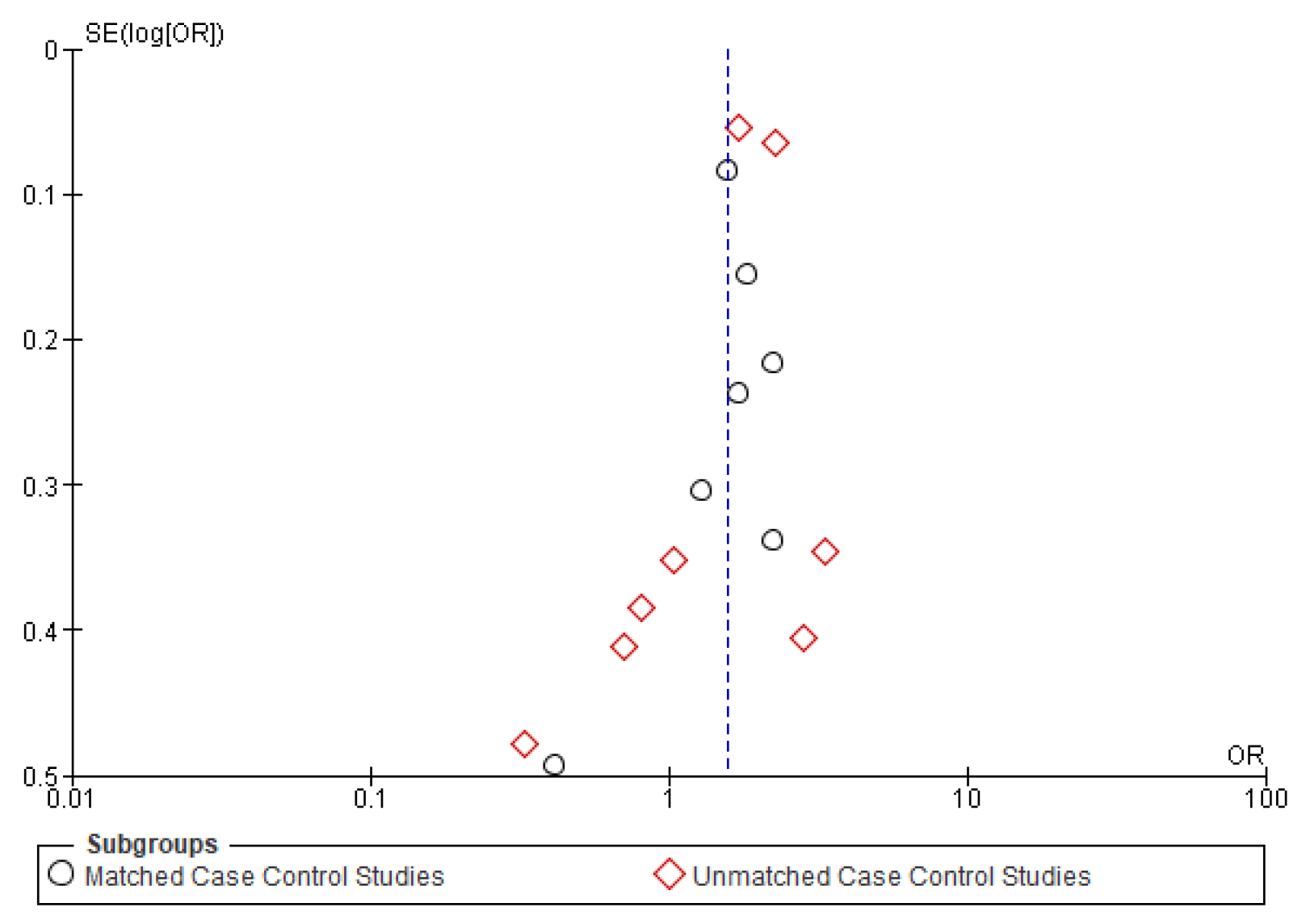
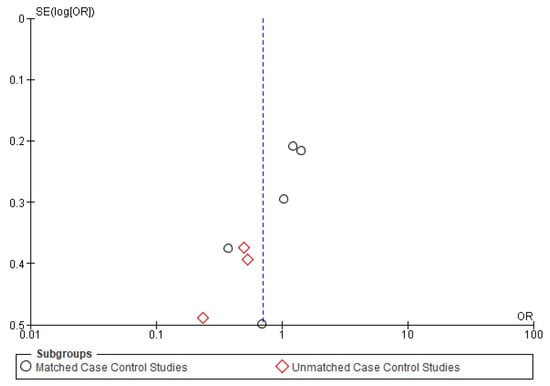
Figure A2.
Funnel plot of disease-free survival.
Figure A2.
Funnel plot of disease-free survival.
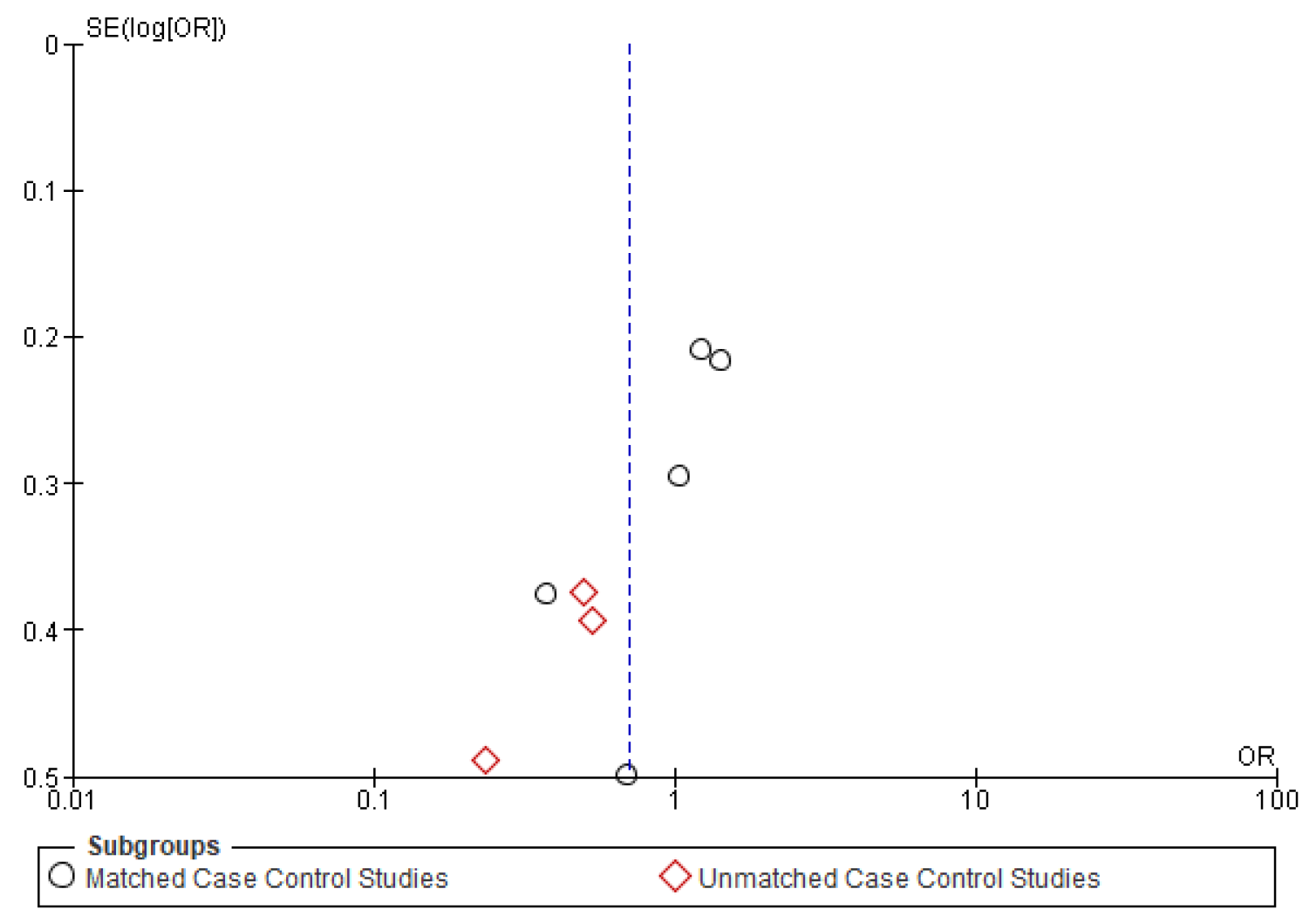
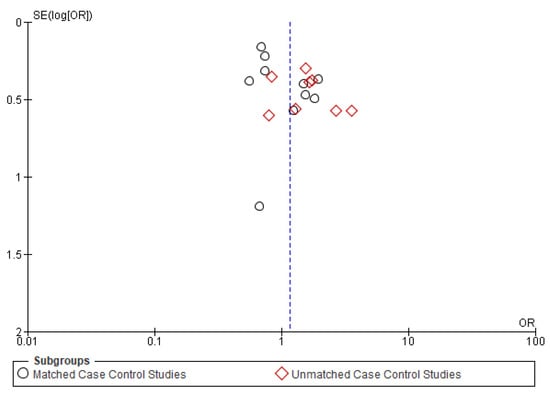
Figure A3.
Funnel plot of recurrence.
Figure A3.
Funnel plot of recurrence.
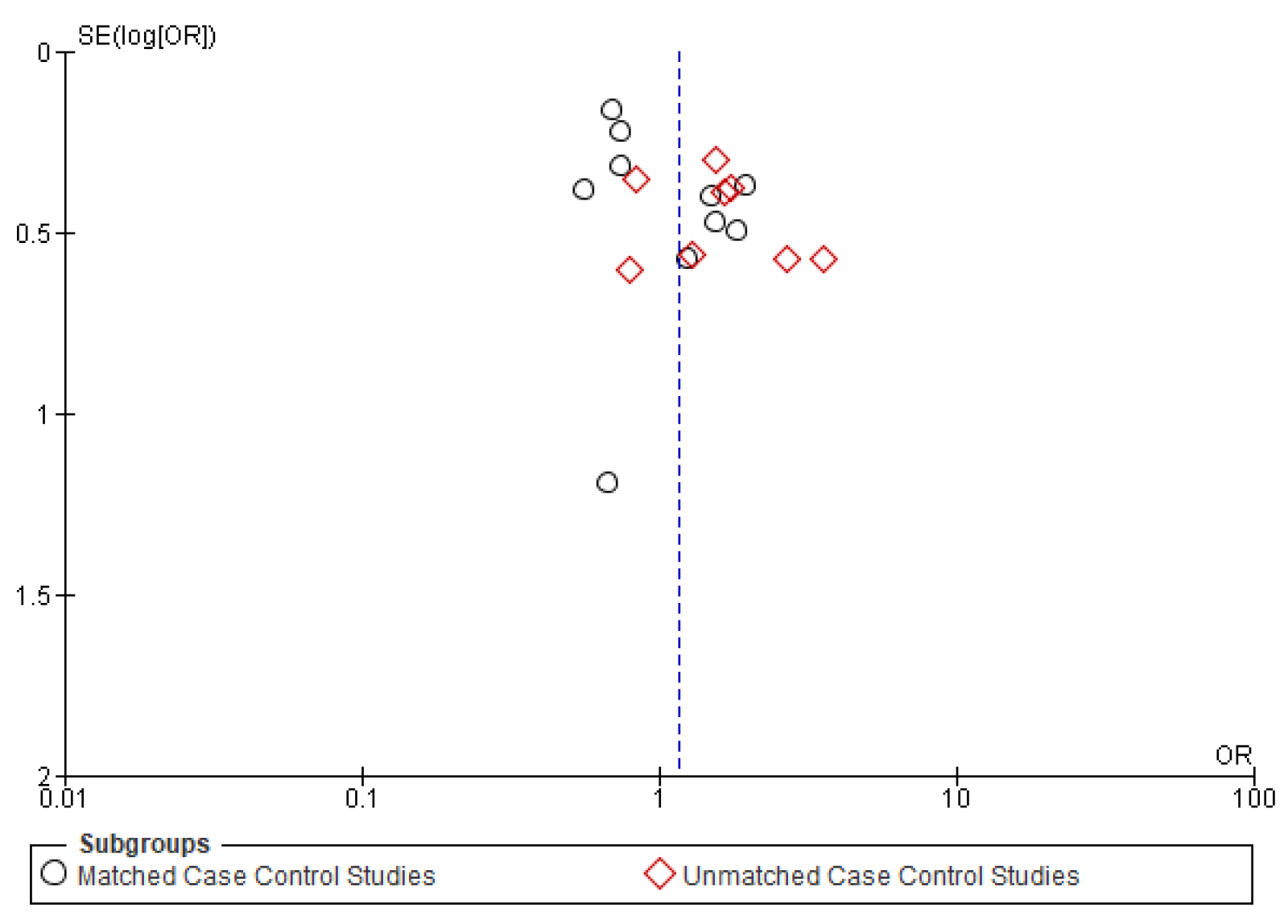
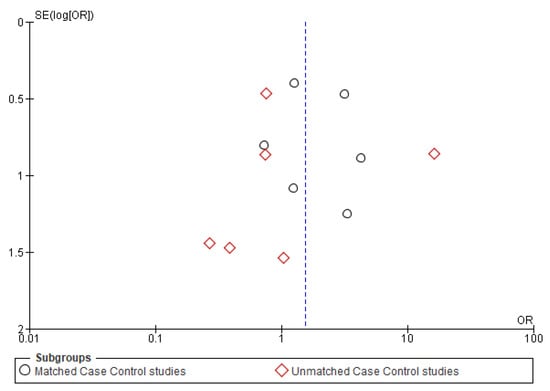
Figure A4.
Funnel plot of distant metastasis.
Figure A4.
Funnel plot of distant metastasis.
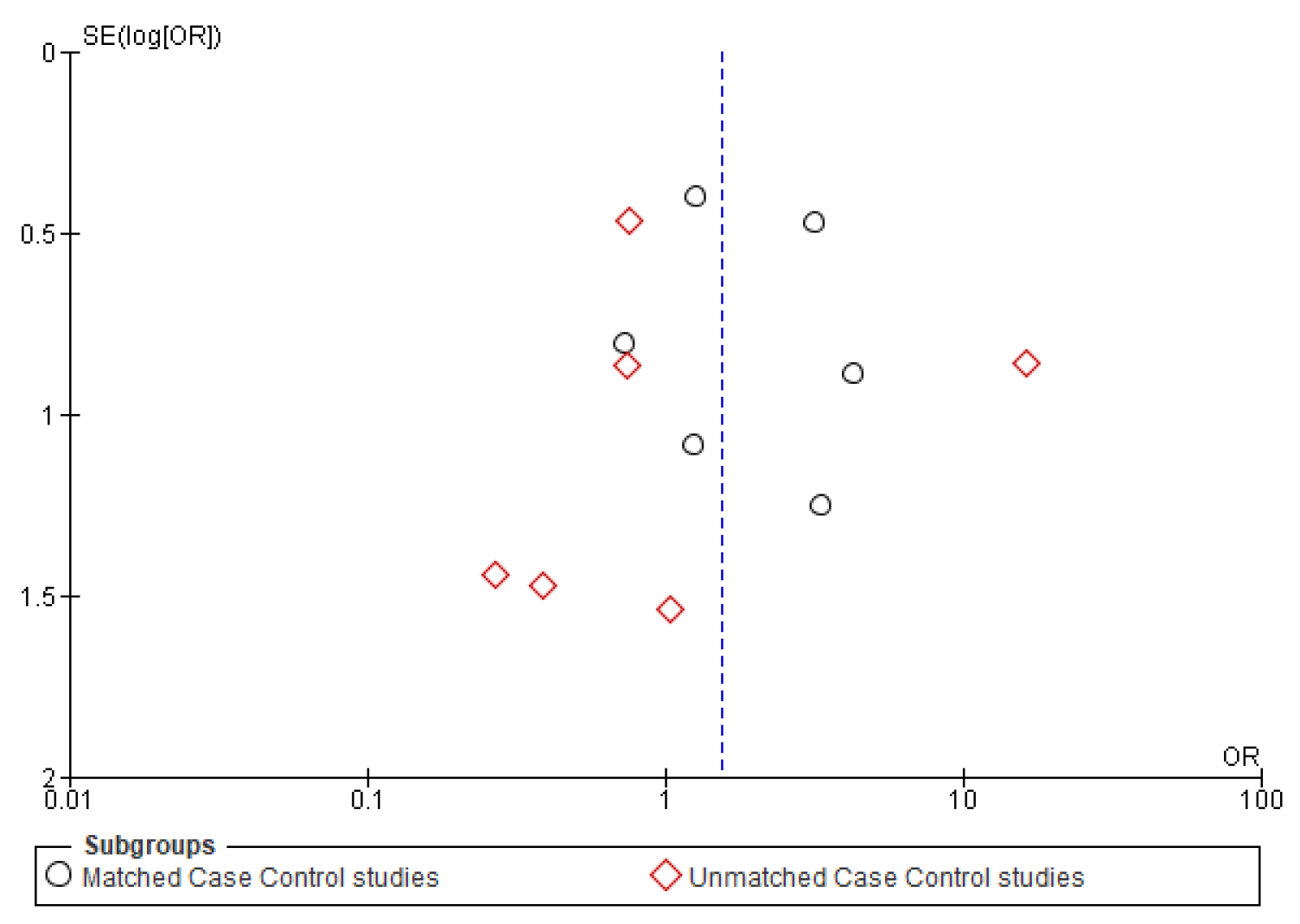
Figure A5.
Funnel plot of second primary.
Figure A5.
Funnel plot of second primary.
References
- Hussein, A.A.; Helder, M.N.; de Visscher, J.G.; Leemans, C.R.; Braakhuis, B.J.; de Vet, H.C.W.; Forouzanfar, T. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: A systematic review. Eur. J. Cancer 2017, 82, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, D.; Kunnath Menon, R.; Veettil, S.K.; George Botelho, M.; Johnson, N.W. Periodontal Diseases as Putative Risk Factors for Head and Neck Cancer: Systematic Review and Meta-Analysis. Cancers 2020, 12, 1893. [Google Scholar] [CrossRef]
- Christianto, S.; Li, K.Y.; Huang, T.H.; Su, Y.-X. The Prognostic Value of Human Papilloma Virus Infection in Oral Cavity Squamous Cell Carcinoma: A Meta-Analysis. Laryngoscope 2019, 48, 76. [Google Scholar] [CrossRef]
- Burke, H.B.; Henson, D.E. Criteria for prognostic factors and for an enhanced prognostic system. Cancer 1993, 72, 3131–3135. [Google Scholar] [CrossRef]
- Piccirillo, J.F.; Feinstein, A.R. Clinical symptoms and comorbidity: Significance for the prognostic classification of cancer. Cancer 1996, 77, 834–842. [Google Scholar] [CrossRef]
- Piccirillo, J.F. Importance of comorbidity in head and neck cancer. Laryngoscope 2000, 110, 593–602. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, J.; Lin, H.; Gold, K.A.; Sturgis, E.M.; Garden, A.S.; Lee, J.J.; William, W.N. Relation between the level of lymph node metastasis and survival in locally advanced head and neck squamous cell carcinoma. Cancer 2016, 122, 534–545. [Google Scholar] [CrossRef]
- Marcus, B.; Arenberg, D.; Lee, J.; Kleer, C.; Chepeha, D.B.; Schmalbach, C.E.; Islam, M.; Paul, S.; Pan, Q.; Hanash, S.; et al. Prognostic factors in oral cavity and oropharyngeal squamous cell carcinoma: The impact of tumor-associated macrophages. Cancer 2004, 101, 2779–2787. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2017; National Cancer Institute: Bethesda, MD, USA. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 10 June 2020).
- McGregor, G.I.; Davis, N.; Robins, R.E. Squamous cell carcinoma of the tongue and lower oral cavity in patients under 40 years of age. Am. J. Surg. 1983, 146, 88–92. [Google Scholar] [CrossRef]
- Carniol, P.J.; Fried, M.P. Head and Neck Carcinoma in Patients under 40 Years of Age. Ann. Otol. Rhinol. Laryngol. 1982, 91, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, J.T.; Strawitz, J.G. Squamous cell carcinoma of the oral cavity in young adults. J. Surg. Oncol. 1982, 19, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-S.; Lai, C.-H.; Lee, C.-P.; Yang, Y.-H.; Chen, P.-C.; Kang, C.-J.; Chang, G.-H.; Tsai, Y.-T.; Lu, C.-H.; Chien, C.-Y.; et al. Mortality in tongue cancer patients treated by curative surgery: A retrospective cohort study from CGRD. PeerJ 2016, 4, e2794. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions|Cochrane Training. Available online: https://training.cochrane.org/handbook/current (accessed on 10 June 2020).
- Fang, Q.G.; Shi, S.; Liu, F.Y.; Sun, C.F. Tongue squamous cell carcinoma as a possible distinct entity in patients under 40 years old. Oncol. Lett. 2014, 7, 2099–2102. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-H.; Kim, M.G.; Park, J.Y.; Lee, J.H.; Kim, M.J.; Myoung, H.; Choi, S.W. Analysis of the outcome of young age tongue squamous cell carcinoma. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 41. [Google Scholar] [CrossRef]
- Mahmood, N.; Muhammad, H.; Ahmed, A.; Jamal, Q.; Saqib, M.; Khan, A. Impact of age at diagnosis on clinicopathological outcomes of oral squamous cell carcinoma patients in Karachi. Pak. J. Med. Sci. 2018, 34, 595–599. [Google Scholar] [CrossRef]
- Sun, Q.; Fang, Q.; Guo, S. A comparison of oral squamous cell carcinoma between young and old patients in a single medical center in China. Int. J. Clin. Exp. Med. 2015, 8, 12418–12423. [Google Scholar]
- Komolmalai, N.; Chuachamsai, S.; Tantiwipawin, S.; Dejsuvan, S.; Buhngamongkol, P.; Wongvised, C.; Chitapanarux, I.; Iamaroon, A. Ten-year analysis of oral cancer focusing on young people in northern Thailand. J. Oral Sci. 2015, 57, 327–334. [Google Scholar] [CrossRef]
- Ur Rahaman, S.; Ahmed Mujib, B. Histopathological correlation of oral squamous cell carcinoma among younger and older patients. J. Oral Maxillofac. Pathol. 2014, 18, 183. [Google Scholar] [CrossRef]
- Kaminagakura, E.; Villa, L.L.; Andreoli, M.A.; Sobrinho, J.S.; Vartanian, J.G.; Soares, F.A.; Nishimoto, I.N.; Rocha, R.; Kowalski, L.P. High-risk human papillomavirus in oral squamous cell carcinoma of young patients. Int. J. Cancer 2012, 130, 1726–1732. [Google Scholar] [CrossRef]
- Kaminagakura, E.; Werneck da Cunha, I.; Soares, F.A.; Nishimoto, I.N.; Kowalski, L.P. CCND1 amplification and protein overexpression in oral squamous cell carcinoma of young patients. Head Neck 2011, 33, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, J.S.; Morris, C.G.; Amdur, R.J.; Mendenhall, W.M. Impact of young age on prognosis for head and neck cancer: A matched-pair analysis. Head Neck 2005, 27, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Verschuur, H.P.; Irish, J.C.; O’Sullivan, B.; Goh, C.; Gullane, P.J.; Pintilie, M. A Matched Control Study of Treatment Outcome in Young Patients With Squamous Cell Carcinoma of the Head and Neck. Laryngoscope 1999, 109, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Pytynia, K.B.; Grant, J.R.; Etzel, C.J.; Roberts, D.; Wei, Q.; Sturgis, E.M. Matched Analysis of Survival in Patients With Squamous Cell Carcinoma of the Head and Neck Diagnosed Before and After 40 Years of Age. Arch. Otolaryngol. Neck Surg. 2004, 130, 869. [Google Scholar] [CrossRef]
- Siriwardena, B.S.M.S.; Tilakaratne, A.; Amaratunga, E.A.P.D.; Tilakaratne, W.M. Demographic, aetiological and survival differences of oral squamous cell carcinoma in the young and the old in Sri Lanka. Oral Oncol. 2006, 42, 831–836. [Google Scholar] [CrossRef]
- Garavello, W.; Spreafico, R.; Somigliana, E.; Gaini, L.; Pignataro, L.; Gaini, R.M. Prognostic influence of gender in patients with oral tongue cancer. Otolaryngol. Neck Surg. 2008, 138, 768–771. [Google Scholar] [CrossRef]
- Kourelis, K.; Tsue, T.; Girod, D.; Tawfik, O.; Sykes, K.; Shnayder, Y. Negative prognostic factors for head and neck cancer in the young. J. BUON 2013, 18, 459–464. [Google Scholar]
- Xu, Q.; Wang, C.; Li, B.; Kim, K.; Li, J.; Mao, M.; Qin, L.; Li, H.; Huang, X.; Xing, R.; et al. The impact of age on oral squamous cell carcinoma: A longitudinal cohort study of 2782 patients. Oral Dis. 2019, 25, 730–741. [Google Scholar] [CrossRef]
- Lacy, P.D.; Piccirillo, J.F.; Merritt, M.G.; Zequeira, M.R. Head and neck squamous cell carcinoma: Better to be young. Otolaryngol. Neck Surg. 2000, 122, 253–258. [Google Scholar] [CrossRef]
- Ansarin, M.; De Berardinis, R.; Corso, F.; Giugliano, G.; Bruschini, R.; De Benedetto, L.; Zorzi, S.; Maffini, F.; Sovardi, F.; Pigni, C.; et al. Survival Outcomes in Oral Tongue Cancer: A Mono-Institutional Experience Focusing on Age. Front. Oncol. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Chen, S.; Lin, Z.; Chen, J.; Yang, A.; Zhang, Q.; Xie, C.; Zhang, X.; Yang, Z.; Chen, W.; Song, M. Older age is a risk factor associated with poor prognosis of patients with squamous cell carcinoma of the oral cavity. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Hyam, D.; Conway, R.; Sathiyaseelan, Y.; Gebski, V.; Morgan, G.; Walker, D.; Veness, M. Tongue cancer: Do patients younger than 40 do worse? Aust. Dent. J. 2003, 48, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Mukdad, L.; Heineman, T.E.; Alonso, J.; Badran, K.W.; Kuan, E.C.; St. John, M.A. Oral tongue squamous cell carcinoma survival as stratified by age and sex: A surveillance, epidemiology, and end results analysis. Laryngoscope 2019, 129, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.R.; Wu, S.P.; Chang, C.M.; Roden, D.F.; Wang, B.; Hu, K.S.; Schreiber, D.; Givi, B. Survival of oral tongue squamous cell carcinoma in young adults. Head Neck 2019, 41, 2960–2968. [Google Scholar] [CrossRef]
- Veness, M.J.; Morgan, G.J.; Sathiyaseelan, Y.; Gebski, V. Anterior tongue cancer: Age is not a predictor of outcome and should not alter treatment. ANZ J. Surg. 2003, 73, 899–904. [Google Scholar] [CrossRef]
- Liao, C.-T.; Wang, H.-M.; Hsieh, L.-L.; Chang, J.T.-C.; Ng, S.-H.; Hsueh, C.; Lee, L.-Y.; Lin, C.H.; Chen, I.-H.; Kang, C.-J.; et al. Higher distant failure in young age tongue cancer patients. Oral Oncol. 2006, 42, 718–725. [Google Scholar] [CrossRef]
- Friedlander, P.L.; Schantz, S.P.; Shaha, A.R.; Yu, G.; Shah, J.P. Squamous cell carcinoma of the tongue in young patients: A matched-pair analysis. Head Neck 1998, 20, 363–368. [Google Scholar] [CrossRef]
- Galvis, M.; Jardim, J.; Kaminagakura, E.; Santos-Silva, A.R.; Fonseca, F.; Almeida, O.; Lopes, M.; Pinto, C.; Kowalski, L.P. Expression of cell cycle proteins according to HPV status in oral squamous cell carcinoma affecting young patients: A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 317–325. [Google Scholar] [CrossRef]
- Mohideen, K.; Krithika, C.; Jeddy, N.; Balakrishnan, T.; Bharathi, R.; Sankari, S. A meta-analysis of oral squamous cell carcinoma in young adults with a comparison to the older group patients (2014–2019). Contemp. Clin. Dent. 2021, 12, 213–221. [Google Scholar] [CrossRef]
- Marchiano, E.; Argirion, I.; Vandenberg, T.; Bhangale, A.D.; Birkeland, A.C.; Prince, M.E.P.; Bradford, C.R.; McHugh, J.B.; Chepeha, D.B.; Sartor, M.A.; et al. Defining the Young Patient with Oral Cavity Cancer: Phenotypic and Genotypic Analysis. SSRN Electron. J. 2021, 100, 1354–1355. [Google Scholar] [CrossRef]
- Sarode, G.; Maniyar, N.; Sarode, S.C.; Choudhary, N.; Mehta, V.; Gopalakrishnan, D.; Yerwadekar, S.; Joshi, S.; Pendyala, G.; Patil, S. Oral Cancer in Young vs Old Individuals: A Systematic Review. J. Contemp. Dent. Pract. 2021, 22, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Ramirez, R.J.; Lee, J.J.; Valenzuela, C.V.; Zevallos, J.P.; Mazul, A.L.; Puram, S.V.; Doering, M.M.; Pipkorn, P.; Jackson, R.S. Survival of Young Versus Old Patients With Oral Cavity Squamous Cell Carcinoma: A Meta-Analysis. Laryngoscope 2021, 131, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, D.; Kunnath Menon, R. Unravelling the molecular signatures in HNSCC: Is the homogenous paradigm becoming obsolete? Oral Oncol. 2018, 82, 195. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.A.; Bentzen, S.M.; Chen, E.X.; Siu, L.L. Surrogate End Points for Median Overall Survival in Metastatic Colorectal Cancer: Literature-Based Analysis From 39 Randomized Controlled Trials of First-Line Chemotherapy. J. Clin. Oncol. 2007, 25, 4562–4568. [Google Scholar] [CrossRef]
- Buyse, M.; Burzykowski, T.; Carroll, K.; Michiels, S.; Sargent, D.J.; Miller, L.L.; Elfring, G.L.; Pignon, J.-P.; Piedbois, P. Progression-Free Survival Is a Surrogate for Survival in Advanced Colorectal Cancer. J. Clin. Oncol. 2007, 25, 5218–5224. [Google Scholar] [CrossRef]
- Nie, R.-C.; Zou, X.-B.; Yuan, S.-Q.; Chen, Y.-B.; Chen, S.; Chen, Y.-M.; Chen, G.-M.; Chen, X.-J.; Luo, T.-Q.; Li, S.-M.; et al. Disease-free survival as a surrogate endpoint for overall survival in adjuvant trials of pancreatic cancer: A meta-analysis of 20 randomized controlled trials. BMC Cancer 2020, 20, 421. [Google Scholar] [CrossRef]
- Santos-Silva, A.R.; Ribeiro, A.C.P.; Soubhia, A.M.P.; Miyahara, G.I.; Carlos, R.; Speight, P.M.; Hunter, K.D.; Torres-Rendon, A.; Vargas, P.A.; Lopes, M.A. High incidences of DNA ploidy abnormalities in tongue squamous cell carcinoma of young patients: An international collaborative study. Histopathology 2011, 58, 1127–1135. [Google Scholar] [CrossRef]
- Tremblay, S.; Pintor dos Reis, P.; Bradley, G.; Galloni, N.N.; Perez-Ordonez, B.; Freeman, J.; Brown, D.; Gilbert, R.; Gullane, P.; Irish, J.; et al. Young Patients With Oral Squamous Cell Carcinoma. Arch. Otolaryngol. Neck Surg. 2006, 132, 958. [Google Scholar] [CrossRef]
- O’Regan, E.M.; Toner, M.E.; Smyth, P.C.; Finn, S.P.; Timon, C.; Cahill, S.; Flavin, R.; O’Leary, J.J.; Sheils, O. Distinct array comparative genomic hybridization profiles in oral squamous cell carcinoma occurring in young patients. Head Neck 2006, 28, 330–338. [Google Scholar] [CrossRef]
- dos Santos Costa, S.F.; Brennan, P.A.; Gomez, R.S.; Fregnani, E.R.; Santos-Silva, A.R.; Martins, M.D.; de Castro-Junior, G.; Rahimi, S.; Fonseca, F.P. Molecular basis of oral squamous cell carcinoma in young patients: Is it any different from older patients? J. Oral Pathol. Med. 2018, 47, 541–546. [Google Scholar] [CrossRef]
- Ho, A.S.; Kraus, D.H.; Ganly, I.; Lee, N.Y.; Shah, J.P.; Morris, L.G.T. Decision making in the management of recurrent head and neck cancer. Head Neck 2014, 36, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Taslim, S.J.; Leemans, C.R.; van der Waal, I.; Karagozoglu, K.H. Follow-up of oral cancer patients: Three uneventful years may be enough. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Rennemo, E.; Zätterström, U.; Boysen, M. Impact of Second Primary Tumors on Survival in Head and Neck Cancer: An Analysis of 2063 Cases. Laryngoscope 2008, 118, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-J.; Chou, H.-W.; Wang, C.-T.; Chung, C.-S.; Lai, M.-S. The Impact of Second Primary Malignancies on Head and Neck Cancer Survivors: A Nationwide Cohort Study. PLoS ONE 2013, 8, e62116. [Google Scholar] [CrossRef]
- Lee, D.H.; Roh, J.-L.; Baek, S.; Jung, J.H.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Second Cancer Incidence, Risk Factor, and Specific Mortality in Head and Neck Squamous Cell Carcinoma. Otolaryngol. Neck Surg. 2013, 149, 579–586. [Google Scholar] [CrossRef]
- Iwatsubo, T.; Ishihara, R.; Morishima, T.; Maekawa, A.; Nakagawa, K.; Arao, M.; Ohmori, M.; Iwagami, H.; Matsuno, K.; Inoue, S.; et al. Impact of age at diagnosis of head and neck cancer on incidence of metachronous cancer. BMC Cancer 2019, 19, 3. [Google Scholar] [CrossRef]
- Sharma, S.; Satyanarayana, L.; Asthana, S.; Shivalingesh, K.K.; Goutham, B.S.; Ramachandra, S. Oral cancer statistics in India on the basis of first report of 29 population-based cancer registries. J. Oral Maxillofac. Pathol. 2018, 22, 18–26. [Google Scholar] [CrossRef]
- Lin, N.-C.; Hsu, J.-T.; Tsai, K.-Y. Difference between Female and Male Patients with Oral Squamous Cell Carcinoma: A Single-Center Retrospective Study in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 3978. [Google Scholar] [CrossRef]
- Venables, C.W.; Craft, I.L. Carcinoma of the tongue in early adult life. Br. J. Cancer 1967, 21, 645–650. [Google Scholar] [CrossRef]
- Kuriakose, M.; Sankaranarayanan, M.; Nair, M.K.; Cherian, T.; Sugar, A.W.; Scully, C.; Prime, S.S. Comparison of oral squamous cell carcinoma in younger and older patients in India. Eur. J. Cancer Part B Oral Oncol. 1992, 28, 113–120. [Google Scholar] [CrossRef]
- Schantz, S.P.; Yu, G.-P. Head and Neck Cancer Incidence Trends in Young Americans, 1973–1997, With a Special Analysis for Tongue Cancer. Arch. Otolaryngol. Neck Surg. 2002, 128, 268. [Google Scholar] [CrossRef]
- Llewellyn, C.; Johnson, N.; Warnakulasuriya, K.A.A. Risk factors for squamous cell carcinoma of the oral cavity in young people—A comprehensive literature review. Oral Oncol. 2001, 37, 401–418. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Knösel, T.; Eichinger, C.; Probst, F.; Troeltzsch, M.; Woodlock, T.; Mast, G.; Ehrenfeld, M.; Otto, S. Clinicopathologic Features of Oral Squamous Cell Carcinoma: Do They Vary in Different Age Groups? J. Oral Maxillofac. Surg. 2014, 72, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Moles, D.R.; Imai, Y.; Speight, P.M. Clinico-pathological features of squamous cell carcinoma of the oral cavity in patients <40 years of age. J. Oral Pathol. Med. 2005, 34, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Pitman, K.T.; Johnson, J.T.; Wagner, R.L.; Myers, E.N. Cancer of the tongue in patients less than forty. Head Neck 2000, 22, 297–302. [Google Scholar] [CrossRef]
- Bell, R.B.; Kademani, D.; Homer, L.; Dierks, E.J.; Potter, B.E. Tongue Cancer: Is There a Difference in Survival Compared With Other Subsites in the Oral Cavity? J. Oral Maxillofac. Surg. 2007, 65, 229–236. [Google Scholar] [CrossRef]
- Weckx, A.; Riekert, M.; Grandoch, A.; Schick, V.; Zöller, J.E.; Kreppel, M. Time to recurrence and patient survival in recurrent oral squamous cell carcinoma. Oral Oncol. 2019, 94, 8–13. [Google Scholar] [CrossRef]
- Agarwal, J.; Krishnatry, R.; Gupta, T.; Murthy, V.; Ghosh-Laskar, S.; Budrukkar, A.; Chaturvedi, P.; Nair, S.; Nair, D.; Kumar, P.; et al. Factors predicting ‘time to distant metastasis’ in radically treated head and neck cancer. Indian J. Cancer 2014, 51, 231. [Google Scholar] [CrossRef]
- Chen, P.-T.; Kuan, F.-C.; Huang, C.-E.; Chen, M.-F.; Huang, S.-H.; Chen, M.-C.; Lee, K.-D. Incidence and Patterns of Second Primary Malignancies Following Oral Cavity Cancers in a Prevalent Area of Betel-nut Chewing: A Population-based Cohort of 26,166 Patients in Taiwan. Jpn. J. Clin. Oncol. 2011, 41, 1336–1343. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).